A Pilot Study of the Signal ReliefTM Patch for the Treatment of Musculoskeletal Pain
Author'(s): Beth Baughman DuPree1*, Jason Wesley2, Estelle Farrell3 and Paul Lewis4
1Redeemer Health, Meadowbrook, PA, Signal Relief, Provo, Utah, US.
2Helios Health AZ, Sedona, Arizona.
3Arizona, Valley of the Sun Institute for Pain Management,Scottsdale, Arizona.
4Proxima Clinical Research Inc., Houston, Texas.
*Correspondence:
Beth Baughman DuPree, Redeemer Health, Meadowbrook, PA,Signal Relief, Provo, Utah, US.
Received: 21 May 2023; Accepted: 07 Jun 2023; Published: 30 Jun 2023
Citation: DuPree BB, Wesley J, Farrell E, et al. A Pilot Study of the Signal Relief TM Patch for the Treatment of Musculoskeletal Pain. Anesth Pain Res. 2023; 7(1): 1-5.
Abstract
The aim of this IRB approved study was to evaluate the efficacy of the non-invasive, drug-free, re-usable patch, Signal ReliefTM, for the management of general musculoskeletal pain. Pain severity was assessed at baseline and upon completion of 7-day treatment schedule using Visual Analog Pain Scale (VAS), Brief Pain Inventory form (BPI) and Pain Interference Score (PIS). Reduction in pain medication use was evaluated via pain medication diary. An optional, exploratory endpoint of Substance P blood serum levels at baseline and end of treatment was also assessed. We enrolled 42 subjects with 2 board-certified physicians, one primary care and one a pain specialist. Thirty-two subjects completed the trial. Seventy five percent (24/32) subjects had a ≥ 30% reduction in pain on VAS. The average reduction in pain per VAS, BPI and PSI for the cohort was 59.7% (-65.6, -53.7); 44% (-53.1, -34.9); and 47.4% (-53.7, -41.1) respectively. Sixty eight percent (22/32) subjects were taking pain medication upon study entry. Reduction in pain medication use demonstrated was a 96.5% decrease for non-opioids and 91.4% decrease for opioids. Substance P levels were collected for 24 subjects. While 50% of subjects (12/24) showed a reduction in Substance P levels there was an overall increase of 11.7% across the entire cohort. Safety assessments included physical examination, monitoring of vital signs, and adverse event reporting per study visit schedule. No adverse events were reported. Our findings suggest that the Signal ReliefTM may be an effective non-invasive, drug-free option for the management of general musculoskeletal pain.
Keywords
Introduction
In 2019, the National Center of Health Statistics reported that 20.4% of adults in the United States have chronic pain and 7.4% have high impact chronic pain (pain that limits life or work activities). Data from the National Health Interview Survey showed that chronic pain is higher in women, persons over the age of 65, and non-Hispanic white adults. The prevalence of chronic pain also increases with age and as the place of residence becomes more rural [1]. While the underlying cause of chronic pain can vary (e.g., inflammation, underlying disease or condition, injury, medical treatment, etc.), it is associated with a decrease in quality of life and depressive and anxiety disorders [2,3].
First-line therapy for chronic pain includes the use of over-the- counter analgesics such as acetaminophen and ibuprofen or COX-2 inhibitors such as celecoxib. When these treatments are ineffective in managing pain, opioids have dominated the treatment paradigm. After the publication of two small studies in the early 1980s demonstrating that opioids were not addictive, opioids became a welcome relief for the management of chronic pain. Unfortunately, this misinformation led to the long-term use of opioids that laid the foundation of our current opioid crisis [4]. In a recent survey, 22.1% of adults with chronic pain reported using opioids over the past three months [5]. Since 1991 the number of drug overdose deaths has quadrupled, and 70% of the 70,630 deaths in 2019 involved an opioid [6]. It is estimated that 4-6 percent of those who misuse opioids transition to heroin and approximately 80% of people who use heroin first misused opioids [7-9]. The Center for Disease
Control (CDC) has initiated an extensive program to help curb the crisis through public education, state funding, and information tracking strategies [10]. The National Institute of Health started the Helping to End Addiction Long-term (HEAL) initiative to provide funding to researchers addressing questions around addiction and to support the development of alternative non-opioid therapies for pain management [11]. The economic impact of opioid addiction was estimated in 2017 to cost the United States $1,021 trillion a year, according to the Center for Disease Control [12].
Therefore, there is an urgent need for safe and effective non-opioid options for pain management. The integration of micrometals in analgesic therapies is well documented. [13,14]. Further, it is believed that addressing the compromised communication pathways in the nervous system, could help mitigate pain. [15] The Signal Relief Patch expands upon these principals by incorporating a conductive particle array that acts as a bio-antenna in the form of a flexible patch.
The goal of this study was to investigate whether this drug-free, non-invasive, re-usable pain patch could benefit patients suffering from general musculoskeletal pain, reducing pain interference with their daily activities as well as reducing their use and dependence on non-opioid as well as opioid pain medications.
Methods
Between January and July of 2022, we conducted a 1-week treatment schedule in 2 outpatient pain clinics in Arizona (Scottsdale and Sedona). Written informed consent was obtained from each subject prior to participating in the study.
Participants were recruited from the principal investigator’s referrals and from advertising on social media. Participants were included if they were ≥ 18 years of age, currently seeking treatment for chronic musculoskeletal pain management, had initial ten-point VAS pain scale score of ≥ 4, willing to refrain from or reduce pain medication use for the study duration, and able to provide informed consent. Participants were excluded if they were receiving other investigational agents or were participating in another clinical trial, in emergent need of pain treatment, had acute injury requiring treatment, not willing to discontinue or reduce pain medication use, currently undergoing physical therapy, starting a new exercise regimen, had an implantable pain device, pacemaker, defibrillator or other neuromodulation device, or were known to be pregnant.
Eligible subjects were provided an innovative, non-invasive bio- antenna that exists as a thin, flexible patch ,Signal Relief TM (see picture 1). The patch contains no drugs, wires, or batteries. The conductive particle array layer in the Signal Relief TM patch exploit the body’s natural electromagnetic field by absorbing electromagnetic energy. Thereby, the intensity of the pain signals reaching the brain is reduced or interrupted, effectively relieving the pain. Patients with chronic musculoskeletal pain and an initial Visual Analog Pain Scale (VAS) score > 4 at baseline received a patch for a 7-day treatment window. A total of 42 subjects were enrolled, 29 females and 13 males with an average age of 61.4 years (28 - 86). Thirty-two subjects completed the trial. Ten subjects were excluded from primary analysis, 7 did not follow up to complete the study, and 3 had an initial VAS score < 4. The 7 subjects that did not complete the study had pain in an area that was difficult to affix the patch to, primarily the neck and shoulder. We are working on a more malleable design to address this. The primary efficacy assessment was the proportion of subjects achieving at least a 30% reduction in reported pain VAS scale from baseline to the end of treatment. Other assessments included proportional change in VAS, Brief Pain Inventory (BPI) and Pain Interference Score (PIS) from baseline to the end of treatment, and average % reduction in non-opioid and opioid pain medication use from baseline to end of treatment. Participant characteristics are summarized in (Table 1).
An optional exploratory endpoint measuring blood serum levels of Substance P (pg/ml) at baseline and day 7 was also evaluated. This evaluation was completed on 24 subjects. Substance P (SP) is secreted by nerves and inflammatory cells such as macrophages, eosinophils, lymphocytes, and dendritic cells and acts by binding to the neurokinin-1 receptor (NK-1R). Safety assessments included physical examination, monitoring of vital signs, and adverse event reporting per study visit schedule.
Results
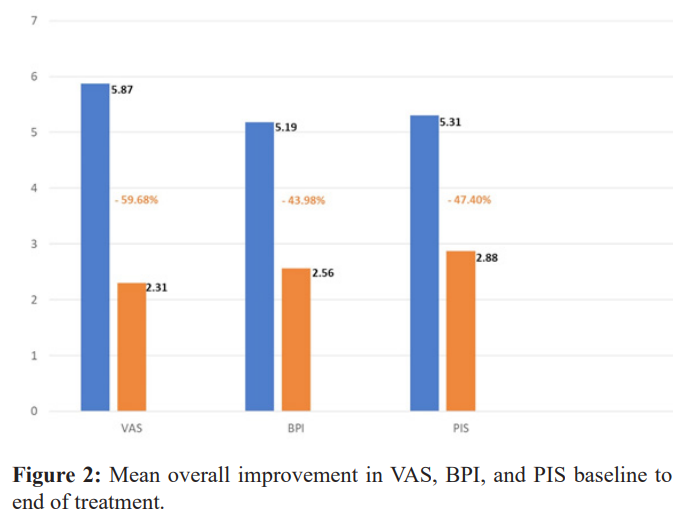
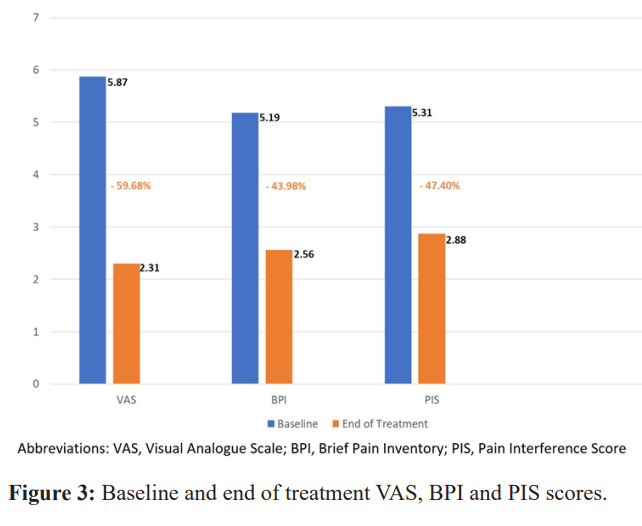
Greater than 90% of study participants received reduction in their pain per VAS score, 30/32 (93.7%). The proportion of subjects achieving our endpoint of at least a 30% reduction in pain per VAS measurement was 75% (24/32). Mean reduction in VAS was 59.7%, with a 95% CI (-65.6, -53.7), mean reduction in BPI was 44.0%, 95% CI (-53.1, -34.9), mean reduction in PIS was 47.4%,95% CI (-53.7, -41.1).
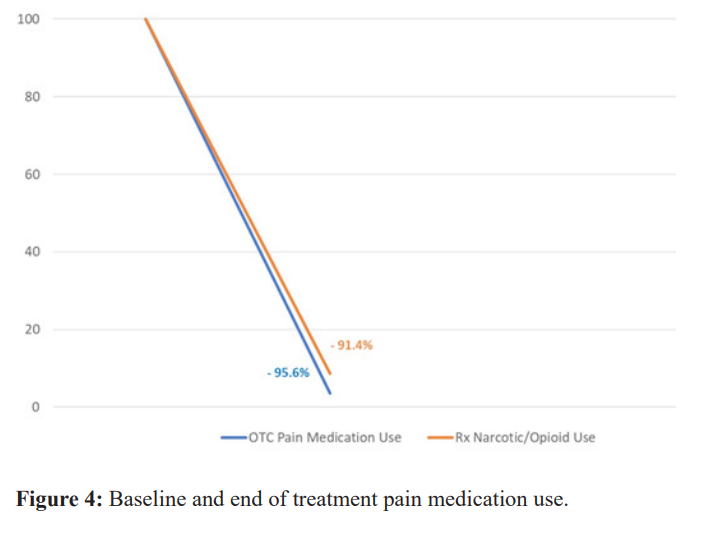
Sixty Nine percent of participants (22/32) were using pain medication upon study entry. Average reduction in non-opioid pain medication use was 96.5% and average reduction in opioid pain medication use was 91.4% from baseline to end of treatment (Figures 2,3, & 4).
In correlating SP to VAS score we found that 12/24 (50.0%) of subjects had a decrease in VAS and decrease in SP as we had anticipated, 11 subjects had a decrease in VAS but an increase in SP. Only 1 participant had an increase in both VAS and SP. Substance P (SP) showed a mean increase of 11.7%, 95% CI (3.2, 20.2). We attribute the overall mean increase in SP values to the fact that over 40% (41.6%) of those who showed an increase in SP had multiple areas of pain. For the purpose of this study, we were only evaluating and treating a single area. Only 1 participant that showed a decrease in SP had more than one area of pain documented. No adverse events were reported.
Discussion
This pilot study is the first of its kind to demonstrate the potential for clinically significant benefits of treatment with this Signal Relief™ pain patch technology. While the mechanism by which this technology affects the pathophysiology of pain is not clearly delineated, there are plausible theories that explain the results demonstrated. It is postulated that the conductive particle layer within this flexible patch design interact with the imperceptible electrical signals from the body’s nervous system that are generated when the body is experiencing pain. The energy from these pain signals is transmitted to the patch like an antenna. This effectively gives the pain signal an opportunity to exit the body through a path of least resistance i.e., ‘off-ramp’, thereby, the intensity of the pain signals reaching the brain is reduced, effectively breaking the pain cycle.
Opioid use for pain treatment dates to the mid-1800s in the United States—but the risk of dependency was recognized even then. Dosage and treatment duration can play a role in a patient's likelihood of experiencing opioid addiction, although studies show that even short-term use of opioids can lead to misuse. Globally a staggering 310 million surgeries are performed annually with 40-50 million occurring in the USA and another 20 million in Europe [16]. Preventing post-operative complications is top of mind for all surgeons when embarking on any surgery procedure
Post-operative pain management begins before the scalpel is placed on the skin. With our current knowledge of the chronic abuse potential of opioids, novel pain management alternatives are needed. Statistically 20% of surgical patients continue to use opioids 3 months after surgery [17]. Post-operative pain management presents an ongoing challenge for surgeons of all surgical subspecialities. Inadequate post-operative pain control leads to delayed rehabilitation, increased complications, and poor quality of life. Balancing pain relief with concerns for potential for opioid abuse and misuse has led to a multimodality approach to pain management [18]. Regional analgesia can be used in the immediate post-operative period, but non-narcotic, non NSAID low risk options for pain management are lacking.
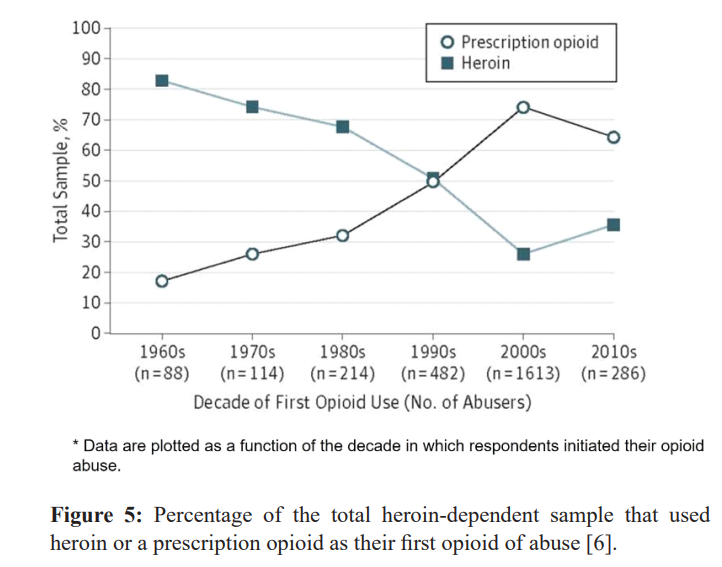
Of those patients prescribed narcotics for pain, 29% will eventually misuse them and can lead to addiction [19]. A study of young, urban injection drug users from 2008 and 2009 found that 86 percent had used opioid pain relievers nonmedically prior to using heroin. Nonmedical use was characterized by three main sources of opioids: family, friends, or personal prescriptions. 80% of heroin addicts began their addiction with a narcotic prescribed by physicians [20,21]. This is in stark contrast to heroin addicts from the 1960’s where most heroin addicts began their addiction with Heroin itself (Figure 5).
Even OTC NSAIDS, aspirin, ibuprofen, and naproxen sodium can have significant symptoms as well as dangerous effects with persistent, long-term use [22,23]. Common gastrointestinal symptoms include gas, feeling bloated, heartburn, stomach pain, nausea, vomiting, diarrhea and/or constipation. NSAIDs can cause more serious complications including gastrointestinal ulcers, serious cardiovascular events, hypertension, acute renal failure, and worsening of preexisting heart failure [24].
The need for non-pharmacological options for pain management is critical. It is important that we continue to advance these novel technologies in the health care space. The average amount of pain reduction achieved in this study was close to 60% (59.7%). Almost 70% of study participants were taking pain medication at study entry, primarily OTC NSAIDS, but 5 participants were taking prescription narcotics or opioids to manage their pain. A significant reduction was demonstrated across both groups, with>90% decrease in medication use for both OTC and prescription medication use. If we were to extrapolate these results into the surgical use case, the benefit could represent a paradigm shift in the opioid crisis. Non-drug options like the Signal Relief™ could help to ‘right-size’ the number of narcotics needed and length of use post-operatively.
Results indicate that the Signal ReliefTM patch may provide a drug-free option for the management of general musculoskeletal pain. The overall reduction in pain score, pain inventory, pain interference, and medication use are very promising. Larger randomized, cross-over, multi-center clinical studies in all areas of pain management are warranted.
References
- Zelaya C, Dahlhamer J, Lucas J, et Chronic Pain and High- impact Chronic Pain Among U.S. Adults, 2019. NCHS Data Brief. 2020; 390: 1-8.
- Nielsen SS, Skou ST, Larsen AE, et Associations of health- related quality of life with sociodemographic characteristics, health, pain, and lifestyle factors, and motivation for changing lifestyle in adults living with chronic pain: a cross-sectional exploratory study. Scand J Pain. 2021; 22: 142-153.
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety J Pain. 2008; 9: 883-891.
- Meldrum ML. The Ongoing Opioid Prescription Epidemic: Historical Context. Am J Public Health. 2016; 106: 1365-
- Dahlhamer JM, Connor EM, Bose J, Lucas JW, Zelaya Prescription Opioid Use Among Adults With Chronic Pain: United States, 2019. Natl Heal Stat Reports Number. 2019;162. Accessed August 27, 2021. https://www.cdc.gov/ nchs/products/index.htm.
- Mattson CL, Tanz LJ, Quinn K, et al. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths— United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021; 70: 202-207.
- Muhuri P, Gofroerer J, Davies C. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United CBHSQ Data Rev. Published online August 2013. Accessed September 10, 2021. https://www.samhsa.gov/data/ sites/default/files/DR006/DR006/nonmedical-pain-reliever- use-2013.htm
- TJ C, MS E, HL S, SP The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71(7):821-826. doi:10.1001/ JAMAPSYCHIATRY.2014.366.
- RG C, RW N, SS M, R Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: A natural history study. Drug Alcohol Depend. 2016;160:127-134. doi:10.1016/J. DRUGALCDEP.2015.12.026.
- CDC’s Role in the Opioid Overdose Epidemic | CDC’s Response to the Opioid Overdose Epidemic | Accessed August 27, 2021. https://www.cdc.gov/opioids/ overdoseprevention/cdc-role.html
- https://heal.nih.gov/about
- Feijun Luo, Mengyao Li, Curtis State-level economic costs of opioid use disorder and fatal opioid overdose-United States, 2017. Centers for Disease Control and Prevention. 2021; 70: 541-546.
- Chen J, Jin T, Zhang H. Nanotechnology in Chronic Pain Front Bioeng Biotechnol. 2020; 8: 682.
- Chakravarthy K V, Boehm FJ, Christo PJ. Nanotechnology: A Promising New Paradigm for the Control of Pain. Pain Med. 2018;19(2):232-243. doi:10.1093/PM/PNX131
- Tang Z, Zhao P, Ni D, et al. Pyroelectric nanoplatform for NIR-II-triggered photothermal therapy with simultaneous pyroelectric dynamic Mat Horiz. 2018; 5: 946-952.
- Dobson Trauma of major surgery: A global problem that is not going away. Int J Surg. 2020; 81: 47-54.
- Santosa KB, Hu HM, Brummett CM, et al. New persistent opioid use among older patients following surgery: A Medicare claims Surgery. 2020; 167: 732-742.
- Oelhaf RC, Del Pozo E, Azadfard M. Opioid Toxicity. 2022 May In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–.PMID: 28613731.
- Blichfeldt-Eckhardt MR, Jensen JM, Møller [Treating post-operative pain]. Ugeskr Laeger. 2017 Jun 26;179(26):V02170090. Danish. PMID: 28648159 J 22.
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002-2004 and 2008-2010. Drug Alcohol 2013;132(1-2):95-100.
- Cicero TJ, Ellis MS, Surratt HL, Kurtz The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821 826.
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J 2009; 103: 1227-1237.
- Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory Expert Opin Drug Saf. 2009; 8: 669- 681.
- Vonkeman HE, van de Laar Nonsteroidal anti- inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum. 2010; 39: 294-312.