A Systematic Review of the Histopathology and Immunochemistry in Duodenal Gangliocytic Paragangliomas with Lymph Node Metastases to Identify Predictors of Malignancy
Author'(s): Dr. Luke Hartford, DVM, MD1, Alexsi Sherazadishvili2*, Dr. Ken Leslie, MD, MHPE, FRCSC3 and Jeremy Parfitt, MD, FRCPC4
1General Surgery Resident, Division of General Surgery, Department of Surgery, Schulich School of Medicine and Dentistry, University of Western Ontario, London, Canada.
2MD Candidate, Class of 2019, Schulich School of Medicine & Dentistry, University of Western Ontario, London, Canada.
3Chief/Chair Division of General Surgery, Department of Surgery, Schulich School of Medicine and Dentistry, University of Western Ontario, London, Canada.
4Associate Professor, Department of Pathology and Laboratory Medicine, Schulich School of Medicine and Dentistry, University of Western Ontario, London, Canada.
*Correspondence:
Alexsi Sherazadishvili, MD Candidate, Class of 2019, Schulich School of Medicine & Dentistry, University of Western Ontario, 1151 Richmond St., London, Ontario, Canada, E-mail: asheraza@uwo.ca.
Received: 19 April 2018 Accepted: 04 May 2018
Citation: Luke Hartford, Alexsi Sherazadishvili, Ken Leslie, et al. A Systematic Review of the Histopathology and Immunochemistry in Duodenal Gangliocytic Paragangliomas with Lymph Node Metastases to Identify Predictors of Malignancy. Gastroint Hepatol Dig Dis. 2018; 1(2): 1-7.
Abstract
Gangliocytic paragangliomas (GP) are rare tumors, most commonly located in the 2nd portion of the duodenum. Their origin is poorly understood and management is uncertain. Typically exhibiting benign behavior, they infrequently metastasize to lymph nodes (LN) and distant sites. Due to their triphasic cellular distribution, tissue diagnosis pre-operatively remains a challenge, as well as prediction of which tumors may metastasize or act more aggressively. A systematic literature search was performed, and data for epidemiology, clinical history, gross pathology, and histopathology of duodenal (DGPs) with LN metastases was collected. Histopathology and Immunohistochemistry (IHC) was described and compared to a review by Okubo et al in an effort to identify predictors of malignancy. It is difficult to obtain a tissue diagnosis and predict malignant behavior of DGPs. Increased tumor size, depth of invasion, angio-lymphatic invasion and IHC findings may warrant further investigation for LN metastases. The presence of LN metastases does not seem to influence the prognosis, but rather the treatment modality.
Keywords
Introduction
Gangliocytic paragangliomas (GPs) were first described by Dahl et al. in 1957 [1]. With only approximately 200 case reports in the medical literature, GPs are rare tumors, most commonly located in the 2nd portion of the duodenum [2]. They have a 5-7% rate of lymph node (LN) metastases. Although these tumors typically behave in a benign fashion, reports of lymph node metastases, rare distant metastasis and a single case of a reported mortality have suggested that it is a tumor of uncertain malignant potential [3].
The pathogenesis of these tumors is still uncertain with several hypotheses about their origin. These tumors have a triphasic cellular distribution, often containing a pre-dominant epithelioid component, as well as spindle and ganglion cells of varying proportions. Tissue diagnosis pre-operatively remains a challenge, as well as prediction of which tumors may metastasize or act more aggressively. The presence of LN metastases is consequential, as currently, the majority of these patients are treated with a pancreatoduodenectomy (PD), instead of local excision. Herein we review the literature, describing the histopathology and immunohistochemistry (IHC) of these tumors. Case reports with the presence of LN metastases will be compared to the case series of Okubo et al., with the significance of angiolymphatic invasion, mitotic rate, nuclear pleomorphism, tumor size, depth of invasion and IHC described and examined as predictors of malignancy [4].
Methods
Literature Search Strategy and Selection
A systematic literary research was performed independently by 2 different investigators. The search terms included the following: “Gangliocytic,” “Paraganglioma,” “Lymph node,” “Paraganglioneuroma,” “Pheochromocytoma,” “Duodenum,” “Pancreatic,” “Ampulla of Vater,” “Duodenal Papilla,” “Sphincter,” “Oddi,” “Neoplasm,” “Cancer,” “Adeno/Carcinoma,” “Sarcoma,” “Tumor,” “Metastasis,” “Malignant,” combined with AND/OR. The references of all selected articles were reviewed. Duplicate studies were eliminated. Agreement between the two reviewers was measured with the Cohen’s kappa coefficient. All retrieved articles were selected if they met inclusion criteria.
Selection Criteria
Selected studies contained patients with duodenal GPs (DGPs), with LN metastases, confirmed by histopathology on the resected specimen. All abstracts/conference proceedings, case reports, reviews, and meta-analyses of all languages were included. No selection on age, sex or ethnicity was adopted.
Data Extraction
Study data included year of publication and patient characteristics, including age, sex, and symptoms. Diagnostic procedures, such as endoscopic biopsy with forceps and endoscopic ultrasound fine needle aspirate (FNA) were recorded. Gross and histopathological data was only included if clearly recorded. Gross pathology features, including tumor location, size, and descriptions were gathered. Histopathology description was recorded including location within the bowel wall, mucosal ulceration, mitotic rate, angiolymphatic invasion, tumor necrosis, nuclear pleomorphism, muscularis propria invasion, and pancreatic involvement in resected specimens. Submucosal location was determined through either imaging by endoscopic ultrasound (EUS) or histopathology in the resected specimen, and IHC results were also summarized. After research ethics board approval, authors of all case reports were contacted on 3 separate occasions and the most current disease specific time interval was included.
Statistical Analyses
Percentages, means, standard deviations and chi square tests of patient and tumor characteristics were reported. These were compared to those cases of DGPs from Okubo et al. [4]. Disease specific survival outcomes were reported in months, using both clinical symptoms and diagnostic imaging, as methods of surveillance.
Results Selection
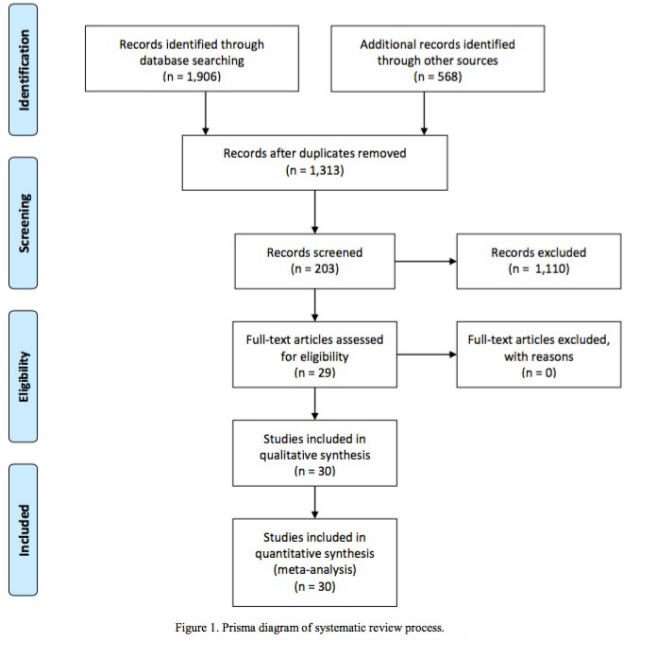
After removing duplicates, 1,276 titles and abstracts were reviewed and 34 articles were selected as potentially eligible for the study, with 29 reports meeting inclusion criteria on further review. Two additional articles were identified after reviewing references. An additional case report, by Schick et al., was added to the data [5]. Agreement between the two investigators, who performed the literature search measured by Cohen’s Kappa Coefficient was 0.944, and disagreements were resolved with discussion involving both investigators. A total of 33 patients with DGPs with LN metastases were identified (Figure 1).
Epidemiology and Gross Pathological Features
The mean age of patients was 48 years, with a range of 16 to 74 years in the 33 patients identified. Male to female ratio was 1:1 (Table 1).
Primary tumor location was the second portion of the duodenum in 28/32 cases (88%), with 21 of reported DGPs described as a sub/peri/ampullary mass. Other tumor locations included the third portion of duodenum, and two cases involving the head of the pancreas [5,6]. On gross appearance 19/23 (83%) had mucosal ulceration and 19/20 (95%) had a polyploid or exophytic appearance. Mean tumor size (maximum diameter) was 3.1 cm (standard deviation SD 1.6 cm), ranging from 1.0 to 9.0 cm (Table 1).
Serum and Urine Tumor Markers
Hormone levels and serum/urine tumor markers were reported in 6 cases. These values were relatively unremarkable, with one case of elevated somatostatin, which normalized post-operatively [7]. One case of elevated pancreatic polypeptide (PP) post-operatively was thought to be indicative or tumor recurrence [8] (Table 2).
Endoscopy, Endoscopic Ultrasound (EUS) and Diagnostic Biopsies
Of 9 reported esophagogastroduodenoscopy (EGD) biopsies, 1 suggested paraganglioma (11%) [8]. The method of biopsy was likely with biopsy forceps, but not recorded. However, EGD was a sensitive diagnostic tool, as in all 23 patients, it successfully identified a polypoid duodenal mass. Endoscopic Ultrasound (EUS) was reported in 11 cases and identified the primary tumor in 11/11 cases (100%), detecting enlarged LNs in 7/11 cases (64%). Of 9 FNA biopsies reported by EUS, GP was correctly diagnosed in 1 case (11%), suspicious/indeterminate in 7 cases (78%), and misdiagnosed as adenocarcinoma in 1 case (11%) (Table 3).
Histopathology
All tumors had a triphasic cellular distribution containing epithelioid cells, spindle cells, and ganglion cells of varying amounts. Epithelioid cells were predominant in the majority of tumors [9]. In 20 cases, there were comments on mitotic rate or proliferative index (Ki-67/MIB-1), with 19 (95%) showing minimal to no mitotic/proliferative activity. In the remaining case, the mitotic activity was not quantified, but the reports stated that the epithelioid cells were sometimes mitotic. In 10 cases, the presence or absence of primary tumor necrosis was reported, which was present in 1 case (10%) [6]. Angiolymphatic invasion was reported in 7/9 (78%) cases by both hematoxylin and eosin (HE) stain, and D240 IHC. Nuclear pleomorphism of epithelioid cells was present in 5/10 (50%) of reported cases. In all 27 cases where it was described, the primary tumor involved the submucosal portion of the duodenal wall, with an infiltrative margin involving the mucosa and/or muscularis propria in 25/27 cases (93%); the pancreatic head was involved in 6/29 cases (21%) [10-15]. There were no reports of encapsulated tumors, and psammoma bodies were rarely described in 1 case [14] (Table 4).
Immunohistochemistry Immunohistochemistry (IHC) findings were documented in 29 cases and are recorded in table 5. The results were compared to Okubo et al., which contain IHC findings from 173 DGPs [4] (Table 5).
|
Year |
Author (first, et al.) |
Age, Sex |
Presenting complaint |
Tumor location |
Mucosal ulceration |
Polyploid /exophytic |
Size (cm) |
|
1981 |
Dookhan [15] |
41, M |
Abdominal discomfort; Partial gastric outlet obstruction |
D2 |
1 |
1 |
2.5 |
|
1985 |
Buchler [24] |
50, M |
UGI bleed |
D2, Peri-ampullary |
0 |
1 |
3 |
|
1987 |
Korbi [10] |
73, F |
Abdominal discomfort, Fever, UGI bleed; Melena |
D2 |
1 |
1 |
9 |
|
1989 |
Burke [25] |
NR |
NR |
NR |
NR |
NR |
NR |
|
1989 |
Burke [25] |
45, M |
NR |
Duodenum |
NR |
NR |
NR |
|
1989 |
Inai [17] |
17, M |
UGI bleed |
D2, Ampulla |
1 |
1 |
2 |
|
1992 |
Hashimoto [26] |
47, M |
Incidental finding |
D2, Ampulla |
1 |
1 |
6.5 |
|
1993 |
Takabayashi [27] |
63, F |
Epigastralgia; Biliary/Pancreatic ductal dilatation |
D3 |
1 |
1 |
3.2 |
|
1996 |
Tomic [7] |
74, F |
Steatorrhea, N/V, Abdominal discomfort |
Head of pancreas |
NR |
NR |
4 |
|
1997 |
Sundararajan [19] |
67, F |
NR |
D2, Ampulla |
1 |
1 |
5 |
|
2003 |
Henry [6] |
50, M |
Obstructive jaundice |
Head of pancreas |
NR |
NR |
3 |
|
2004 |
Bucher [11] |
31, F |
Anemia; Subclinical jaundice |
D2, Peri-ampullary |
NR |
NR |
3 |
|
2005 |
Wong [8] |
49, F |
Melena, RUQ discomfort |
D2, Ampulla |
1 |
1 |
1.4 |
|
2007 |
Witkiewicz [28] |
38, F |
RUQ discomfort |
D2, Peri-ampullary |
0 |
1 |
1.5 |
|
2009 |
Mann [29] |
17, F |
Duodenal obstruction, N/V, Abdominal discomfort |
Junction D2-D3 |
1 |
NR |
3 |
|
2010 |
Saito [37] |
28, M |
UGI bleed |
D2, Ampulla |
1 |
1 |
1.7 |
|
2010 |
Uchida [30] |
67, F |
Anemia |
D2 |
1 |
1 |
4.2 |
|
2010 |
Okubo [4] |
61, M |
Epigastralgia; UGI bleed |
D2, Ampulla |
1 |
1 |
3 |
|
2011 |
Fiscaletti [31] |
61, M |
RUQ discomfort, Weight loss |
D2 |
NR |
1 |
1.2 |
|
2011 |
Rowsell [14] |
52, F |
Incidental Liver Metastasis on Imaging |
D2, Peri-ampullary |
0 |
1 |
1 |
|
2011 |
Ogata [2] |
16, M |
Anemia; UGI bleed; Dyspnea; Fever |
D2, Ampulla |
1 |
1 |
3.5 |
|
2012 |
Barret [12] |
51, F |
Anemia, UGI bleed |
D2, Ampulla |
1 |
1 |
2.5 |
|
2013 |
Dustin [22] |
56, F |
Epigastralgia, Weight loss |
D2, Peri-ampullary |
0 |
NR |
1.8 |
|
2013 |
Amin [9] |
57, M |
N/V, Abdominal discomfort |
D2, Ampulla |
NR |
NR |
3 |
|
2013 |
Park [16] |
54, F |
UGI bleed |
D2 |
NR |
NR |
2 |
|
2014 |
Li [3] |
47, M |
Epigastralgia |
D2, Peri-ampullary |
1 |
NR |
3 |
|
2014 |
Shi [13] |
47, M |
LLQ discomfort; Weight loss |
D2, Ampulla |
1 |
1 |
4 |
|
2015 |
Choi [32] |
41, M |
UGI bleed |
D2, Periampullary |
1 |
NR |
2.5 |
|
2015 |
Wang [33] |
49, M |
Epigastralgia |
D2 |
1 |
NR |
4 |
|
2015 |
Dowden [34] |
59, F |
Abdominal discomfort, Weight loss |
D2, Ampulla |
NR |
NR |
2.8 |
|
2015 |
Sun [35] |
40,F |
Abdominal discomfort |
D2, Ampulla |
NR |
1 |
2 |
|
2016 |
Hu [36] |
65, M |
UGI bleed |
D2, Peri-ampullary |
1 |
1 |
3 |
|
2016 |
Schick [5] |
35, F |
Abdominal discomfort; Dyspepsia |
D2, Ampulla |
1 |
0 |
3.2 |
Table 1: Patient Demographics and Characteristics of Duodenal Gangliocytic Paragangliomas.
|
Author |
Pre-operative |
Post-operative |
|
Buchler [11] |
NR |
Serotonin (-), 5HIAA (-), Gastrin (-) |
|
Ogata [2] |
Serum markers WNL |
NR |
|
Tomic [7] |
Somatostatin (+++) Calcitonin, VIP, PP, Gastrin, Serotonin level WNL |
Somatostatin normalized |
|
Uchida [30] |
Dopamine, A/NoA, Urine catecholamines, CEA, Ca19-9 WNL |
NR |
|
Amin [9] |
Somatostatin and CG WNL; PP NR |
PP (+++); Gastrin, Insulin, Glucagon, VIP, 5-HIAA, Catecholamine, CG WNL; |
|
Saito [37] |
Glucagon, Gastrin, Urinary 5 - HIAA, A/NoA; Met/NoMet; VMA WNL |
NR |
Table 2: Recorded Serum and Urine Tumor Markers of Duodenal Gangliocytic Paragangliomas.
NR = Not recorded; WNL = Within Normal Limits; VIP = Vasoactive Intestinal Peptide; PP = Pancreatic Polypeptide; A/NoA = Adrenaline/Noadrenaline; CG = Chro- mogranin A; 5-HIAA = 5 Hydroxyindoleacetic acid; CEA = Carcinoembryonic antigen; Met/NoMet = Metanephrine/Nometanephrine; VMA = Vanillylmandelic acid; (+++) = strongly positive; (-) = negative.
|
Endoscopic biopsy |
Endoscopic U/S biopsy |
|
1/9 correct dx |
1/9 correct dx; 1/9 incorrect dx; 2/9 suspicious but not dx; 5/9 inconclusive. |
Table 3: Biopsy Diagnostic Results of Duodenal Gangliocytic Paragangliomas. dx = diagnosis; U/S = Ultrasound.
|
Author (first, et al.) |
Tumor location |
Mitotic rate |
Angio-lymphatic invasion |
Tumor Necrosis |
Epithelioid pleomorphism |
Submucosal location |
Muscularis propria Invasion |
Mucosal ulceration |
Pancreatic involvement |
|
Dookhan [15] |
D2 |
NR |
NR |
NR |
NR |
1 |
1 |
1 |
0 |
|
Buchler [24] |
Periampullary |
0 |
NR |
0 |
0 |
1 |
1 |
0 |
0 |
|
Korbi [10] |
D2 |
1 |
NR |
NR |
1 |
1 |
0 |
1 |
0 |
|
Burke [25] |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
0 |
|
Burke [25] |
Duodenum |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
Inai [17] |
Ampulla |
NR |
1 |
NR |
0 |
1 |
1 |
1 |
1 |
|
Hashimoto [26] |
Ampulla |
0 |
1 |
NR |
1 |
1 |
0 |
1 |
0 |
|
Takabayashi [27] |
D3, ampulla |
NR |
NR |
NR |
NR |
1 |
1 |
1 |
0 |
|
Tomic [7] |
Pancreas |
0 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
Sundararajan [19] |
Ampulla |
0 |
NR |
0 |
0 |
1 |
1 |
1 |
0 |
|
Henry [6] |
Pancreas |
NR |
NR |
NR |
NR |
0 |
0 |
NR |
1 |
|
Bucher [11] |
Periampullary |
NR |
NR |
NR |
NR |
0 |
0 |
NR |
1 |
|
Wong [8] |
Ampulla |
NR |
NR |
NR |
NR |
1 |
1 |
1 |
0 |
|
Witkiewicz [28] |
Periampullary |
2/10 hpf |
NR |
0 |
1 |
1 |
1 |
0 |
0 |
|
Mann [29] |
D2-D3 |
NR |
1 |
NR |
NR |
1 |
1 |
1 |
0 |
|
Saito [37] |
Ampulla |
MIB-1 2.2% |
1 |
NR |
NR |
1 |
0 |
1 |
0 |
|
Uchida [30] |
D2 |
NR |
NR |
NR |
NR |
1 |
0 |
1 |
0 |
|
Okubo [4] |
Ampulla |
0 |
1 |
0 |
0 |
1 |
1 |
1 |
0 |
|
Fiscaletti [31] |
D2 |
2/10 hpf |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
Rowsell [14] |
Periampullary |
Ki-67 < 1% |
NR |
NR |
NR |
1 |
0 |
0 |
0 |
|
Ogata [2] |
Ampulla |
0 |
1 |
NR |
NR |
1 |
0 |
1 |
0 |
|
Barret [12] |
Ampulla |
0 |
NR |
0 |
NR |
1 |
1 |
1 |
0 |
|
Dustin [22] |
D2 |
0 |
NR |
0 |
1 |
1 |
1 |
0 |
0 |
|
Amin [9] |
Ampulla |
Ki-67 < 1% |
NR |
0 |
0 |
NR |
NR |
NR |
NR |
|
Park [16] |
D2 |
NR |
NR |
NR |
NR |
1 |
0 |
NR |
0 |
|
Li [3] |
Periampullary |
0 |
NR |
0 |
1 |
1 |
1 |
1 |
1 |
|
Shi [13] |
Ampulla |
Ki-67 < 1% |
NR |
NR |
NR |
1 |
1 |
1 |
1 |
|
Choi [32] |
Periampullary |
< 1/50 hpf |
NR |
NR |
NR |
1 |
1 |
1 |
0 |
|
Wang [33] |
D2 |
0 |
NR |
0 |
NR |
1 |
1 |
1 |
0 |
|
Dowden [34] |
Ampulla |
0/10 hpf |
0 |
NR |
NR |
1 |
1 |
NR |
0 |
|
Sun [35] |
Ampulla |
NR |
0 |
NR |
NR |
1 |
1 |
0 |
0 |
|
Hu [36] |
D2 |
NR |
NR |
NR |
NR |
1 |
0 |
1 |
0 |
|
Schick [5] |
Ampulla |
Ki-67 < 1 % |
1 |
1 |
NR |
1 |
1 |
1 |
1 |
Table 4: Histopathology. NR = Not reported; D2 = 2nd portion of duodenum; D3 = 3rd portion of the duodenum; hpf = high power field; 1 = present; 0 = absent.
|
Immunohistochemistry |
Hartford et al. (2018) Epithelioid cells |
Okubo et al. (2011) Epithelioid cells |
Hartford et al. (2018) Spindle-shaped cells |
Okubo et al. (2011) Spindle-shaped cells |
Hartford et al. (2018) Ganglion-like cells |
Okubo et al. (2011) Ganglion-like cells |
|
NSE |
90.9% (10/11) |
93.9% (77/82) |
45.5% (5/11) |
84.0% (63/75) |
45.5% (5/11) |
84.0% (63/75) |
|
Chromogranin A |
90.9% (20/22) |
67.4% (60/89) |
4.7% (1/21) |
7.2% (6/83) |
0.0% (0/21) |
15.4% (12/78) |
|
Synaptophysin |
95.2% (20/21) |
90.0% (36/40) |
30.0% (6/20) |
64.7% (22/34) |
85.7% (18/21) |
94.3% (33/35) |
|
S-100 |
4.2% (1/24) |
3.8% (4/104) |
96.0% (24/25) |
94.2% (98/104) |
8.3% (2/24) |
9.3% (9/97) |
|
Cytokeratins |
84.6% (11/13) |
48.3% (28/58) |
0.0% (0/12) |
4.0% (2/50) |
0.0% (0/12) |
3.9% (2/51) |
|
Somatostatin |
81.8% (9/11) |
81.8% (63/77) |
0.0% (0/11) |
8.7% (6/69) |
54.5% (6/11) |
44.1% (30/68) |
|
Pancreatic polypeptide |
71.4% (5/7) |
89.7% (70/78) |
0.0% (0/7) |
0.0% (0/72) |
57.1% (4/7) |
22.2% (16/72) |
|
Serotonin |
20.0% (1/5) |
22.0% (13/59) |
0.0% (0/5) |
0.0% (0/55) |
20.0% (1/5) |
16.7% (9/54) |
|
Insulin |
16.7% (1/6) very weak |
2.2% (1/46) |
0.0% (0/6) |
0.0% (0/41) |
0.0% (0/6) |
0.0% (0/42) |
|
Glucagon |
0.0% (0/6) |
6.4% (3/47) |
0.0% (0/6) |
0.0% (0/44) |
0.0% (0/6) |
2.4% (1/42) |
|
Gastrin |
0.0% (0/3) |
5.9% (4/68) |
0.0% (0/3) |
0.0% (0/64) |
0.0% (0/3) |
0.0% (0/63) |
|
VIP |
40.0% (2/5) |
12.1% (4/33) |
40.0% (2/5) |
12.9% (4/31) |
20.0% (1/5) |
9.7% (3/31) |
Table 5: Immunohistochemistry (IHC): Comparison of Hartford et al. cases with lymph node metastasis to Okubo et al. review.
NOTE: The numbers 1-31 are used in Table 4 and correspond to the descending order of authors in Tables 1 & 4. Weak positive and partially positive findings are not included in this table.
Compared to Okubo et al. [5]
NSE = Neuron Specific Enolase; VIP = Vasoactive Intestinal Peptide
Surgical Management and Outcomes
Pancreatoduodenectomy (PD) was the definitive surgery in 27/31 cases. Local excision had a high rate re-operation 4/7 (57%). Distant metastases were present in 6 cases, including liver, mesentery, pelvis, retroperitoneum, and bone. Reported clinical follow up showed quite good disease specific survival. Local recurrence was reported in 1 case, with another case likely, but not definitively identified [15]. The recorded mean and median times post-operatively with no evidence of disease was 34 and 24 months respectively. There were 3 mortalities (9%) recorded; one from post-operative complications, one from metastatic disease, and one of unknown cause at 156 months [2,6,10].
Discussion
We examined the gross pathology, histopathology, and IHC characteristics of 33 cases of DGP with LN metastases, exploring significant factors associated with LN or distant metastases. These rare triphasic neoplasms are most commonly located in the 2nd portion of the duodenum, but have also been described in the jejunum, appendix, esophagus, spinal cord and lung.
Serum and Urine Tumor Markers
Of the tumor markers reported, only somatostatin was confirmed to be elevated pre-operatively and drop post-operatively in one case [7]. More information is needed to determine the significance of this, as well as of PP. While there are few reports of functioning tumors, this contributes to the possibility of misdiagnosis of these tumors as neuroendocrine tumors (NETs) and conversely duodenal NETs having GP, but reported otherwise.
Diagnostic Biopsies
Although EGD is sensitive in detecting a polyploid/ulcerated lesion in the duodenum, it is not specific in diagnosing DGPs, and the submucosal location of these tumors results in a high proportion of false negative biopsies by forceps. FNAs by EUS may be more specific, but are often indeterminate for a diagnosis of GP. This likely reflects the relative difficulty for pathologists to diagnose a rare tumor with a triphasic cellular distribution with limited sampling of a submucosal lesion. A core tissue sample may be more useful but is difficult to obtain given the location of the tumor.
Histopathology and Immunohistochemistry
On histopathology, epithelioid, spindle, and ganglion cells were present in all primary tumors. All three cellular components were also identified within the LN metastases. The epithelioid cell component predominated in the primary tumor, as well as in metastases. The presence of only epithelioid cells in some LN metastases may represent the dominance of that component or under sampling.
GPs with LN metastases do not appear to show increased mitotic rate or necrosis compared with those without LN metastases. Nuclear pleomorphism does not seem to correlate with LN metastases. Angiolymphatic invasion was identified in 7/9 reported cases (78%), and further investigations including D240 IHC would be helpful to further define the role of angiolymphatic invasion as a predictor of LN metastases.
The muscularis propria was involved in 18/28 (64%) with 7/27 cases (26%) reported to have extension into the subserosa or adjacent connective tissues. This is not significantly different from the 108 cases of GP where depth of tumor invasion was described in Okubo et al. [4] (P = .50). In their study, 62/108 cases (57%) had invasion of the musuclaris propria, while extension into connective tissue beyond the muscularis propria was seen in 4/108 cases (4%), and 12 of these 108 cases had lymph node metastasis. We support their evidence that increased tumor size, extension beyond the submucosal layer and depth of invasion are significant risk factors for LN spread (Table 4).
IHC was similar to those without LN metastases, which is consistent with both Okubo et al. and Park et al. [4,16]. However, in comparing GPs without LN metastases to GPs with LN metastases: epithelioid cells more frequently had uptake of chromogranin A (20/22 (91%) vs 60/89 (67%), P = .03) and cytokeratin (11/13 (85%) vs 28/58 (48%), P = .02). In cases of GPs with LN metastases, spindle cells had less frequent uptake of neuron specific enolase (NSE) (5/11 (46%) vs 63/75 (84%), P = .004) and synaptophysin (6/20 (30%) vs 22/34 (65%), P = .01). Ganglion cells from GPs with LN metastases also had less frequent uptake of NSE (5/11 (46%) vs 63/75 (84%), P = .004). More study is needed to determine whether IHC is able to predict a more aggressive course of DGPs (Table 5).
Outcomes
In our review of the literature of patients with DGP, there were three mortalities. In one case, death was thought to be directly secondary to the tumor; in one case, death from cardiopulmonary failure occurred as a complication of a PD procedure; in another case, death of an unknown cause occurred after 13 years [2,6,10]. Although there appears to be relatively good 1 and 5-year disease specific survival rates, analysis is limited by lack of extended follow up data. Only two cases showed evidence of local recurrence and 5/33 cases showed evidence of distance metastasis. This continues to suggest that GP, even with evidence of local spread with LN metastases, typically behaves in a benign fashion.
Origin
Despite extensive IHC and ultrastructural studies, the histogenesis of GPs is still debatable, and authors have been unable to reconcile the combination of endocrine, ganglion and spindle cells observed in a single tumor [3]. The tumor components are of different embryologic origins, the first being of endodermal origin and the others originating from neural crest tissue. All components normally occur in the duodenum, and given the relatively benign nature, some contend that it is a hamartoma [12]. Others postulate it is a hyperplastic or neoplastic lesion from ectopic cells of the ventral primordium of the pancreas, possibly explaining its relatively consistent location in the second portion of the duodenum [2,15,16]. Also suggested is an ectodermal origin, originating from pluripotent stem cells of the crytps of Lieberkuhn or celiac ganglion [17-21]. GPs discovered distant to the duodenum and pancreas, such as lung, esophagus, appendix, and spinal cord, as well as the presence of rare metastatic lesions, argue against GPs being a true hamartoma [20].
Although regarded by some as a neuroendocrine tumor, which may rarely show hormone secreting function, neuroendocrine tumors lack ganglion and spindle cells [22]. While the epithelioid component is often immunopositive for PP, this is not specific to pancreatic neuroendocrine tumors [16].
Comments
Many articles did not include all data examined. Therefore, data was only included if recorded clearly leading to a small sample size in many data categories and led to many different proportion characteristics. As there is a tendency to only publish positive results, published diagnostic modalities, as well as patient and tumor characteristics of DGP cases may be biased leading to a type I error. For this reason, sensitivity, specificity, and predictive values of tests were not calculated. As the majority of GPs are treated with local excision, this does not allow examination of the nodes, the number of cases with LN metastases may be underestimated. With such a rare diagnosis, the only articles obtained were case reports, abstracts, and reviews. As such, all studies met criteria for level 4 evidence, and therefore all recommendations were grade C.
Conclusion
Pre-operative tissue diagnosis of DGP is difficult due to the rarity, triphasic cellular distribution, and submucosal location of the tumor.
It is difficult to predict malignant behavior of DGPs by histopathology or IHC;. There is low-grade evidence to warrant further investigation for LN involvement with increased tumor size, depth of invasion and presence of angiolymphatic invasion. More information is needed to determine if IHC may assist in identification of malignant DGPs. More evidence is needed to confirm the utility of serum hormone and tumor markers, such as somatostatin and PP, in the role of diagnosis and post-operative surveillance.
Despite LN and distant metastasis, disease specific survival has been shown to be relatively good in our review, the presence of LN metastases does not seem to influence the prognosis, but rather treatment modality. PD is likely the most effective method of ensuring complete tumor excision and removal of affected LNs. Pathologists and treating clinicians should continue to be aware of the rare potential for metastases, and a multidisciplinary approach should be utilized in the approach and treatment of this rare diagnosis.
References
- Dahl EV, Waugh JM, Dahlin Gastrointestinal ganglioneuromas; brief review with report of a duodenal ganglioneuroma. Am J Pathol. 1957; 33: 953-965.
- Ogata S, Horio T, Sugiura Y, et al. Duodenal gangliocytic paraganglioma with regional lymph node metastasis and a glandular Pathol Intl. 2011; 61: 104-107.
- Li B, Li Y, Tian XY, et Malignant gangliocytic paraganglioma of the duodenum with distant metastases and a lethal course. World J Gastroenterol. 2014; 20: 15454-15461.
- Okubo Y, Wakayama M, Nemoto T, et Literature survey on epidemiology and pathology of gangliocytic paraganglioma. BMC cancer. 2011; 11:187.
- Schick B, Hartford L, Leslie K, et Periampullary gangliocytic paraganglioma with lymph node metastases: a case report. Can J Pathol. 2017; 9: 60-70.
- Henry C, Ghalel-Mechaoui H, Bottero N, et al. Gangliocytic paraganglioma of the pancreas with bone metastasis. Ann 2003; 128: 336-338.
- Tomic S, Warner Pancreatic somatostatin-secreting gangliocytic paraganglioma with lymph node metastases. Am J Gastro. 1996; 91: 607-608.
- Wong A, Miller AR, Metter J, et Locally advanced duodenal gangliocytic paraganglioma treated with adjuvant radiation therapy: case report and review of the literature. World J Surg Onc. 2005; 3: 15.
- Amin SM, Albrechtsen NW, Forster J, et al. Gangliocytic paraganglioma of duodenum metastatic to lymph nodes and liver and extending into the retropancreatic Pathologica. 2013; 105: 90-93.
- Korbi S, Kapanci Y, Widgren Malignant paraganglioma of the duodenum. Immunohistochemical and ultrastructural study of a case. Ann Pathol. 1986; 7: 47-55.
- Bucher P, Mathe Z, Bühler L, et al. Paraganglioma of the ampulla of Vater: a potentially malignant neoplasm. Scand J 2004; 39: 291-295.
- Barret M, Rahmi G, van Huyen J PD, et Duodenal gangliocytic paraganglioma with lymph node metastasis and an 8-year follow- up: a case report. Eur J Gastroenterol Hepat. 2012; 24: 90-94.
- Shi H, Han J, Liu N, et al. A gangliocytic partially glandular paraganglioma with lymph node Diag Pathol. 2014; 9: 63.
- Rowsell C, Coburn N, Chetty Gangliocytic paraganglioma: a rare case with metastases of all 3 elements to liver and lymph nodes. Ann Diag Pathol. 2011; 15: 467-471
- Dookhan DB, Miettinen M, Finkel G, et Recurrent duodenal gangliocytic paraganglioma with lymph node metastases. Histopathology. 1993; 22: 399- 401.
- Park HK, Han HS. Duodenal Gangliocytic Paraganglioma With Lymph Node Metastasis. Arch Pathol Lab Med. 2016; 140: 94-98.
- Inai K, Kobuke T, Yonehara S, et al. Duodenal gangliocytic paraganglioma with lymph node metastasis in a 17-year-old Cancer. 1989; 63: 2540- 2545.
- Perrone T, Sibley RK, Rosai Duodenal gangliocytic paraganglioma: An immunohistochemical and ultrastructural study and a hypothesis concerning its origin. Am J Surg Pathol. 1985; 9: 31- 41.
- Sundararajan V, Robinson-Smtih T, Lowy Duodenal gangliocytic paraganglioma with lymph node metastasis: a case report and review of the literature. Arch Pathol Lab Med. 2003; 127: 139-141.
- Scheithauer BW, Nora FE, Lechago J, et Duodenal gangliocytic paraganglioma: clinicopathologic and immunocytochemical study of 11 cases. Am J Clin Pathol. 1986; 86: 559-565.
- Lukash WM, Hyams VJ, Nielsen OF. Neurogenic neoplasms of the small bowel: Benign nonchromaffin paraganglioma of the Am J Dig Dis. 1966; 11: 575-579.
- Dustin SM, Atkins KA, Shami VM, et The cytologic diagnosis of gangliocytic paraganglioma: a case report. Diag Cytopathol. 2013; 41: 650-653.
- Phillips B, Ball C, Badenoch D, et al. Oxford centre for evidence-based medicine levels of evidence. BJU Int. 2001; 107: 870.
- Büchler M, Malfertheiner P, Baczako K, et al. A Metastatic Endocrine-Neurogenic Tumor of the Ampulla of Vater with Multiple Endocrine Immunoreaction Malignant Digestion. 1985; 31: 54- 59.
- Burke AP, Helwig EB. Gangliocytic paraganglioma. Am J Clin 1989; 92: 1-9.
- Hashimoto S, Kawasaki S, Matsuzawa K, et al. Gangliocytic paraganglioma of the papilla of Vater with regional lymph node Am J Gastro. 1992; 87: 1216-1218.
- Takabayashi N, Kimura T, Yoshida M, et A case report of duodenal gangliocytic paraganglioma with lymph node metastasis. Jap Soc Gasro Surg. 1993; 26: 2444-2448.
- Witkiewicz A, Galler A, Yeo CJ, et Gangliocytic paraganglioma: case report and review of the literature. J Gastro Surg. 2007; 11: 1351-1354.
- Mann CM, Bramhall SR, Buckels JA, et al. An unusual case of duodenal obstruction-gangliocytic paraganglioma. J HBP 2009; 16: 562-565.
- Uchida D, Ogawa T, Ueki T, et al. A case of gangliocytic paraganglioma with lymphoid metastasis. Jap J Gastro. 2010; 107: 1456-1465.
- Fiscaletti M, Fornelli A, Zanini N, et al. Segmental groove pancreatitis and duodenal gangliocytic paraganglioma with lymph node metastasis: a newly described Pancreas. 2011; 40: 1145-1147.
- Choi SB, Park PJ, Han HJ, et Acase of duodenal gangliocytic paraganglioma with regional lymph node metastasis. Office J Int HPB Assoc. 2014; 16: 614-615.
- Wang B, Zou Y, Zhang H, et Duodenal gangliocytic paraganglioma: report of two cases and review of literature. Int J Clin Exp Pathol. 2015; 8: 9752-9759.
- Dowden JE, Staveley-OCarroll KF, Kimchi ET, et Ampullary gangliocytic paraganglioma with lymph node metastasis. Am Surgeon. 2015; 81: E359-360.
- Sun Y, Kindelberger D, Xu H, et Periampullary gangliocytic paraganglioma with lymph node involvement: A case report. Am J Clin Pathol. 2015; 144: 347.
- Hu W, Gao S, Chen D, et Case Report: Duodenal gangliocytic paraganglioma with lymph node metastases: a case report and review of literature. Int J Clin Exp Pathol. 2016; 9: 4756-4760.
- Saito J, Hirata N, Furuzono M, et al. A case of duodenal gangliocytic paraganglioma with lymph node metastasis. Jap J 2010; 107: 639-648.