Antioxidant and Hepatoprotective Activity of Leaf Extract of Smilax Anceps Wild
Author(s): Shorinwa Olusayo A* and Shatange Dorothy D
Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
*Correspondence:
Shorinwa Olusayo Aderonke, Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria, Tel: +234 803 313 0810.
Received: 01 Dec 2021 Accepted: 29 Dec 2021 Published: 04 Jan 2022
Citation: Shorinwa OA, Shatange DD. Antioxidant and Hepatoprotective Activity of Leaf Extract of Smilax Anceps Wild. Diabetes Complications. 2022; 6(1); 1-8.
Abstract
Introduction: Smilax anceps leaf is used for general healing of the body in traditional medicine. The aim of this study was to investigate the in-vitro antioxidant potential and in-vivo hepatoprotective activity of the ethanol leaf extract of Smilax anceps against carbon tetrachloride induced hepatotoxicity.
Materials and Methods: Preliminary phytochemical screening and acute toxicity were determined. In-vitro antioxidant activity of the plant was evaluated using 2, 2-diphenyl-1-picryl-hydrazyl (DPPH), reducing power activity, nitric oxide scavenging, and lipid peroxidation scavenging activity assays. In- vivo hepatoprotective activity was determined using albino rats, divided into groups of six comprising of 5 animals each vis the control, CCl4, extract 250,500 and 1000mg/kg, and silymarin 100mg/kg. Treatments were done for seven days and carbon tetrachloride dissolved in olive oil was administered on the seventh day. Biochemical analysis was performed to assay the levels of aspartate aminotransferase (AST), alanine amino transaminase (ALT), alkaline phosphatase (ALP), albumin (ALB), total protein (TP) and Total bilirubin (TB) with histological examination of the liver. Phytochemical screening showed the presence of saponins, flavonoids, tannins, carbohydrates and steroids/ terpenoids.
Results: The extract showed significant (P<0.01) scavenging activity against DPPH, reducing power activity, nitric oxide scavenging, and lipid peroxidation. There was a statistically significant (P<0.05) decrease in total bilirubin (TB), alanine amino transaminase (ALT) and alkaline phosphatase (ALP).
Conclusion: The findings of this study showed that the ethanol leaf extract of S. anceps possesses significant antioxidant and hepatoprotective which might be due to the presence of flavonoids.
Keywords
Introduction
Nature has provided man with multitude of life saving medicines as most of what we use and consume on daily basis are products from interactions with nature [1]. All medicinal preparations were derived from plants, whether in simple form of raw plant materials or in the refined form of crude extracts, mixtures etc. Recent estimates suggest that several thousands of plants have been known with medicinal applications in various cultures [2].
The effects of free radicals on man have long been related to the aging process and has been considered to be an underlying factor in the development of diseases such as diabetes, cancers, cardiovascular disease, stroke etc. Several studies have shown that free radicals alter the equilibrium between pro-oxidants and antioxidants resulting in modifications in the genomes, proteins, carbohydrate, lipid peroxidations and lipids in biologic systems. Plants however, have been shown to be endowed with phytochemicals that possess properties such as antioxidants, antimalarial, antibacterial, etc [3]. In recent times, it’s important to note that pharmacological investigations of medicinal plants have been the stepping stone towards the isolation and development of lead compounds with good safety and efficacy profile. Oxidative stress can be defined as a distortion in the balance between the production of pro-oxidants and the antioxidant system responsible for the homeostasis of an organism [4].
Several types of reactive oxygen species and reactive nitrogen species are generated in the body from free radicals or non-radicals which are the pro-oxidants. They attack macromolecules such as proteins, carbohydrates, lipids etc. leading to cellular damage. Antioxidants are often produced endogenously to counter their effects however, when there is a distortion in the homeostasis balance between pro-oxidants and antioxidants, exogenous sources of antioxidants are needed to restore the biological system back to normal. These antioxidants include vitamin A, C and E with some some other compounds that contain phenolics such as flavonoids, tannins etc [5].
Plants have severally been reported to possess these antioxidants in abundance, majority of which are yet to be investigated. Smilax anceps is used traditionally for general healing, debility etc [6]. Smilax anceps is a climbing shrub that is up to 5 m long, tough, fibrous, armed with numerous hooked prickles and paired coiled tendrils at the base of the leaf petioles. Leaves alternate, ovate, elliptic to almost circular, 4-14 cm long, more or less thickly leathery, 2-3 pairs of veins along each side of the midrib and net- veining between these veins; margin entire; petiole 0.5-2.5 cm long, thickened, channeled above [7]. Smilax is a genus from the family of plants known as the Smilacaceae which consist of over 350 species. The genus are climbers with tendrils for climbing as well as a long thin and thorny stem. Plants of this genus are known to have antioxidants, immunomodulatory, antibacterial, diuretic and antifungal properties [8]. Smilax zeylarica roots and rhizomes have been reported to possess hepatoprotective activity [9], the leaf and fruits were found to have antimicrobial and antioxidant activity [10] while the stem extract demonstrated good antioxidant and cytotoxic activity [11]. Hirota et al, [12] have also reported that Smilax larvata possess antioxidants, anti-nociceptive and anti-inflammatory properties. The consumption of exogenous antioxidants mitigates the destruction caused by oxidative stress through slowing or restriction of the processes involved in oxidative stress, by foraging the free radicals or acting as reducing agents [13]. Therefore, this study aims to investigate the in-vitro antioxidant property as well as the in-vivo hepatoprotective activity of the ethanol leaves extract.
Materials and Methods
Plant Material Collection
The leaves of Smilax anceps were collected from Ibaa in Emohua local Government area of Rivers State, Nigeria. Identification of the plant was done by Dr. Oladele of the Department of Forestry and Wildlife while authentication was done by Dr. Ekeke Chimezie of the Department of Plant Science and Biotechnology, University of Port Harcourt. A voucher specimen was deposited in the Herbarium with the code (UPH/V/1285) at the Department of Plant Science, University of Port Harcourt.
Extraction of Plant Material
The leaves of the plant were hand shredded into smaller pieces and air-dried under room temperature for two weeks. The air-dried leaves were pulverized by grinding with the aid of an electric grinder out of which 500g was macerated with 3.5L of absolute ethanol for 72 hours with intermittent shaking of the macerating jars. Filtration was done using whatmann filter paper, the filtrate was concentrated further with the aid of rotary evaporator and subsequently evaporated to dryness using a water bath at 45oC. The obtained yield was stored in airtight container and refrigerated.
Animals Used
Thirty adult albino rats and eighteen albino mice of weight ranging of either sex were maintained at the animal house of the Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Choba, Rivers State were used for the study. The animals were housed in cages of five animals each at normal room temperature. The animals were allowed to acclimatize for two weeks before the commencement of the study. The animals were fed on standard feed (Broiler finisher - Guinea feeds) and water ad libitum.
Drugs and Chemicals
Silymarin 70mg tablet (silybon-70) Micro labs. limited India.; Olive oil (Goya) Goya En Espana, S.A.U. Seville, Spain.; Absolute ethanol (JHD Guagdong Guanghua Sci-Tech.co. Ltd. Shantou, Guandong, China); Carbon tetrachloride (Sigma-Aldrich,St. Louis, Missouri, USA); Diethyl ether. (JHD Guagdong Guanghua Sci-Tech.co. Ltd. Shantou, Guandong, China).
Phytochemical Screening
Phytochemical screening was conducted on the ethanol extract of Smilax anceps according to the method described by [14].
In-vitro Antioxidant Assay
2,2, diphenyl, picryl,1, hydrazyl (DPPH) scavenging activity Three milliliters of each of the diluted extracts were put in the test tube and 1 mL of a methanol solution of DPPH (0.1 mM) was added. The mixture was kept in the dark at room temperature for 30 min and absorbance was measured at 517 nm against a blank. The same procedure was used for the ascorbic acid used as standard. The following equation was used to determine the percentage of the radical scavenging activity of the extract.
Scavenging effects (%) = 100 × (A0- As)/A0
Where A0 is the absorbance of the blank and As absorbance of the sample [15].
Nitric oxide scavenging activity
The reaction mixture contained 2 mL of sodium nitroprusside (10 mM) in 0.5 mL phosphate buffer (0.5 M; pH 7.4), 0.5 mL of various concentrations (100,200,400,600,800,1000 μg/mL) of the extracts were added to make a final volume of 3 mL which was rhen incubated for 60 min at 37°C. Griess reagent [α-napthyl- ethylenediamine (0.1%) and sulphanilic acid (1%) in H3PO4 (5%)] was added to the mixture after incubation. The pink chromophore generated during diazotization of nitrite ions with sulphanilamide and subsequent coupling with α-napthylethylenediamine was measured spectrophotometrically at 540 nm. Ascorbic acid was used as a positive control. All evaluations were done in triplicates. The scavenging ability (%) of the nitric oxide was calculated using the formula:
Scavenging effects (%) = 100 × (A0- As)/A0
Where A0 is the absorbance of the blank and As absorbance of the sample [15].
Reducing power assay
Different concentrations of extracts in 1 mL of distilled water were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferrocyanide. The mixtures were incubated at 50°C for 20 minutes. Aliquots of 2.5 mL of trichloroacetic acid (10%) were added to the mixtures and centrifuged at 3000 rpm for 10 min. The supernatant of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%). The absorbance was measured at 700 nm [15].
Lipid peroxidation assay
Excised rat liver was homogenized in buffer and then centrifuged to obtain liposome. To make 10 % liver homogenate, excised rat liver was minced using glass Teflon homogenizer in ice cold phosphate buffered saline (50 mm, pH 7.4). The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was used as liposome for in vitro lipid peroxidation assay. The process of homogenization and filtration was carried out in ice cold condition. Firstly, 0.5 mL of supernatant, 1 mL of 0.15 M potassium chloride, and 0.3 mL of extractives or standard at different concentrations (100-1000μg/mL) were mixed. Peroxidation was initiated by the addition of 300 μL of 0.5 mM FeCl. The mixture was incubated at 37 °C for 30 min and the reaction was stopped by adding 2 mL of ice-cold TBA-TCA-HCl-BHT solution. The TBA-TCA-HCl solution was prepared by dissolving 1.68 mg TCA and 41.60 mg TBA in 10 ml of 0.125 M HCl. 1 mL BHT solution (1.5 mg/mL ethanol) was added to 10 mL TBA-TCA HCl solution. The reaction mixture was heated for 60 min at 90°C and then cooled on ice and centrifuged at 3000 rpm for 5 min. The supernatants were removed and the intensity of the pink colored complex was measured using a spectrophotometer at 532 nm. The degree of lipid peroxidation was assayed by estimating the TBARS (TBA-reactive substances) content. A control experiment was performed in the presence of distilled water without the extract. The percentage of lipid peroxidation inhibition in the samples was calculated using the following formula:
% lipid peroxidation inhibition = [(A0 – AS) /A0] × 100
Where A0 is the absorbance of the control (300 μL of distilled water), and AS is the absorbance of extractives/standard. The % of inhibition was plotted against concentration, and from the graph, IC50 was calculated. The experiment was performed in triplicate and repeated three times at each concentration [16].
Total antioxidant activity by Ferric Reducing Antioxidant Power assay (FRAP)
The fresh FRAP reagent consisted of 500 mL of acetate buffer (300 mM; pH 3.6), 50 mL of 2,4,6- Tri (2-pyridyl)-s-triazin (TPTZ) (10 mM), and 50 mL of FeCl3•6H2O (50 mM). The colorimetric measurement was performed at 593 nm and the measurement was monitored up to 12 min on 75 μL of each extract and 2 mL of FRAP reagent [15].
Total phenol determination
The reaction mixture contained 200 μL of extract, 800 μL of freshly prepared diluted Folin Ciocalteu reagent and 2 ml of sodium carbonate (7.5%). The final mixture was diluted to 7 mL with deionized water and kept in the dark at ambient conditions for 2 h to complete the reaction. The absorbance was measured at 765 nm. Ascorbic acid was used as standard and the results were expressed as mg/g [15].
Total flavonoid content
This was determined using aluminium chloride (AlCl3). The extract at 0.1 ml was added to 0.3 ml distilled water followed by 0.03 ml of NaNO2 (5%). After 5 min at 25°C, 0.03 ml of 10% AlCl3 was added and 5 minutes afterward, 0.2 ml of 1 mM NaOH was added to the reaction mixture. After a further 5 minutes, the mixture was diluted to 1 mL with water prior to the measurement of the absorbance at 510 nm. The results were expressed in mg/100g [15].
Experimental Procedure
Acute Toxicological Evaluation
Eighteen albino mice of either sex were used in the study. The animals were deprived of food a night prior to the study. This was done in two phases of 9 animals each. The 9 animals in the first phase were grouped into three units of three animals each. The route of administration used for the study was oral and the doses for groups 1, 2, and 3 were 10 mg, 100mg and 1000 mg per kg of the ethanol extract of Smilax anceps respectively. The animals were observed closely for twenty-four hours for any sign of toxicity or death. The 9 animals in the second phase were grouped into three as in phase one and each group received different doses of the ethanol extract vis 1600 mg, 2900 mg and 5000 mg respectively [17].
Experimental Design
The method described by Nwidu et al, [18] and modified with the report of Sadeghi et al, [19] was used in this study. Albino rats of either gender were selected into six groups, each comprising five rats (two males and three females). The protocol used for the study was as follows:
Group A, served as the negative control and received 0.2ml/ kg distilled water once daily for seven days. Group B (positive control) received 0.2ml/kg distilled water while groups C, D and E were administered with 250, 500 and 1000 mg/kg ethanol extract, respectively, once daily for seven days while group F was treated with silymarin 100 mg/kg once daily by oral gavage for seven days. On the seventh day, groups B to F were treated with freshly prepared carbon tetrachloride in olive oil (1 ml/kg body weight in 1:1 intraperitoneally), one hour after administration of the last treatment dose. The body weights of all rats were recorded at the beginning and at the end of the experiment.
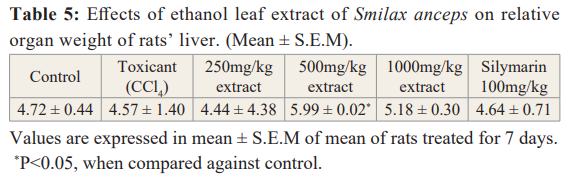
Relative Organ Weight
On the 8th day of the dosing period, all the animals were euthanized under diethyl ether induced anaesthesia. The liver was carefully dissected out and weighed in grams (absolute organ weight). The relative organ weight of each animal was then calculated using the equation:
Relative organ weight = Absolute weight (g)/ Body weight of rat on sacrifice day (g) × 100.
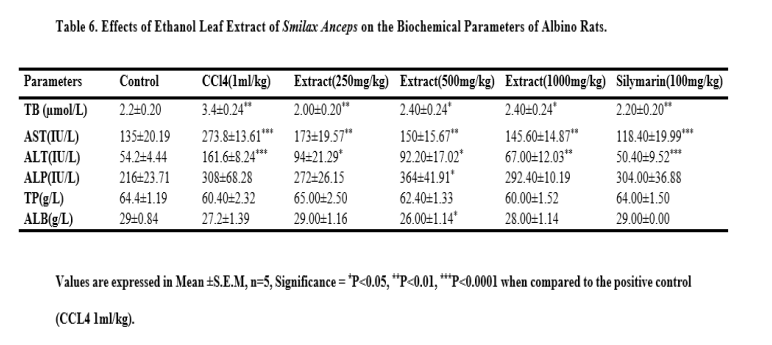
Assessment of Liver Function Parameters
Twenty- four hours after the administration of the last dose of carbon tetrachloride, Smilax anceps leaf extract and silymarin, treated animals were anaesthetized using diethlyl ether. Blood was collected by cardiac puncture. The serum was separated from the whole blood by centrifugation at 3000rpm for 10 minutes. The serum was evaluated for the alanine amino transferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP), albumin (ALB), total protein (TP) and total bilirubin (TB) levels.
Histopathology
The harvested liver tissues were dissected and representative tissue blocks of 3mm thick were taken for histological processing each with identifying label in a tissue cassette. On the average, the liver measured 4.2 x 2.0 x 1.7 cm. Compression effects arising from specimen containers were noted. The formalin fixed tissue blocks were dehydrated through ascending grades of alcohol, de-alcoholised in xylene, infiltrated and embedded in molten paraffin wax. Sections were cut at 3µm on a rotary microtome. Deparaffinised sections were then stained with the standard H&E staining technique and the slides mounted in distyrene plasticizer xylene (DPX). Sections on slide were examined and photomicrography images captured with ×40 objective lens [18].
Statistical Analysis
All the data obtained were expressed in mean ± SEM. Statistical analysis of data was done using one- way analysis of variance (ANOVA) followed by the post hoc test using graph pad prism version 5. This was done to determine the significance between the control group and the treated groups. P-values were considered statistically significant at a value of P<0.05.
Results
Phytochemical Screening
The qualitative phytochemical screening of the leaf extract of S. anceps indicated the presence of tannins, flavonoids, steroids/ triterpenes, saponins and carbohydrate while alkaloids and anthraquinones were absent.
Quantification of phytochemical constituents of ethanol leaf extract of Smilax anceps
The quantified total phenol content of S. anceps leaf extract was 66.54 ± 0.43 mg/100g, total flavonoid was 134.64 ± 1.77 mg/100g and total antioxidant capacity was 90.33 ± 0.76 mg/100g.
Acute Toxicity Result
The acute toxicity test result of the ethanol leaf extract of Smilax anceps showed that the median lethal dose (LD50) was greater than 5000 mg/kg, as no death was recorded above this dose.
In-vitro Antioxidant Activity Result
The DPPH radical scavenging activity of the extract showed a proportionate increase with increase in concentration as shown in table 1. The mean IC50 value of S. anceps extract for DPPH radical was 281.83 µg/ml.
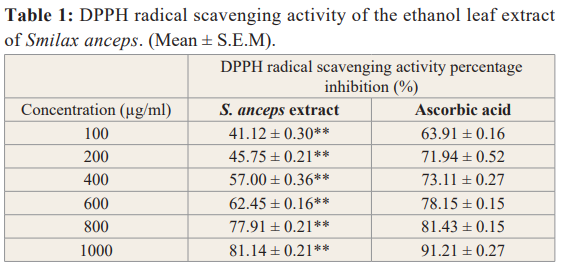
Values are expressed in Mean ± S.E.M, n=3, Significance =**P<0.01 when compared to the standard (Ascorbic acid).
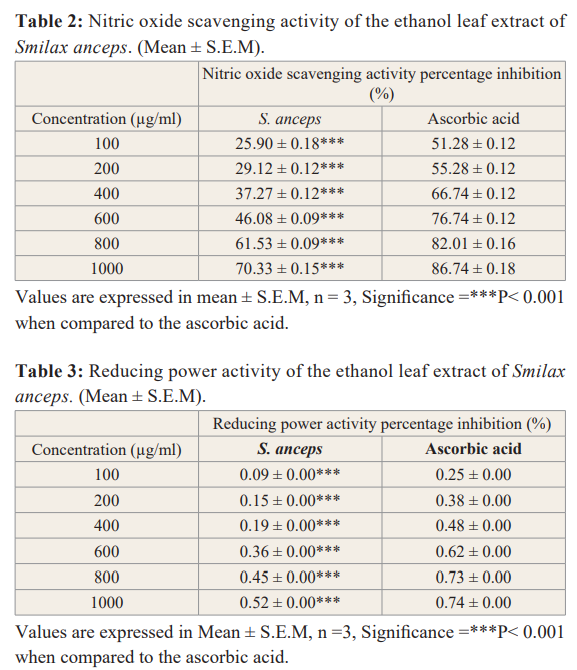
Nitric oxide radical scavenging activity of the extract had an IC50 value of 614.37 µg/ml while that of the standard (ascorbic acid) was 32.93 µg/ml. The extract inhibitory activity increases as the concentration increases as indicated in the table 2 below.
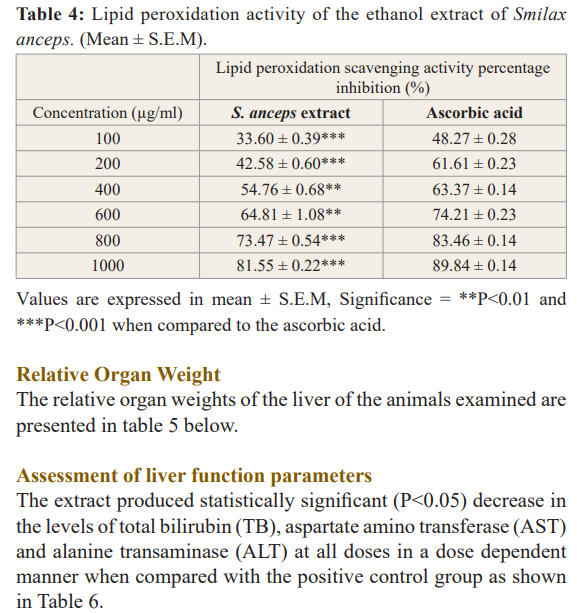
The reducing ability of the extract against free radicals was found to increase as the concentration of the extract increases as presented in table 3. S. anceps leaf extract had a mean IC50 value of 99,925 µg/ml which is comparable to that of ascorbic acid with mean IC50 was 82,919 µg/ml.
s.anceps leaf extract exhibited a significant protective inhibition of lipid peroxidation relatively with the standard (ascorbic acid).
There was an increase in protection of the cell membrane as the extract concentration increases with an IC50 value of 354.66 µg/ ml (Table 4).
Histopathology Result
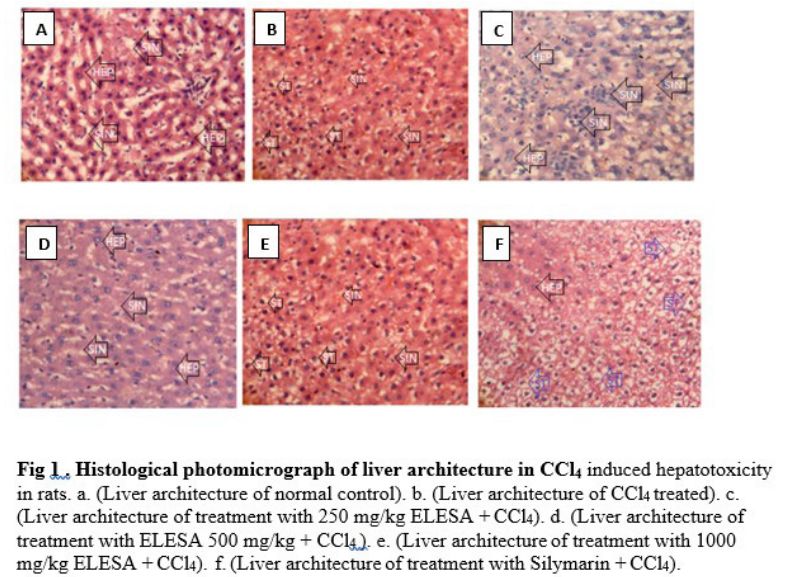
There were no histopathological changes in the liver tissues of the control group, normal hepatic histo-architecture with mild microvesicular fatty changes were observed. The extract treated groups showed histopathological changes which ameliorates with increase dose administration. The silymarin treated group also showed mild pathological changes; mild fatty depletion against a backdrop of moderate fatty changes (Figure 1).
Normal hepatocytes with sinusoids containing von Kuppfer cells were observed in the histology of normal control rats. The carbon tetrachloride treated liver tissue showed inflammatory cells and congested capillaries. However, pretreatment with S. anceps leaf extract exhibited a degree of protection through normal hepatocytes with microvesicular steatosis which ameliorated with increase in dose of the extract from 250 mg/kg to 500 and 1000 mg/ kg respectively. The silymarin treated group showed hepatocytic steatosis of different stages.
Discussion
Medicinal plants have from time immemorial remained the stepping stone towards the isolation of lead compounds in drug discovery and synthesis. Free radicals otherwise known as reactive oxygen species are known to damage biological cell membranes inducing lipid peroxidation, DNA damage and alteration in cell function [20]. They have been reported to also promote an imbalance in the redox system of biological cells and have been implicated in the pathogenesis of some chronic diseases such as cancer, Alzheimer disease and atherosclerosis.
Phytochemical screening results revealed the presence of plant secondary metabolites such as saponins, tannins, flavonoid, steroids/terpenoids and carbohydrates. The plant lacked the presence of alkaloids and anthraquinones.
The median lethal dose of the ethanol leaf extract of Smilax anceps was found to be greater than 5000mg/kg from the result of the acute toxicity evaluation. This therefore implies that the extract has a wide therapeutic window [17].
Phenolic compounds are powerful chain breaking antioxidants. The total phenolic constituent of Smilax anceps was 66.4mg/g when compared to ascorbic acid. The presence of this phenolic constituent may contribute to it antioxidant properties. This can be linked to the report of Muruhan et al., [21] which attributed the biological properties of Solanum surattense to the phenolic constituents. The total flavonoid and total antioxidant capacity were 134.64mg/g and 90.33mg/g respectively which is good and can be related to the report of Ozsoy et al., [22] that Smilax excelsa possessed reasonable total phenol and total flavonoids constituents.
The DPPH scavenging activity of the extract increases as the dose increased which was comparable to that of ascorbic acid. The extract also exhibited effective scavenging activity against nitric oxide generated free radicals. Smilax anceps ethanol leaf extract was also observed to possess good reducing power activity with the ability to donate electrons, hence promoting stability by inhibiting the initiation of chain reactions while offering a good degree of protection against lipid peroxidation [23]. Antioxidants obtained from plant sources have been reported to offer some degree of protection against cell damage by mitigating the effects of free radicals’ activity [24]. Thus, Smilax anceps leaf extract has potent antioxidant activity which may be attributed to the presence of phenolic compounds such as tannins and flavonoids. This can be corroborated to the reports of Senguttuvan et al. [25], which stated that the alcoholic and aqueous extract of the root and leaves of Hypochacus radicata showed prominent antioxidant properties due to the presence of flavonoids, tannins, and phenolics as its phytoconstituents.
Carbon tetrachloride as a known toxicant induces liver damage during its metabolism through the generation of free radicals. This leads to a sequence of events which may ultimately cause lipid peroxidation and interference with the integrity of the cell membrane, hence causing leakage of liver biomarkers and cell constituents into the blood [26].
In this study, the biochemical analysis revealed that the treatment with the ethanol leaf extract of Smilax anceps at all the examined doses significantly reduced the levels of aspartate aminotransferase (AST), alanine amino transaminase (ALT), alkaline phosphatase (ALP) and total bilirubin (TB) in all the groups compared to the group exposed to carbon tetrachloride only. Increase in serum liver enzymes is indicative of liver damage and S. anceps leaf extract was able to restore the enzymes levels to near normal in comparison with the normal control group which was not exposed to carbon tetrachloride. There was a significant decrease in albumin at 500 mg/kg of S. anceps while there was no significant difference in total protein at all the extract doses. This implies that the extract possesses hepatoprotective activity against carbon tetrachloride induced hepatic damage. This can be related to the report of Sadeghi et al., [19] which stated that the hydro-alcoholic fruit extract of Rosa canina exhibited hepatoprotective activity and that of Jevadevi et al, [27] which stated that Ipomoea staphylina leaves showed potent antioxidant and hepatoprotective activity against carbon tetrachloride induced hepatic damage.
Histological examination confirmed the results obtained from the biochemical analysis which is a reflection of S. anceps hepatoprotective activity. Histological examination of the liver sections of the control animals showed normal hepatocytes (fig.1a). Exposure to carbon tetrachloride induced inflammatory cells and congested capillaries (fig.1b). However, pretreatment with the ethanol extract of Smilax anceps showed amelioration of carbon tetrachloride induced hepatotoxicity (figs.1c, 1d and 1e) The extract was most effective at the dose of 1000 mg/kg which showed microvesicular steatosis. (fig. f). The silymarin treated group showed normal hepatocytes with microvesicular steatosis. The phytoconstituents of this plant such as the phenolics may be responsible for the antioxidant and hepatoprotective activity [28,29].
Conclusion
Thus, it could be said that the ethanol extract of Smilax anceps leaf seems to possess potent antioxidant and hepatoprotective activity which is dose-dependent.
Conflicts of Interest
The authors hereby declare that no conflict of interest exist.
Funding
There was no external funding in this study.
References
- Hance J. What does nature give us? 2011. ( https://news.com/2011/04/what-does-nature-give-us-a-special-earth-day-article/ ) access date: 8/8/2018.
- Ciddi Natural Products Derived from Plants as a Source of Drugs. J of Adv Pharm Tech Res. 2012; 3: 200-201.
- Kaushik P, Kaushik D, Khokra In vivo antioxidant activity of plant Abutilon indicum. J Pharm Edu Res. 2011; 2: 136- 138.
- Betteridge What is Oxidative Stress? Metabolism. 2000; 49: 3-8.
- Kelly FJ. Use of antioxidant in prevention and treatment of disease. J Intl Fed Clin Chem. 1998; 10: 21-23.
- Burkill The useful plants of West Tropical Africa, Vol.3. Royal Botanic Gardens, Kew, UK. 1985.
- Richard Encyclopedia of life, 2017, Miles Kelly Publishing Limited (2017). 512 pages. ISBN 10: 1786173271 ISBN 13: 9781786173270.
- Raúl SC, Beatriz HC, Joseoziel LG, et Phenolic compounds in genus Smilax (Sarsaparilla). In M Soto-Hernandez, M Palma-Tenango, M del Roasario Garcia-Mateos, Eds., Phenolic compounds – Natural sources, importance and applications. Intechopen, Rijeka. 2017; 233-260.
- Murali A, Ashok P, Madhavan Screening of methanol extract of roots and rhizomes of Smilax zeylarica for hepatoprotective effects against carbon tetrachloride induced hepatic damage. J Exp Integrative Med. 2012; 2: 237-244.
- Dhanya Shree VS, Arbin A, Saema Noorain GK, et al. Preliminary phytochemical analysis, antimicrobial and antioxidant activity of Smilax zeylanica L. (Smilacaceae). J Drug Delivery Therapeutics. 2018; 8: 237-243.
- Uddin MN, Ahmed T, Pathan S, et al. Antioxidant and cytotoxic activity of stems of Smilax Zeylanica in vitro. J Basic Clin Physiol Pharmacol. 2015; 26: 453-463.
- Hirota BCK, Paula CS, de Oliveira VB, et al. Photochemical and Antinociceptive, Antiinflammatory and Antioxidant Studies of Smilax larvata (Smilacaceae). Evid-based Complement Alternat Med. 2016; 2016: 9894610.
- Baiano A, del Nobile MA. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 2015; 56: 2053-2068.
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3rd edition, Chapman and Hall, London, UK, 1998.
- Moukette BM, Pieme CA, Njimou JA, et In-vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol Res. 2015; 48: 15.
- Rahman T, Hosen I, Islam TMM, et Oxidative stress and human health. Adv Biosci Biotech. 2012; 3: 997-1019.
- Lorke D. A new approach to acute toxicity testing. Arch Toxicol. 1983; 54: 275-289.
- Nwidu LL, Oboma YI, Elmorsy E, et al. HEPA to protective effect of hyrdromethanolic leaf extract of Musanga cecropioides (Urticaceae) on carbon tetrachloride-induced liver injury and oxidative J Taibah Univ Med Sci. 2018; 13: 344-354.
- Sadeghi H, Hosseinzadeh AS, Akbartabar TM, et al. Hepatoprotective effect of Rosa canina fruit extract against carbon tetrachloride induced hepatotoxicity in Avicenna J Phytomed. 2016; 6: 181-188.
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. The American J Clin Nutri. 1993; 57: 715-724.
- Muruhan S, Selvaraj S, Viswanathan In vitro antioxidant activity of Solanum surattense leaf extract. Asian Pacific J Trop Biomed. 2013; 3: 28-34.
- Ozsoy N, Can A, Yanardag R, et al. Antioxidant activity of Smilax excelsa L. Leaf extract. Food Chem. 2008; 110: 571-
- Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica ) leaves. Food Chem. 2007; 102: 1223-1240
- Wong C, Li H, Cheng K, et al. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006; 97: 705-711.
- Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of the leaf and root parts of the medicinal herb Hypochaeris radicata Linn.for in vitro antioxidant activity. Asian Pacific J Trop Biomed. 2014; 4: S359-S367.
- Halliwell Β, Gutteridge JΜ. Role of free radicals and catalytic metal ions in human diseases: an Methods Enzymol. 1990; 186: 1-85.
- Jeyadevi R, Arul Ananth D, Sivasudha T. Hepatoprotective and antioxidant activity of Ipomoea staphylina Linn. Clin Phytosci. 2019; 5: 1-11.
- Duh PD, Tu YY, Yen Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT- Food Sci Technol. 1999; 32: 269-277.
- Yang J, Li Y, Wang F, et Hepatoprotective effects of apple polyphenols on carbon tetrachloride induced acute liver damage in mice. J Agric Food Chem. 2010; 58: 6525-6531.