Breast Cancer Chemotherapy In Pointe Noire
Author'(s): Ngatali, C.F.S1*, Moukassa, D3 and Nkoua-Mbon, JB2
1Department of Oncology and Internal Medicine, Loandjili General Hospital (Congo).
2Medical Oncology Department, Teaching Hospital ofBrazzaville (Congo).
3Hospital General, Edith Lucie Bongo (Congo).
*Correspondence:
Ngatali Christian, Loandjili General Hospital and Faculty of Health Sciences, Brazzaville.
Received: 28 Jan 2022; Accepted: 18 Feb 2022; Published: 25 Feb 2022
Citation: Ngatali CFS, Moukassa D, Nkoua-Mbon JB. Breast Cancer Chemotherapy In Pointe Noire. Cancer Sci Res. 2022; 5(1): 1-5.
Abstract
Objective: The objective of our study was to determine chemotherapy and these frequent side effects of breast cancer in Pointe-Noire.
Patients and Methods: This was a retrospective descriptive study that took place in the cancerology and internal medicine department during the period from January 1, 2019 to December 31, 2021. Were included in our study: patients with and histological diagnosis, and having received at least one course of chemotherapy and having presented hematological toxicity: anemia and/or neutropenia. Patients having status performances less than 2. The variables studied were: Age, level of study, stage of extension, type of chemotherapy, type of toxicity. L4ANA The khi2 was used and results were statistically significant for a value of P<5%.
Results: The average age of the patients was 50.35 ± 13.6 years. The extremes were 27 years and 79 years old. The most represented age group was the age group from 37 to 46 years with 18 cases or 33.33%. The most represented level study in our study was the primary level 70%, followed by secondary level 26% and the upper or superior level 11%. Metastatic stage of location was represented in 16.6% of cases, the local stage was represented in 16.7% of cases. The most commonly chemotherapy used was FAC protocol in 50% of cases, followed by FAC+DOCETAXEL in 47% of cases, AC protocol was used in 3% of cases The majority of patients had received more than 3 courses of chemotherapy in 83% of cases case. Frequent toxicities were fatigue 100%, alopecia 100%, nausea 100%, hematological 95%, vomiting 20%. The most used chemotherapy was FAC type chemotherapy in 19 cases, 4 cases, 2 cases respectively at the locoregional, metastatic and local stages.
Conclusion: Chemotherapy for breast cancers in our context with limited resources is of the anrhracyclines (AC, FAC) and taxanes (docetaxel) type. These chemotherapies are responsible for haematological toxicities, fatigue, nausea, vomiting and alopecia.
Keywords
Introduction
Breast cancer is the leading cancer and the leading cause of cancer death in women worldwide [1-6]. In low income countries, breast cancer is often diagnosed at advanced stages and has a poor prognosis [6]. Drug therapies used for breast cancer are classically classified into three categories: endocrine or hormonal therapy, targeted therapies, including anti HER2, and chemotherapy. Various combinations of these classes of drugs are given as either an adjuvant or neoadjuvant regimens. The adjuvant therapy follows the primary treatment by surgery with or without radiation to decrease the risk of distant recurrence in an approach that proved to improve outcomes and survival rates [7,8]. In contrast, neo-adjuvant regimens are given before surgery to downsize or downstage the tumor in the cases of locally advanced or large tumors[8,9]. Chemotherapy is an essential component in many cases of breast cancer, and different classes of cytotoxic agents are being used. The main chemotherapy classes used are anthracyclines, anti-microtubules (taxanes), alkylating agents (cyclophosphamide), antimetabolites (5-fluorouracil, capecitabine), platinum compounds (cisplatin), and others. These drugs are usually given in multiple-drug regimens, which proved to be superior to single agents in terms of efficacy and safety[10]. However, in cases of advanced breast cancer, some chemotherapies are given as single agents.
Neoadjuvant chemotherapy (NACT), or preoperative chemotherapy, was introduced in the treatment of locally advanced breast cancer in the 1980's.
The major advantage of NACT is downsizing the tumor and downstaging the axilla. By downstaging, possible metastases to axillary lymph nodes are reduced. Downsizing breast tumors facilitates conversion of inoperable tumors to operable ones and enables the surgeon to offer minor surgery or breast-conserving surgery (BCS) instead of mastectomy [11-13].
Thereby, the possibility of mastectomy and immediate breast reconstruction (IBR) is enhanced. The increased utilization of NACT has resulted in 16–17% of the patients converting from mastectomy to breast conserving surgery and mastectomy followed by radiationtherapy [12,14]. Chemotherapy causes many side effects that can reduce.
In Congo, to our knowledge, no study has been carried out, so we set ourselves the objective of determining the types of chemotherapy and their side effects in patients with breast cancer in Pointe-Noire.
Patients and Methods
This was a cross-sectional descriptive study that took place in the cancerology and internal medicine department during the period from January 1, 2019 to December 31, 2021, i.e. a period of 3 years. The following were included in our study: patients with a histological diagnosis of cancer and an extension assessment made of a thoraco-abdominal CT scan and/or a chest X-ray and an abdominal ultrasound, all patients who had hematological toxicity (anemia and/ or neutropenia) discovered by complete blood count; patients who have received at least one cycles of chemotherapy for breast cancer in a neoadjuvant or adjuvant setting; patients having status performances less than 2. The type of chemotherapy used was alone or in combination or séquential. chemotherapy and radiation Chemotherapy was neoadjuvant or adjuvant or sequential.
The data was collected from the records of patients hospitalized in the department for breast cancer.
The variables studied were:
- socio-demographic: age, level of study, socio-economic level
- clinics: internship extension
- type of chemotherapy
- toxicity
The stage of extension was grouped in local (stage 0 and I), locoregional or advanced (stage II and III) and metastatic for stage IV.
The collection of data was made from a previously written survey sheet, containing the different variables studied. Data entry was done using the Excel version 2016 software. Qualitative variables were represented in terms of number and percentage. Quantitative variables were represented effective and on average. The statistical analysis and the data processing were carried out by the Excel 2016 software and the graphpad prism version 7 software. The statistical test used was the chi square test.
Results
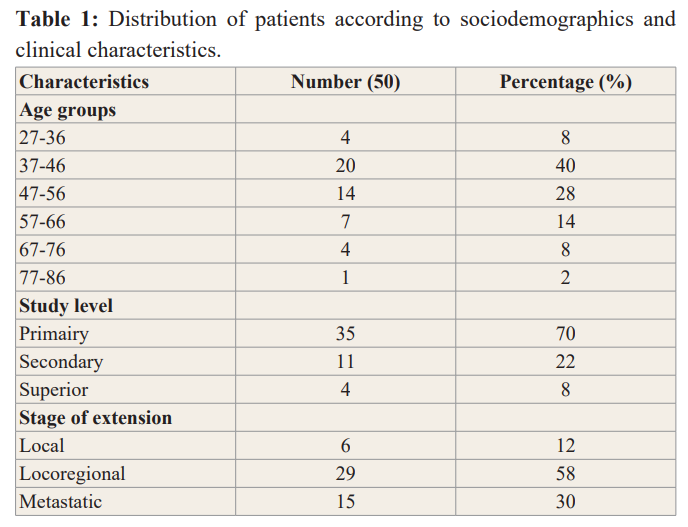
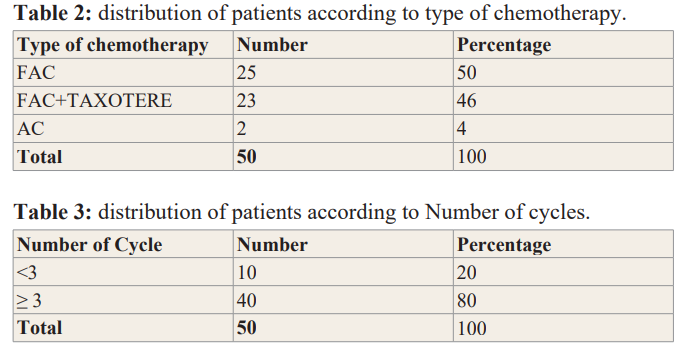
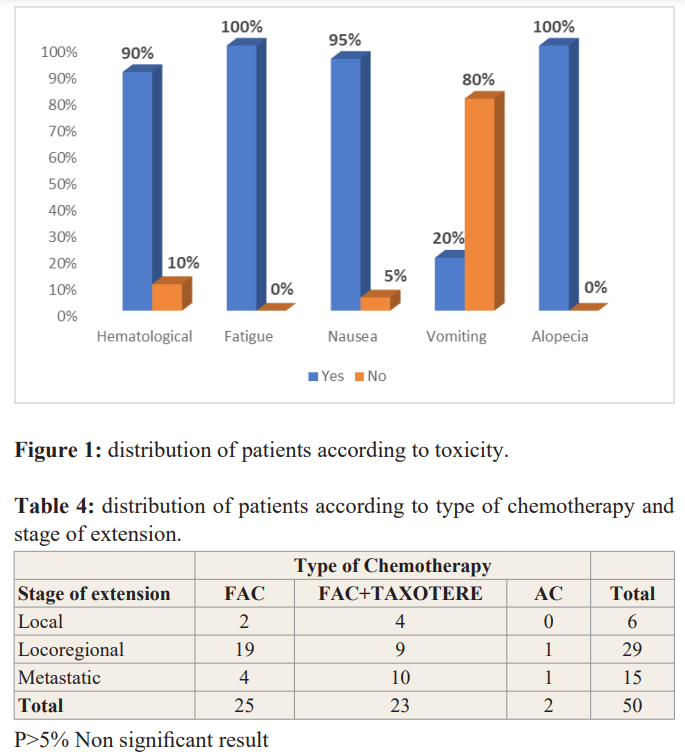
70 files had met the inclusion criteria. The average age of the patients was 51 ± 12 years. The extremes were 27 years and 79 years old. The most represented age group was the age group from 37 to 46 years with 24 cases or 34% Table1. The most represented level study in our study was the primary level 70%, followed by secondary level 22% and the upper or superior level 8%Table1. The majority of patients presented at locoregional stage in 58% of cases, local stage was represented in 6% of cases while the metastatic stage was represented in 30% of cases. Table1. The most used chemotherapy was anthracyclines (FAC) in 50% of cases followed by fac +docetaxel regimens in 46% and AC regimens in 6% Table 2. Most of the patients received more than 3 Cycles of chemotherapy in 80% of cases, 20% received less than 3 Cycles Table 3. Frequent toxicities were fatigue 100%, alopecia 100%, nausea 100%, hematological 95%, vomiting 20% Figure 1. The bivariate analysis showed that the most used chemotherapy was FAC type chemotherapy in 19 cases, 4 cases, 2 cases respectively at the locoregional, metastatic and local stages. FAC + docetaxel sequential chemotherapy was the second most used chemotherapy in 10 cases, 9 cases, 4 cases respectively for the metastatic, locoregional and local stages Table 3.
Discussion
The average age of the patients in our study was 51 ± 12 years, the extremes were 27 years and 79 years. Breast cancer affects women at a relatively young age. The average age was relatively young, this average age of the patients in our study was found by most authors in Africa with averages of 47.5 ± 12.36; 47.97 respectively described by Ngowa J et al. [15], Cameroon and Mensah et al Ghana [16]. On the other hand, in developed countries in the USA, for example, the average age of breast cancer patients was 61 years with the extremes of 55 years and 64 years [17]. In Saudi Arabia the average age found was also relatively young 47.16 ± 12.15 [18]. The age group most represented in our study was 37 to 46 years of age, followed by the age group 47 to 56, the younger age groups. These age groups were close to the age groups of the majority of African countries [15,16]. In the US, the most affected age group was 54 at age 65 [17]. The age groups of patients in both developed and US countries are quite high age groups, unlike age groups in African countries, especially those in developmental pathways. This could be explained on the one hand by the composition of the African population which is a young population and on the other hand by the probable increase in the incidence of breast cancer in this age group but also by the development diagnostic and therapeutic techniques. In fact, the population of developed countries is aging and age is a risk factor for breast cancer. The level of study in our study was primary in our study in 70% of cases, followed by the secondary level 22% of cases. In developing countries, patients had a primary level of education or were illiterate [19]. In developed countries, particularly in the US, the highest level of education is the highest level [20,21].
Type of chemotherapy
The chemotherapy protocols used in our study were protocols based on anthracyclines (FAC and AC) but also based on taxanes. These treatments were used in a neoadjuvant or adjuvant setting.
The benefit of adjuvant chemotherapy was well established by the 1980s. A meta-analysis including 40 adjuvant chemotherapy trials in over 13,000 breast cancer patients showed multiagent chemotherapy reduced the annual odds of death by about one quarter in the initial 5 years after treatment for women under 50 [22]. Although many of these early data supported the use of the combination of cyclophosphamide, methotrexate, and fluorouracil (CMF), advanced breast cancer studies in the 1980s suggested greater activity of anthracycline-containing regimens based on higher response rates and response durations [23]. Subsequently, multiple randomized trials compared adjuvant anthracycline-based chemotherapy regimens with CMF and suggested a diseases free Survival and overall survival benefit with adjuvant anthracyclines [24-26].
One study established the anthracycline-and taxane chemotherapy protocol as a standard for adjuvant treatment. Notably, the absolute benefit is relatively small, indicating only a small subset of patients with invasive breast cancer derive benefit from adjuvant anthracyclines when compared with other adjuvant chemotherapy regimens [27]. Other authors have used anyhracyclines in 54% of cases [28].
In our study 80% of the patients had received more than 3 cures in our study, indeed this could be explained by the fact that they arrived at the advanced stages.
Toxicity
Anthracycline-associated adverse events are considered as a significant limiting factor in utilizing these powerful cytotoxic agents. These events include mainly cardiotoxicity that might occur as acute toxicity manifested by arrhythmias or depressed ejection fraction, particularly in the left ventricle (LVEF) or might be chronic that develops years after the anthracycline use [29]. In addition to their cardiac effects, hematological toxicity, gastrointestinal toxicity, and febrile neutropenia events are all among the dose-limiting side effects of anthracyclines [29].
In our study we observed the following adverse effects: fatigue, alopecia, nausea, vomiting, and hematological toxicity. According to Montemurro et al [30] symptom incidence after the third cycle was highest for fatigue (78%), nausea (73%), dysgeusia (51%), anorexia (53%), and constipation (49%). Toxicity results were presented for all chemotherapy regimens combined, not separately for the chemotherapy regimens in the sample—5- fluorouracil/epirubicin/ cyclophosphamide (64%), doxorubicin/ cyclophosphamide or epirubicin/cyclophosphamide (22%), or docetaxel/cyclophosphamide (14 %). In general, few studies report toxicities for individual chemotherapy regimens [31], and these were mostly for single-symptom studies such as fatigue [32,33].
Type of chemotherapy and stage of extension
The bivariate analysis showed that the most used chemotherapy was FAC type chemotherapy in 19 cases, 4 cases, 2 cases respectively at the locoregional, metastatic and local stages. FAC+ docetaxel sequential chemotherapy was the second most used chemotherapy in 10 cases, 9 cases, 4 cases respectively for the metastatic, locoregional and local stages. But this result ws not signufucant. First this result of the use of fac or anthracylices in our context of limited resources can be explained by the high cost of taxane-based chemotherapy. And the second reason was that most of patients was in advanced stage.
Conclusion
Chemotherapy for breast cancers in our context with limited resources is of the anrhracyclines (AC, FAC) and taxanes (docetaxel) type. The most commly chemotherapy used in our limited resources context is anthracycines based regims. These chemotherapies are responsible for haematological toxicities, fatigue, nausea, vomiting and alopecia. The cost reduction taxanes regimens will help patients to receive more chemotherapy based on taxanes.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020 GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J 2021; 71: 209-249.
- Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA A Cancer Journal for Clinicians. 2011; 61: 69-90.
- Bray F, Ferlay J, Soerjomataram I, et Global Cancer Statistics GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer Journal for Clinicians. 2018; 68: 394-424.
- Gullatte M, Phillips J, Gibson L. Factors Associated with Delays in Screening of Self-Detected Breast Changes in African-American Women. Journal of National Black Nurses 2006; 17: 45-50.
- Brahim NA, Odusanya Knowledge of Risk Factors, Beliefs and Practices of Female Healthcare Professionals towards Breast Cancer in a Tertiary Institution in Lagos Nigeria. BMC Cancer. 2009; 9: 76.
- Lauby-Secretan B, Scoccianti C, Loomis D, et International Agency for Research on Cancer Handbook Working Group. Breast-Cancer Screening Viewpoint of the IARC Working Group. The New England Journal of Medicine. 2015; 372: 2353-2358.
- Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer an BMC Med. 2015; 13: 195.
- Carels N, Spinassé LB, Tilli TM, et al. Toward precision medicine of breast cancer. Theor Biol Med Model. 2016; 13:
- Joseph K, Vrouwe S, Kamruzzaman A, et Outcome analysis of breast cancer patients who declined evidence- based treatment. World J Surg Oncol. 2012; 10: 118.
- González Neira Pharmacogenetics of chemotherapy efficacy in breast cancer. Pharmacogenomics. 2012; 13: 677- 690.
- Thompson AM, Moulder-Thompson Neoadjuvant treatment of breast cancer. Ann Oncol. 2012; 23: x231-x236
- Ataseven B, von Minckwitz G. The impact of neoadjuvant treatment on surgical options and Ann Surg Oncol. 2016; 23: 3093-3099.
- Pathak M, Deo SS, Dwivedi S, et al. Role of neoadjuvant chemotherapy in breast cancer patients systematic review and meta-analysis. Indian J Med Paediatr 2019; 40: 48.
- González Neira Pharmacogenetics of chemotherapy efficacy in breast cancer. Pharmacogenomics. 2012; 13: 677- 690.
- Jean Dupont Kemfang Ngowa, Jean Marie Kasia, Jean Yomi, et Breast Cancer survival in Cameroun Analysis of a cohort of 404 patients at Yaoundé General Hospital. Advances in Breast Cancer Research. 2015; 4: 44-52.
- Mensah A, Yarney J, Nokoe S, et al. Survival Outcomes of Breast Cancer in Ghana: An Analysis of Clinicopathological Open Access Library Journal. 2016; 3: 1-11.
- Hope S, Rugo, Melanie Majure, et al. Neoplasme of breast Cancer Edition. 2017; 9: 1368-1438.
- Al Isawi Breast Cancer in Western Iraq: Clinicopathological Single Institution Study. Advances in Breast Cancer Research. 2016; 5: 83-89.
- Gueye M, Gueye S, Diallo M, et Sociodemographic Factors Associated with Delays in Breast Cancer. Open Journal of Obstetrics and Gynecology. 2017; 7: 455-463.
- Dieterich M, Stubert J, Reimer T, et al. Influence of life style factors on breast cancer risk. Breast cancer Basel. 2014; 9: 407-414.
- Cuzick Assessing risk for breast cancer. Breast Cancer Res. 2008; 10: S13.
- Early Breast Cancer Trialists' Collaborative Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988; 319: 1681-1692.
- Aisner J, Weinberg V, Perloff M, et al. Chemotherapy versus chemoimmunotherapy CAF v CAFVP v CMF each +/- MER for metastatic carcinoma of the breast A CALGB study Cancer and Leukemia Group J Clin Oncol. 1987; 5: 1523-1533.
- Ejlertsen B, Mouridsen HT, Jensen MB, et Improved outcome from substituting methotrexate with epirubicin Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer. 2007; 43: 877-884.
- Coombes RC, Bliss JM, Wils J, et Adjuvant cyclophosphamide methotrexate and fluorouracil versus fluorouracil epirubicin and cyclophosphamide chemotherapy in premenopausal women with axillary node positive operable breast cancer Results of a randomized trial. The International Collaborative Cancer Group J Clin Oncol. 1996; 14: 35-45.
- Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide epirubicin and fluorouracil chemotherapy compared with cyclophosphamide methotrexate and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group J Clin 1998; 16:2651-2658.
- Ami N Shaha, William J Gradishar. Adjuvant Anthracyclines in Breast Cancer What Is Their Role. Oncologist. 2018; 23: 1153-1161.
- Kirsten A Nyrop, Allison M Deal, Shlomit S Shachar, et al. Patient-Reported Toxicities During Chemotherapy Regimens in Current Clinical Practice for Early Breast Oncologist. 2019; 24: 762-771.
- Schneider BP, Shen F, Gardner L, et Genome-Wide Association Study for Anthracycline Induced Congestive Heart Failure. Clin Cancer Res. 2017; 23: 43-51.
- Montemurro F, Mittica G, Cagnazzo C, et al. Self evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA 2016; 2: 445-452.
- Hall E, Cameron D, Waters R, et al. Comparison of patient reported quality of life and impact of treatment side effects experienced with a taxane-containing regimen and standard anthracycline based chemotherapy for early breast cancer 6 year results from the UK TACT trial CRUK/01/001. Eur J 2014; 50: 2375-2389.
- Jong N, Candel MJ, Schouten HC, et Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004; 15: 896-905.
- Junghaenel DU, Cohen J, Schneider S, et Identification of distinct fatigue trajectories in patients with breast cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015; 23: 2579-2587.