Chemical Meningitis Following Spinal Analgesia with Levobupivacaine in Labor and Delivery: A Case Report
Author'(s):Anamarija Predrijevac1, Alan šustiÄ?2, Igor AntonÄiÄ?3, Siniša Dunatov3, Željko Župan2, Janja KuhariÄ?2,Boban DangubiÄ?2 and Vlatka Sotošek TokmadŽiÄ?2*
1Faculty of Medicine, University of Rijeka, Croatia, Brace Branchetta 20, 51000 Rijeka, Croatia.
2Department of Anaesthesia, Resuscitation and Intensive Care Medicine, Faculty of Medicine, University of Rijeka, Croatia,Brace Branchetta 20, 51000 Rijeka, Croatia.
3Department of Neurology, Faculty of Medicine, University of Rijeka, Croatia, Brace Branchetta 20, 51000 Rijeka, Croatia.
*Correspondence:
Vlatka Sotošek tokmadžiÄ?, MD, PhD, Department of Anaesthesia,Resuscitation and Intensive Care Medicine, Faculty of Medicine,University of Rijeka, Brace Branchetta 20, 51000 Rijeka, Croatia,Tel: +38551651182; E-mail: vlatkast@medri.uniri.hr.
Received: 14 October 2017; Accepted: 05 November 2017
Citation: Predrijevac A, šustiÄ? A, AntonÄiÄ? I, et al. Chemical Meningitis Following Spinal Analgesia with Levobupivacaine in Labor and Delivery: A Case Report. Anesth Pain Res. 2017; 1(1): 1-3.
Abstract
Chemical meningitis is a very rare but potentially devastating complication of spinal anaesthesia and analgesia. It can be provoked by intrathecal application of substances, such as local anaesthetics, or may occur as a result of the anaesthesia technique used. We describe, until now published, a case of 20-year-old primipara who received spinal analgesia with levobupivacaine for labor and delivery and developed generalized epileptic seizures and high fever. Laboratory tests showed an increased white blood cell count, elevated neutrophil granulocytes, and elevated C-reactive protein; the cerebrospinal fluid (CSF) analysis showed increased levels of proteins, lactate, leukocytes, and erythrocytes. A brain computed tomography (CT) and CT angiography scan did not reveal any pathological alteration. Microbiological analysis of CSF and blood cultures did not show any pathogen growth, and the patient was treated with antibiotics and corticosteroids. The patient later fully recovered and was discharged from the hospital.
Keywords
Introduction
Spinal anaesthesia is one of the most reliable and versatile techniques available for providing anaesthesia and analgesia for labor and delivery. Although it has been used since the 1800s, its frequency of use decreased due to complications and the invention of the epidural technique. It became popular again with the development of newer, beveled needles. Spinal anaesthesia has many advantages, including extremely rapid onset of pain relief and the ability to retain motor control, but it is not free of side effects [1,2]. The most common side effects of spinal analgesia are hypotension and post-dural puncture headache [3]. These effects are usually mild, well tolerated, and transient [4]. More serious side effects, such as meningitis or seizures, are rare but potentially fatal. When meningitis, either chemical or bacterial, or epilepsy occurs, they must be treated without delay. We describe, in English literature until now unpublished, a case of chemical meningitis following spinal analgesia with levobupivacaine.
Case Report
A 20-year-old primipara at 39 weeks of pregnancy received spinal analgesia for labor and delivery. The technique and possible complications of spinal analgesia were explained to her, and she signed the informed consent form. The primipara was in a sitting position. An aseptic washing solution containing iodine (Antiseptica, Pulheim, Germany) was applied, and the excess solution was removed. Spinal anaesthesia was performed at the L2- to-L3 level using an atraumatic 26-gauge spinal needle (B Braun, Melsungen, Germany). When the spinal space was detected, 1.5 mL of 0.5% levobupivacaine (Fressenius Kabi, Bad Homrung, Germany) and 2.5 μg of sufentanil (Renaudin, Itxassou, France) were applied intrathecally. Within five minutes, the patient felt pain relief and in forty minutes, she delivered a healthy baby boy. The next day she began to complain of a headache, neck and shoulder pain, and neck stiffness. Pain in the frontal area of the head was more intense when she stood up. Analgesic therapy (diclofenac, Voltaren, Pliva, Zagreb, Croatia) and hydration with crystalloid fluids were prescribed to the patient, and she was advised to stay in bed. She felt well, did not have a headache or other symptoms, and was discharged from the hospital 2 days after the delivery.
On the third day postpartum, while breast-feeding; she had two generalized epileptic seizures at her home. Her husband called an emergency medical service that transported the patient to the emergency room at the Clinical Hospital Centre Rijeka, Rijeka, Croatia. During transportation, she had another generalized seizure and urinated herself. On arrival at the emergency room, she was conscious but disoriented. Neurological examination revealed neck stiffness. Inspection of the spinal puncture site did not reveal any signs of infection. Laboratory tests showed an increased white blood cell count, elevated neutrophil granulocytes, and elevated C-reactive protein. Multi-sliced computed tomography (MSCT, Avanto 1.5 T, Siemens, Forcheim, Germany) and computed tomography (CT, Sensation 16, Siemens, Forcheim, Germany) angiography of the brain were normal. The results of the cerebrospinal fluid (CSF) analysis are shown in Table 1.
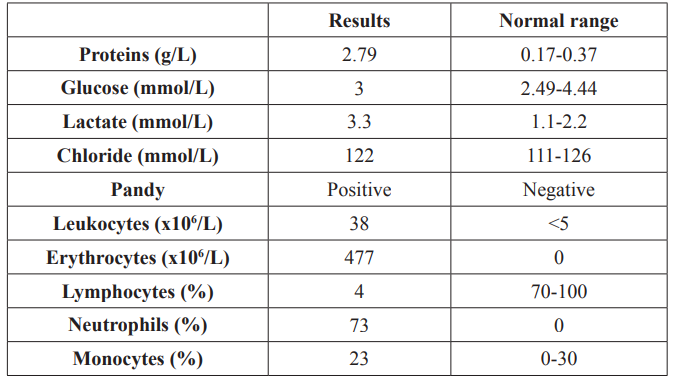
The patient was then admitted to the neurological intensive care unit, where she was treated with continuous infusion of 0.01 mg/ kg/h of tramadol (Stada, Bad Vilbel, Germany) and 50 μg/kg/h of midazolam (Roche, Basel, Switzerland).
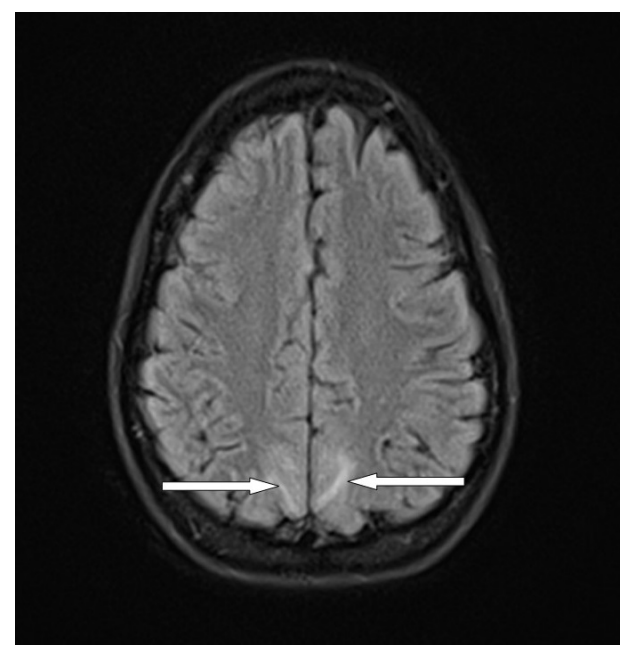
Blood cultures were taken and intravenous triple antimicrobial therapy with 2 g meropenem (Sandoz International GmbH, Holzkirchen Germany) three times per day, 1 g vancomycin (Xellia Pharmaceuticals ApS, Copenhagen, Denmark) three times per day, and 750 mg acyclovir (GlaxoSmith Kline, Bredford, UK) three times per day was started. The next day the patient was somnolent, respiratory and hemodynamically stable, and febrile up to 38.7°C. Two days after admission to the neurological intensive care unit, she had a generalized convulsive seizure followed by altered consciousness, and during the night, she developed status epilepticus. She was febrile up to 39°C, and was then endotracheally intubated and mechanically ventilated. Continuous analgosedation with midazolam and sufentanil was administered intravenously. Microbiological analysis of CSF and blood cultures did not show any growth of pathogens, and 125 mg methylprednisolone (Pfizer Manufacturing, Rijksweg, Belgium) was administered intravenously. Electroencephalography (EEG) showed slow activity in the fronto-temporo-central area of the brain. The patient was mechanically ventilated for the next 4 days. She was hemodynamically stable, occasionally tachycardic, and sub- febrile. Analgosedation was discontinued 6 days after admission to the neurologic intensive care unit; the patient was weaned from the ventilator and extubated. She was alert, complained of a mild headache, and had neck stiffness. The remainder of her examination was unremarkable, without neurological deficits. Brain magnetic resonance imaging (MRI, Avanto 1.5 T, Siemens, Germany) showed postictal edematous changes (Figure 1). During the clinical course of her illness, the patient fully recovered and was discharged from the hospital. Oxcarbazepine (Trileptal, Novartis Farma, Naples, Italy) at a dose of 300 mg twice per day was prescribed. Clinical examination of the patient three months after hospital discharge showed no neurological deficits, and the results of all laboratory tests were within normal ranges.
Discussion
Neurologic complications after spinal anaesthesia in obstetric patients are fortunately rare, and often occur as a complication of neuraxial block. They can be classified into three groups: 1) those related directly to anaesthesia, 2) those unrelated to anaesthesia, and 3) those in which anaesthesia is incidental but possibly a contributing factor [5].
Chemical meningitis after spinal anaesthesia was previously considered to be related to antiseptics and cleansing agents adhering to syringes and needles used for this technique with incidence reported 0.2% [6]. There are several case reports of chemical meningitis and epileptic seizures following spinal or epidural anaesthesia with bupivacaine [6-13]. To the best of our knowledge, this is the first report of generalized epileptic seizures after the administration of levobupivacaine. Levobupivacaine is a long-acting, amide-type local anesthetic with a clinical profile closely resembling that of bupivacaine. However, preclinical toxicity and safety data show an advantage for levobupivacaine over bupivacaine [14]. Anesthetics from the amide group may lead to central nervous system (CNS) symptoms, such as seizures or an altered mental state. An animal study demonstrated that the small, moderate thorn-like complexes in the amygdaloid nucleus can lead to generalized seizures; they result from local anesthetics in the amide group and are dose-dependent [7].
Headache and neck and shoulder pain were the first symptoms experienced by the patient. These symptoms responded well to pain medications, bed rest, and hydration. On the third day postpartum, she had three generalized epileptic seizures, was disoriented, and had neck stiffness. Her blood tests and negative CSF cultures substantiated the diagnosis of chemical meningitis.
At present, levobupivacaine is considered one of the safest local anesthetics, with only transient neurological symptoms. Cranial migration of the anesthetic agent through the subarachnoid space and physiologic changes occurring in pregnant women are among the theories that explain involvement of CNS symptoms after spinal anaesthesia [6].
There are several case reports of seizures following spinal anaesthesia. Kim et al. [13], reported a case of a gravida developing generalized tonic-clonic seizures following spinal anaesthesia with bupivacaine for a Cesarean section. The factors that caused the seizure could not be precisely identified, but the authors considered that the physiological and anatomical changes due to pregnancy increased the level of bupivacaine and simultaneously increased blood absorption of the anesthetic, a direct effect of the local anesthetic on the cerebral cortex. Those changes, coupled with and anxiety or fear, were responsible for the seizure.
Javed et al. [6], have reported a case of chemical meningitis following epidural anaesthesia with bupivacaine in a gravida. The factors that caused chemical meningitis were also unknown, but they considered two theories that could explain the meningitis are the diffusion of the anesthetic agent into the subarachnoid space or across the epidural space, and the physiological and anatomic changes that occur in pregnant women.
Although levobupivacaine is safe for spinal anaesthesia, it can induce seizures. In the case presented here, we presume that levobupivacaine could be the culprit for the development of generalized seizures, and most probably chemical meningitis. The possible factors for such dramatic clinical symptoms could be activation of microglial cells induced by levobupivacaine or other particles contained in the solution applied intrathecally in a predisposed patient, such as a postpartum woman.
Conclusion
In conclusion, we described a patient with generalized epileptic seizures following spinal analgesia with levobupivacaine. Our study demonstrates that chemical meningitis and generalized epileptic seizures should be among the differential diagnoses of complications occurring in patients undergoing epidural or spinal anaesthesia, especially in certain populations such as pregnant women, although these agents have been mostly associated with transient neurological symptoms [6]. As unrecognized chemical meningitis and/or generalized epileptic seizures can lead to severe morbidity and mortality, early diagnosis and appropriate treatment are essential for a successful recovery.
References
- Palmer Continuous spinal anaesthesia and analgesia in obstetrics. Anesth Analg. 2010; 111: 1476-1479.
- Morgan Spinal anaesthesia in obstetrics. Can J Anaesth.1995; 42: 1145-1163.
- http://www.intechopen.com/books/topics-in-spinal-anaesthesia/complications-in-spinal-anaesthesia
- Gogarten W. Spinal anaesthesia for obstetrics. Best Pract Res Clin 2003; 17: 377-392.
- Horlocker Complications of regional anaesthesia and acute pain management. Anesthesiol Clin. 2011; 29: 257-278.
- Javed F, Grosu HB, Minkin R. Chemical meningitis following epidural anaesthesia with bupivacaine: case report and review of Chest. 2009; 136: 36S-d-37S.
- Akil E, Varol S, Güzel A, et al. Status epilepticus induced by intrathecal bupivacaine use: a case report. J Clin Exp Inv. 2014; 5: 108-111.
- Tateno F, Sakakibara R, Kishi M, et al. Bupivacaine-induced chemical J Neurol. 2010; 257: 1327-1329.
- Galante D. An unusual cause of seizures during subarachnoid anaesthesia in a patient undergoing transurethral resection of the prostate: a case report. Minerva Anestesiol. 2009; 75: 221-
- Antwi-Kusi A, Sam Awortwi W, Serwaa Hemeng Unusual complication following spinal anaesthesia for caesarean section. Open J Anesth. 2013; 3: 275-277.
- Hiller A, Karjalainen K, Balk M, et Transient neurological symptoms after spinal anaesthesia with hyperbaric 5% lidocaine or general anaesthesia. Br J Anaesth. 1999; 82: 575-579.
- Abrão J, Bianco Mde P, Roma W, et al. Spinal myoclonus after subarachnoid anaesthesia with bupivacaine. Rev Bras 2011; 61: 619-623.
- Kim HJ, Kwon MY, Kang HJ, et al. Generalized tonic-clonic seizure following spinal anaesthesia for Cesarean section with bupivacaine: a case Anesth Pain Med. 2011; 6: 393-396.
- Foster RH, Markham A. Levobupivacaine: a review of its pharmacology and use as a local Drugs. 2000; 59: 551-579.