Childhood Obesity and Its Associations with Morbidity and Mortality in Adult Life
Author'(s): Kakleas Kostas, Soldatou Alexandra*, Karavanaki Kyriaki
National and Kapodistrian University of Athens, Faculty of Medicine, Diabetes and Metabolism Clinic, 2nd Department of Pediatrics, “P&A Kyriakou” Children’s Hospital, Athens, Greece.
*Correspondence:
Soldatou Alexandra MD, PhD, Assistant Professor of Pediatrics, 2nd Department of Pediatrics, National and Kapodistrian University of Athens, Faculty of Medicine, “P&A Kyriakou” Children’s Hospital, Athens, Greece, Tel: +30-213-2009474; Fax: +30-210-7774383; E-mail: alex_soldatou@hotmail.com.
Received: 01 November 2018 Accepted: 03 December 2018
Citation: Kakleas Kostas, Soldatou Alexandra, Karavanaki Kyriaki. Childhood Obesity and Its Associations with Morbidity and Mortality in Adult Life. Diabetes Complications. 2018; 2(4): 1-12.
Abstract
Childhood and adulthood obesity has reached epidemic levels. Unfortunately, obesity tracks from childhood into adulthood and its persistence rises with age among obese children. Childhood obesity is associated with comorbidities, which are present even in childhood, including hypertension, endothelial dysfunction, insulin resistance and type 2 diabetes (T2D), fatty liver disease, obstructive sleep apnea and endocrine, musculoskeletal and psychosocial problems. Early onset obesity is a risk factor for morbidity and mortality later in life. The main causes of death of obese children and adolescents in adulthood include cardiovascular diseases, T2D and its complications, obstructive sleep apnea and cancer. Morbidity and mortality increase proportionately to the severity and duration of childhood obesity. Furthermore, body mass index (BMI) is associated with projected life expectancy. Decreased life expectancy is observed among obese, but also among lean individuals, with the best life expectancy being observed among normal weight and overweight people. Life expectancy has halted at present and further decrease due to the obesity epidemic is expected, if proper measures are not taken. The association between childhood obesity and adult morbidity and mortality strongly suggests that more effective prevention and treatment of childhood obesity should be pursued.
Keywords
Introduction
Epidemiology
The incidence of obesity and overweight among children and adolescents at increasingly younger ages has reached alarming rates globally the last 25 years. Pediatric obesity rates have doubled or tripled between 1970 and 1990 in Australia, Brazil, Canada, France, Greece, Japan, the U.K. and the U.S.A [1]. There are over 155 million overweight children worldwide. According to the NHANES study, the prevalence of childhood and adolescent obesity has significantly increased over the last 15 years and is associated with increasing age, race/ethnicity, female gender and low socioeconomic status [2].
The International Obesity Taskforce recommends the use of international age and gender specific growth charts that allow comparison of obesity rates among countries. Based on these charts, children are overweight when the Body Mass Index (BMI) is above the 85th percentile, and obese above the 95th. The World Health Organization defines overweight when the BMI Z-score is above 2 and obese when it is above 3 [3]. Obesity seems to track into adulthood; 25% of obese children and 75% of obese adolescents will reportedly eventually become obese adults [4].
Complications of Obesity
Even in childhood and adolescence, obesity can cause immediate complications, often severely affecting almost all organs/systems (Table 1).
Cardiovascular complications include hypertension, left ventricular hypertrophy and endothelial dysfunction. In addition, a higher percentage of overweight/obese children develop insulin resistance/type 2 diabetes (T2D), dyslipidemia and metabolic syndrome [5]. Indeed over 50% of children with BMI at the 97% percentile already have one or more elements of metabolic syndrome [6].
Gastrointestinal disorders frequently observed are fatty liver infiltration and gastroesophageal reflux. Other effects of obesity, related to the respiratory system are obstructive sleep apnea [7], asthma [8] and Pickwick syndrome [9]. Direct effects on the reproductive hormonal axis of leptin and insulin of obesity may cause early puberty in girls, while mostly delayed puberty and less frequently early puberty in boys [10,11]. Advanced skeletal maturation may ensue due to increased aromatization of androgens into estrogens in the fatty tissue [12]. Obesity in adolescent females has also been associated with the development of polycystic ovaries syndrome [13], characterized by central obesity, hyperandrogenism, insulin resistance and menstruation abnormalities.
Excess weight-bearing may inadvertently cause musculoskeletal disorders, such as fractures, weight-bearing intolerance and lower extremity long bone misalignment. Severe orthopedic complications include Blount disease, slipped capital femoral epiphysis, osteoarthritis, etc [14]. To the contrary, obesity increases bone density, through the association of increased fatty tissue with increased concentrations of bone minerals in the spine and extremities [15]. Obesity may be related to significant psychosocial problems, such as depression and poor quality of life [16]. Finally, there is a long-term risk of malignant tumours (esophageal, colorectal, endometrial, breast, renal and prostate) [17].
The prevalence of complications caused by obesity in childhood and adolescence are mentioned in Table 1 [18].
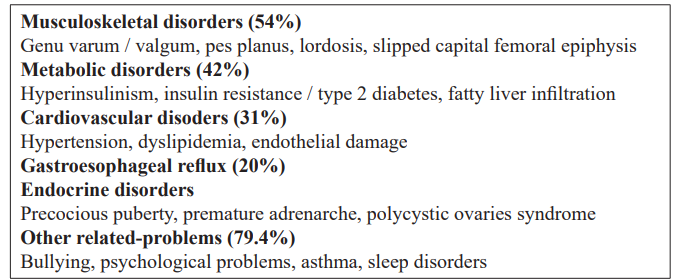
Table 1: Childhood obesity consequences in childhood (Maggio et al, Pediatrics 2014) [18].
Pediatric Obesity and Cardiovascular and metabolic complications in adults
Overweight/obese children are at increased risk of cardiovascular disease in adulthood (OR=1.7- 2.6) with the risk being higher for males [19]. The Harvard Growth Study [20], a long-term prospective study, showed that obesity in adolescence had a stronger negative effect on health than in adulthood. In addition, obesity in adolescence conferred increased mortality due to all causes, particularly due to cardiovascular disease in men, and increased morbidity from cardiovascular disease and atherosclerosis in both genders.
The Muscatine Study [21], another prospective population study, demonstrated that obesity in childhood predicts obesity in adulthood and calcification of coronary arteries. Obesity characteristically causes insulin resistance and metabolic syndrome.
Insulin Resistance (IR)
Insulin resistance is defined by the inability of insulin to promote glucose uptake in skeletal muscles and the liver to convert it to glucagon. Central obesity is associated with insulin resistance and the development of cardiovascular disease [22], and seems to be more prevalent in childhood than adolescence [23].
Obesity predisposes to the development of insulin resistance and T2D. The increasing prevalence of T2D in children and adolescents is worrisome, as it previously affected adults almost exclusively [24]. In a study from the US impaired glucose tolerance (IGT) was reported in 22.1% of obese children and T2D in 2.4% of them [25]. However, the frequencies of IGT and T2D are further increased among obese high-risk populations (African-American and Hispanic children and adolescents) (19.5% and 39.8% respectively) and are markedly increased among those morbidly obese (IGT:27.3% and T2DM:52.4%) [25].
Metabolic Syndrome
Metabolic syndrome is a constellation of: a. Glucose intolerance, Dyslipidemia, c. Cardiovascular disorders (hypertension), d. Hemostatic dysfunction e.Endothelial dysfunction, f. Reproductive disorders (polycystic ovaries syndrome). A modified definition of metabolic syndrome in children includes at least 3 of the criteria shown in Table 2.
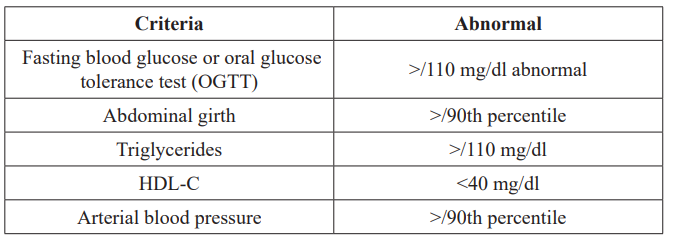
Table 2: A modified definition of metabolic syndrome in children with at least 3 criteria (Cook S et al, Arch Pediatr Adolesc Med 2003) [6].
Atherogenic Dyslipidemia
Atherogenic dyslipidemia, defined by high total triglycerides and low HDL cholesterol levels, is an additional aggravating factor for the development of endothelial dysfunction [26].
Post-mortem examinations of patients 2 – 39 years old revealed a strong correlation of BMI, systolic and diastolic blood pressure (BP), total cholesterol levels, triglycerides, LDL- C and HDL- C with the degree of damage in the coronary arteries and the aorta [27].
Factors Associated with Endothelial Dysfunction
Factors associated with endothelial dysfunction are: a. Non- preventable, such as advancing age, male gender, menopause, family history of cardiovascular disease, homocystenemia and low birth weight, and b. Preventable, such as obesity, tobacco use, stress, sedentary lifestyle, dyslipidemia, arterial hypertension, glucose intolerance, T2D and insulin resistance [28].
Clinical manifestations of atherosclerosis:
Precocious atherosclerosis in children presents with: a. coronary fatty streaks in 50% of adolescents and b. fibrous plaques observed in 8% of children and 12% of adolescents [29]. The presence of aortic fatty streaks and/or fibrous plaques was strongly associated with elevated total, LDL and VLDL cholesterol and low HDL cholesterol levels and with elevated systolic blood pressure [29].
The LIFE Child Obesity study [30], a long-term prospective population study, demonstrated that children and particularly adolescents have a high risk for endothelial dysfunction due to their propensity for central obesity and increased visceral fat.
Risk of cardiovascular disease
Arterial hypertension, endothelial dysfunction and decreased arterial elasticity, structural and functional alterations in the left heart ventricle may cause precocious development of cardiovascular complications with potential long-term implications [31,32].
According to the Bogalusa Heart Study [5], a large long-term population study, parameters predictive of endothelial damage in adult life included increased LDL cholesterol, increased BMI and left ventricular hypertrophy in childhood. In addition, left ventricular hypertrophy was associated with mildly decreased myocardial function.
The Generation R prospective Study [33] indicated that overweight and obese children have echocardiographic evidence of myocardial adaptation as early as the age of 2 years.
Furthermore, Shah et al. [34] found that obese adolescents and adolescents with T2D present subclinical alterations of myocardial structure (remodeling) prior to developing left ventricular hypertrophy and cardiac malfunction.
Non-alcoholic fatty live disease (NAFLD)
NAFLD ranges from different degrees of fat accumulation in the liver and elevated transaminases, to fatty liver or steatohepatitis with or without fibrosis, to cirrhosis and its complications, while infrequently it is associated with development of hepatocellular carcinoma [35]. The NAFLD diagnostic criteria are: infiltration of more than 5% of hepatocytes confirmed by liver histology, in the absence of alcohol use, viral, autoimmune or drug-induced liver disease [36,37]. Based on autopsy data, fatty liver was present in 9-13% of children and adolescents, 23% of which had histological changes consistent with steatohepatitis [38]. In a study of obese children that underwent gastric bypass surgery 33% suffered from fatty liver and 20% from steatohepatitis [39]. In most studies, two thirds of obese adults and half of obese children have fatty liver [40,41]. The risk of suffering from NAFLD increases 4.14 and 5.98 times among overweight and obese adolescents compared to age-matched children [42]. Apart from obesity, T2D and insulin resistance are highly associated with NAFLD [43,44].
Although liver biopsy is the gold standard for the diagnosis of NAFLD, elevation of ALT and AST in conjunction with abdominal ultrasound can serve as a non-invasive, cheap, easily available screening method when steatosis exceeds 20% of hepatocytes [45-48]. The steatosis score, determined by ultrasonography, was strongly associated with the fatty liver grade determined by hepatic histology [49]. CT scan is not indicated due to the risk of irradiation, but magnetic resonance imaging and magnetic resonance spectroscopy are excellent diagnostic tools to quantify the amount of liver steatosis [50].
NAFLD can progress to steatohepatitis, hepatic fibrosis and ultimately cirrhosis and hepatic cancer [50,51]. The presence and severity of fibrosis have been associated with higher ΒΜΙ or larger waist circumference [52,53].
The presence of necro-inflammation at liver biopsy is associated with possible rapid progression of fibrosis [43]. BMI, lipid profile, insulin resistance, and fasting glucose are clinical predictors of NAFLD in children [54]. Moreover, waist circumference alone seems to correlate with liver fibrosis [55]. Based on the age, waist circumference and triglyceride level, a Pediatric NAFLD fibrosis index algorithm has been developed to predict progression of disease [56]. Predictive molecules are currently under research and not yet clinically applicable [57].
Treatment includes lifestyle modification and healthy weight reduction via diet and exercise [58,59]. Both metformin and anti- oxidant drugs (vitamin E) have been used in children who failed lifestyle modification.
Obesity and respiratory system
Obesity affects the respiratory system through the development of asthma and obstructive sleep apnea.
Obesity and asthma
Obesity is associated with the presence, severity and poor control of asthma [60-63]. Children had an increased risk of developing asthma in the first 6 years of life if they had been obese earlier in life [64].
Obesity exerts a direct mechanical effect on the respiratory system. Increased fat on the chest wall and abdomen increases the weight of the thoracic wall on the lungs, reduces efficient respiration and restricts diaphragm movement [65]. Obese individuals who suffer from asthma breathe faster and in lower tidal volumes [66]. Pulmonary function tests in obese patients revealed decreased total lung capacity, vital lung capacity, and residual volume [67].
Obese individuals’ thriving inflammatory process contributes to the development of asthma. Leptin levels are associated with poor asthma control, and severe exercise induced bronchoconstriction, whereas adiponectin exerts protective, anti-inflammatory effects against asthma severity and bronchoconstriction [68-70]. The main mechanism is through shifting of the T-helper balance by adipokines from a Th3 response, observed in atopic asthma, to a Th3 response, observed in obese asthmatic individuals [71,72].
Obesity can increase the risk for asthma in adults by approximately 50% [73]. BMI is reportedly a better predictor of adult-onset asthma than metabolic syndrome in women, whereas insulin resistance is a better predictor of asthma-like symptoms than BMI regardless of gender [74,75].
Obesity and obstructive sleep apnea
Obstructive sleep apnea (OSA) is defined by recurrent upper- airway obstruction during sleep, resulting in a cycle of hypoxemia, increased respiratory effort, and frequent arousals [76]. The worldwide prevalence of OSA is 2% of women and 4% of men [76], and 45-98% of obese adults depending on the severity of obesity [77]. The prevalence of OSA in obese children is 46% compared to 33% in the general pediatric population [9]. Apart from the effects of chest and abdomen fat on lung mechanics, fat deposition in the surrounding tissues results in a narrower lumen and increased collapsibility of the upper airway, predisposing to apnea [78]. On the other hand, OSA can predispose to weight gain via reduced activity and increased appetite [79].
The “Obesity hypoventilation syndrome” (OHS) is a novel condition associated with obesity and defined by the triad of obesity, daytime hypoventilation with hypercapnia, hypoxemia and sleep-disordered breathing [80]. In 90% of cases of OHS, sleep-disordered breathing presents as OSA and in 10% purely as sleep hypoventilation, characterised by sustained levels of low saturation without obvious apnoeic and hypopneic effects [81,82]. The prevalence of OHS among adults is estimated to be 0.15% to 0.3% [83].
The three main factors for the development of OHS are abnormal respiratory function, sleep disordered breathing and diminished respiratory drive [84]. Obesity leads to low lung volumes, reduction of chest wall and lung compliance and increased airway resistance [85]. This in turn causes small airway collapse during expiration and increased work of breathing [86]. Patients with OHS display increased upper airway resistance both in the sitting and lying position, whereas patients with OSA only in the supine position. Faster breathing in small tidal volumes among obese patients increases dead space and further worsens gas exchange [86]. These patients also have disordered breathing during sleep, leading to hypoxemia and hypercapnia, with decreased respiratory responsiveness. Exposure to long periods of hypoxia during wakefulness and sleep results in development of hypoxia-related disorders, such as pulmonary hypertension, congestive heart failure and cor pulmonale [84].
Obesity and renal disease
Obesity has been associated with renal disease in children and adults [87], and subsequently end-stage renal disease [88]. Renal grafts donated by obese individuals showed lower glomerular filtration rates (GFR) and higher allograft dysfunction rates compared to those from lean individuals [89].
Insulin has an effect on GFR, increases albumin excretion in kidneys, increases the activity of the renin angiotensin aldosterone system and enhances the effects of angiotensin II in mesangial cells of kidneys [90-93]. Decreased insulin sensitivity and hyperinsulinemia in obese individuals result in hypertension through renal sodium reabsorption and sympathetic nervous system stimulation. High blood pressure induces renal injury and nephron loss. Furthermore, insulin stimulates the production of pro-inflammatory cytokines such as interleukin-1 (IL-1), Tumour necrosis factor-a (TNF-a), C-reactive protein (CRP), leptin and resistin that contribute in podocyte remodeling, loss of split pore diaphragm integrity, basement membrane thickening and glomerular mesangial expansion [94].
Obese children have been found to have microalbuminuria, an early index of obesity-related nephropathy. Csernus et al. found increased levels of urine albumin and b2-microglobulin in obese children [95]. Ferris et al. demonstrated that the level of microalbuminuria was strongly related to the severity of obesity in adults [96]. However, others found no differences between obese and healthy children [97].
Finally, obesity and renal disease may be found in syndromes like Alport and Bardet-Biedl [98]. Prematurity and born small for gestational age are risk factors for obesity as well as reduced nephron mass and subsequent progression to renal disease [99].
Obesity-autoimmunity
Autoimmune conditions clearly associated with obesity are rheumatoid arthritis, psoriasis, psoriatic arthritis and multiple sclerosis [100-102]. There is a two-fold increased risk of multiple sclerosis in obese individuals during childhood and late adolescence and an up to 6-fold risk of psoriasis [103,104]. Obesity is also associated with increased severity of rheumatoid arthritis and psoriasis and reduced response to treatment [105]. Other associated autoimmune diseases include T1D, Hashimoto thyroiditis and inflammatory bowel disease [106,107].
Recent studies have revealed that adipose tissue produces pro- inflammatory mediators, “adipokines”, which are either cytokines, such as interleukin-6 (IL-6) and TNF-a, or specific molecules such as leptin and adiponectin [108]. Leptin exerts its actions through the OB-Rb receptor, expressed in different tissues, including the immune system [109,110]. Specifically, leptin stimulates proliferation of naïve T-cells and promotes differentiation of T-cells to Th3 cells [109,110]. When uncontrolled, this pro-inflammatory activity could facilitate the development of autoimmunity.
On the other hand, adiponectin has anti-inflammatory properties through the reduction of the maturation and proliferation of macrophages, T-cells and B-cells, the inhibition of the production of inflammatory cytokines (IFNγ, TNFα, IL-6) and the promotion of the proliferation of regulatory T-cells [110]. Both patients with autoimmune conditions, as well as obese patients, have reportedly reduced levels of adiponectin.
Resistin or adipocyte-secreted factor and visfatin, both produced by adipose tissue, increase the production of pro-inflammatory cytokines (Il-1β, IL-6, IL-12, TNF-a), while visfatin additionally acts as a chemotactic factor and promoter of the activation of T-cells [111]. Both molecules are upregulated by inflammatory mediators and have been found increased in obese individuals.
High-fat, high-salt diets popular among obese individuals can increase Th37 cells that promote autoimmunity [112] and reduce the levels of vitamin-D, which inhibits the differentiation of T-cells to Th3 and Th37 cells [113]. Furthermore, these diets can change the gut flora resulting in modulation of extra-intestinal immune responses and dysregulation of the Th37/Treg balance [114]. These theories are still controversial and additional research is required.
Obesity-cancer in adults
The association between obesity and cancer is well-established. Estimations indicate that obesity accounts for 15% of cancer cases in the U.S. [115]. Specifically, obesity is a causative factor for esophageal, colon, uterine, kidney and post-menopausal breast cancer and a significant risk factor for pancreatic, prostate cancer and non-Hodgkin’s lymphoma [116,117]. Obesity and overweight account for 15-20% of all cancer deaths in the USA [118].
The presence of obesity increases mortality from cancer; women with a BMI>/40 kg/m2 have a three-fold higher mortality rate from breast cancer than women with BMI </20 kg/m2 [3]. Furthermore, cancer risk increases in parallel with the BMI in both genders [116]. Colditz et al. found that cancer is associated with obesity in 14% of cases in men and 16-20% in women [17].
There is evidence of a connection between central obesity during childhood or adolescence and the risk for colorectal cancer development or death later in life [118-120]. Furthermore, the risk for colorectal cancer is increased in men, as it has been associated with increased waist/hip ratio and central obesity [121].
Increased BMI between the ages of 7-13 years has also been associated with risk for papillary thyroid cancer [122]. Recently childhood obesity between 9-13 years was highlighted as a risk factor for the future development of adenocarcinoma of esophagus [123] and squamous esophageal carcinoma in women [124]. This could be attributed to the increased prevalence of gastroesophageal reflux in obesity [125].
Although adult obesity is an established risk factor for breast cancer in post-menopausal women and associated with large tumour size, metastasis and poor prognosis [126] the impact of childhood obesity on breast cancer risk for pre-menopausal women is controversial. Some studies found that increased BMI before puberty increases the risk for premenopausal breast cancer in adulthood [127-131]. Inversely others found a protective effect [132], while another study found no association between BMI at 18 years and future development of breast cancer [133].
Obesity causes a chronic inflammatory state [134] associated with cancer [135,136]. Adipose tissue can produce free-fatty acids, TNF-a, Il-6, leptin, adiponectin and other cytokines and hormones [137]. Through the production of these hormones adipose tissue participates in the body homeostasis, lipid metabolism and regulation of insulin sensitivity. In obese individuals enlarged adipocytes suffer from insufficient oxygenation, resulting in increased production of TNF-a, IL-6, IL-1b and MCP-1 [138]. These cytokines attract macrophages to adipose tissue [139] that in turn produce cytokines, prostaglandins and angiogenetic factors. In addition, free fatty acids are secreted from enlarged adipocytes, enter the circulation and deposit in other tissues causing increased insulin resistance and T2D [140].
Existing evidence suggests that lipogenesis and carcinogenesis may be associated through the triglyceride/free fatty acid metabolism. Indeed, tumour cells share common characteristics with adipocytes, such as growth factor production, angiogenesis and tissue invasion and migration [141-143]. Free-fatty acids, TNF-a, Il-6 and Il-1b promote the activation of NFkB [140]. NFkB, a transcription factor found in many tumours, promotes the expression of genes to enhance cell proliferation, apoptosis, angiogenesis and metastasis. The activation of this factor has also been associated with insulin resistance and increased levels of leptin, insulin and IGF-1 [144]. In the context of obesity, increased insulin levels result in increased levels of IGF-1. Insulin activates the NFkB [145] and the phosphatidylinositol-3 kinase and mTOR pathways that have also been found in tumours [146]. In addition, increased levels of IGF-1 could result in activation of similar to insulin pathways, which affect transcription factors controlling genes responsible for cell proliferation and metastasis [147]. In animal models increased IGF-1 levels have been associated with colon, pancreatic and breast cancer [148-150].
Obese individuals also have increased levels of leptin and reduced levels of adiponectin. Leptin affects cellular proliferation, angiogenesis and immunomodulation and the leptin receptor has similar homology to class I cytokines that signal through the janus kinase/signal transducer and activator transcription pathway, which is impaired in cancer [151]. In vitro studies have also shown that leptin has a proliferative effect in breast and prostate cancer [151].
Accumulating evidence shows that weight reduction in obese individuals reduces the incidence of cancer, probably through the reduction of predisposing hormones and inflammatory proteins [152]. However, obese individuals frequently regain weight, thus minimising the protective effect of weight loss in future cancer development [152-156].
Childhood obesity: morbidity and mortality in adult life Childhood obesity has been associated with increased risk of cardiovascular disease and T2D (HR=1.5–5), 30% overall increased risk of cancer, twofold risk of asthma and allergies, polycystic ovaries syndrome (HR=1.5), precocious puberty, increased risk for disability related pension and sudden death [157].
The third Harvard Growth Study, which included children from the USA born between 1922 and 1935, found that childhood obesity increases the risk of death from breast cancer and all causes in women, whereas it increases the risk of death from ischemic heart disease in men [128].
In a Norwegian study of adolescents, the presence of high BMI was associated with increased risk of death from ischemic heart disease in both genders, but did not increase risk of death from breast cancer [158]. In a similar study in Native Indian Americans born between 1945 and 1984, rates of death from endogenous causes were more than double among children in the highest BMI quartile compared with those in the lowest [159]. A study in Welsh children born between 1937-1939 found an association between childhood BMI and mortality from all causes, but no association between high BMI in childhood and mortality from cardiovascular disease [160]. A Swedish study found a positive association between weight gain during puberty and young adulthood with the risk of death from all causes later in life [161]. The main limitations of the aforementioned studies are that possible confounding lifestyle factors were not taken into account and obesity was relatively uncommon at the time.
As previously mentioned, the association between childhood weight gain and premenopausal breast cancer is controversial [128,162-164]. Inconsistency in the literature regarding the effect of high BMI during childhood and risk for mortality from ovarian cancer also exists [128,158]. This can be attributed to different study populations with wide age ranges, variable reproductive histories of female participants, and heterogeneous definitions of overweight or obesity.
In a study conducted by Bjorge et al. [158], overweight and obese adolescents had increased mortality risk later in adult life from cardiovascular diseases, T2D and cancer (rectal, oesophageal, endometrial, breast). Specifically, male adolescents with high BMI had increased risk for development of cardiovascular disease in adult life (OR=2.9) and T2D (OR=1.8 and 3.6 for overweight and obese respectively), whereas for overweight and obese women the OR for increased mortality risk was 2.6 and 5.6 respectively. The risk for respiratory disease was increased for obese (OR=4.1) as well as overweight men (OR=2.7) and women (OR=2.5). There was also increased risk for sudden death (men OR=2.2, women OR=2.7). Mortality risk increased in parallel with BMI increase in both genders, and morbidity and mortality from obesity increased proportionately to the duration and the severity of obesity [116].
Interventions on body weight and effect on mortality
All the aforementioned studies used a measurement of BMI at some point of life to predict the future risk for mortality without adjusting for midlife BMI, whereas BMI trajectories overtime are more predictive of mortality. The third Harvard Growth Study suggested that the risk for mortality was higher when obesity occurred prior to puberty [128]. Early pubertal timing was predictive of higher adult BMI and risk for obesity in adult life. In addition, puberty is associated with psychosocial changes, predisposing to earlier development of obesity [10,165]. Hirko et al. found that the presence of obesity at young adulthood (21 years) was associated with increased risk of all causes mortality, the leading cause being cardiovascular disease, but not from cancer. The risk was independent of the presence of obesity in middle adulthood. Possible confounders, such as smoking, alcohol, physical activity, age and sex were included in the analysis. Participants who were obese at 21 years, but non-obese at 40-65 years had increased relative mortality risk attributed to weight loss from illness or lifestyle change following a significant adverse obesity-related event or chance [166].
In a Finnish study, females with an increased BMI from infancy to childhood or a lower BMI in infancy and childhood, which increased at the age of 8 years, had an increased risk of premature mortality later in life [167]. However, men with average BMI during the first two years of life, followed by a subsequent decrease, had a higher risk of cancer mortality. Having included socio-economic status and lifestyle factors in the analysis, no effect of adulthood BMI was found [167].
Consequently, it is becoming apparent that increased BMI during childhood and adolescence, irrespective of normalization of weight status in adulthood, increases the long-term risk for premature mortality. However, all these studies used BMI as an indicator of obesity, although it is not the ideal measure of adiposity in children [168,169]. In addition, trajectories have been based on the U-shaped association of BMI in adulthood and premature mortality, not necessarily applicable in childhood and early adolescence [170]. In addition, these studies were retrospective and in most weight and height were self-reported. Finally, adult obesity may be a confounding factor, since it is a strong independent predictor for future mortality and obese children are more likely to remain obese adults [171,172].
Mortality rates according to BMI
An increased risk of mortality has been equally found not only among obese, but also among lean individuals, following a U-shaped pattern (Figure 1) [173]. Specifically, individuals with BMI: 23-28 kg/m2 have a lower mortality risk compared to overweight or underweight individuals, while the lowest mortality risk was observed in those with a BMI of 25 kg/m2.
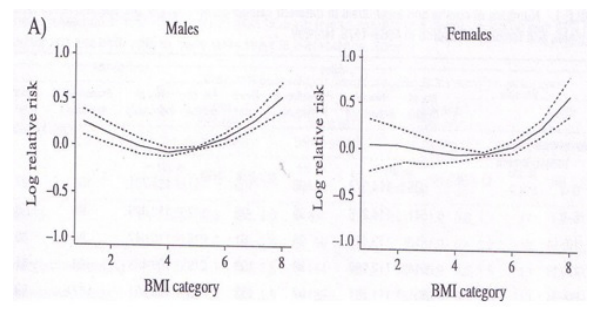
Figure 1: Life expectancy decrease among lean and overweight/obese (adapted from Bjorge T et al, Am J Epidemiol 2008) [173].
Based on long-term studies of U.S. population regarding the causes of death, lean and obese individuals had increased rates of all-cause mortality and increased mortality from other conditions except cancer and cardiovascular disorders [174,175].
Overweight individuals have lower rates of mortality from other conditions except cancer and cardiovascular disorders, but increased rates of mortality from T2D and renal disease [158]. However, obesity correlated positively with increased overall mortality and significantly increased mortality from cardiovascular disorders, certain cancer types, T2D and its complications, renal disease, obesity hypoventilation syndrome (OHS) and sudden death [158]. Specifically, the risk of death from cardiovascular disease and T2D is higher for women and for obese, while the risk for death from OHS is higher for men and for obese, and finally the risk for sudden death and cancer is similar for both genders [158].
According to Reilly JJ et al. [157], the longer the duration of childhood obesity, the greater the risk for comorbidities in adulthood. Furthermore, the cardiovascular risk and the risk for premature mortality and comorbidities in adulthood increase in parallel with the severity of childhood obesity.
Childhood obesity and life expectancy
During the course of the 20th century, life expectancy gradually increased to 65 years, and projected life expectancy in the USA by 2060 was estimated at 100 years [176]. However, over the past 20 years life expectancy has halted at 65 years (Figure 2). This halt is attributed to the AIDS epidemic, the development of resistant microorganisms, the influenza epidemic, the atmospheric pollution, sedentary lifestyle, smoking, stress and obesity [176].
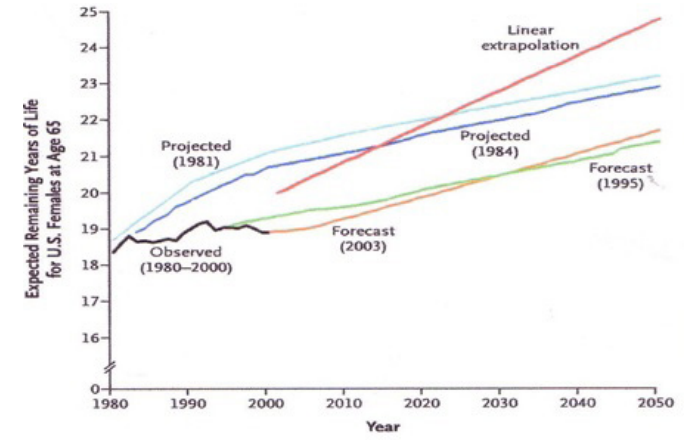
Figure 2: Life expectancy at 65 years (1980-2005) (adapted from Olsshansky SJ et al, N Engl J Med 2005) [176].
According to Olsshansky SJ et al., life expectancy in the USA has decreased by 0.3–0.75 years due to obesity. Moreover, the gradual increase in diabetes-related deaths and the obesity epidemic, overall life expectancy is estimated to decrease further by 2-5 years. Among overweight individuals, life expectancy is expected to decrease by 3 years and among obese by 1-8 years [176].
Similarly Preston SH [177] noted that the life expectancy increase, observed in the USA, has ceased in view of the obesity epidemic and concluded that unless drastic measures are taken to battle obesity, current young adults are expected to live less healthy and shorter lives than their parents.
Prevention of Obesity in Children and Adolescents
The prevention of childhood and adolescent obesity should commence during endometrial life through the avoidance of excessive maternal weight gain and the achievement of tight glycaemic control of diabetes during pregnancy. In addition, avoidance of excessive weight gain [178] and continuation of breastfeeding for at least 6 months is critical in the first few months of life. Adoption of healthy eating habits by all family members, regular exercise (at least 30 min /day), restricted TV viewing and Internet use (max 2 hours / day) and school education on healthy eating are of paramount importance [179,180].
Obesity in Childhood and Adolescence Life style interventions
The management of childhood and adolescent obesity is based on reducing daily calorie intake by 20–30% to lose 10% of body weight in 6 months. The diet should be based on the principles of the Mediterranean diet and feasible at school. The loss of weight should be coupled by physical activity, ideally 2–3 sessions of intense physical activity per week in addition to regular twice weekly physical education classes [181].
Conclusion
Childhood obesity often tracks into adult life. Precocious manifestations of atherosclerosis, left ventricular hypertrophy, sleep apnea, fatty liver infiltration, endocrine disorders, musculoskeletal and psychosocial problems are observed in obese children and adolescents. Obese children are at increased risk of morbidity and mortality from cardiovascular disorders, T2D and its complications, various types of cancer and sudden death in adulthood. The risk of cancer increases proportionately to the severity of obesity and the presence of central obesity. Morbidity and mortality increase proportionately to the severity and duration of childhood obesity. Decreased life expectancy is observed among obese, but also among lean individuals, while the best life expectancy is observed among normal weight and overweight people. It is estimated that life expectancy will be decreased by 2-5 years among obese individuals. Due to obesity the current generation of children and adolescents are entitled to shorter life expectancies than their own parents. Therefore, the importance of the prevention and management of obesity with the adoption of healthy eating habits from the entire family and an active lifestyle is highlighted.
References
- Hansen AR, Duncan DT, Woo Baidal JA , et An increasing trend in health-care professionals notifying children of unhealthy weight status NHANES 1999-2014. Int J Obes. 2016; 40: 1480-1485.
- van Stralen MM, te Velde SJ, Singh AS, et EuropeaN Energy balance Research to prevent excessive weight Gain among Youth ENERGY project Design and methodology of the ENERGY cross-sectional survey. BMC Public Health. 2011; 31: 11:65
- Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international BMJ 2000; 320: 1240–1243.
- Herman KM, Craig CL, Gauvin L, et Tracking of obesity and physical activity from childhood to adulthood the Physical Activity Longitudinal Study. Int J Pediatr Obes. 2009; 4: 281- 288.
- Srinivasan SR, Bao W, Wattigney WA, et Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors the Bogalusa Heart Study. Metabolism. 1996; 45: 235-240.4
- Cook S, Weitzman M, Auinger P, et al. Dietz WH Prevalence of a metabolic syndrome phenotype in adolescents: findings from NHANES-III. 1988–1994. Arch Pediatr Adolesc 2003; 157: 821-827.
- Piper Obesity hypoventilation syndrome The big and the breathless. Sleep Medicine Reviews. 2011; 15: 79-89.
- Brüske I, Flexeder C, Heinrich J. Body mass index and the incidence of asthma in Curr Opin Allergy Clin Immunol. 2014; 14: 155-160.
- Romero-Corral A, Caples SM, Lopez-Jimenez F, et Interactions between obesity and obstructive sleep apnea implications for treatment. Chest. 2010; 137: 711-719.
- Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men a systematic review and meta-analysis. International Journal of Obesity 2013; 37: 1036-1043.
- Burt Solorzano CM, McCartney Obesity and the pubertal transition in boys and girls. Reproduction. 2010; 140: 399-410.
- De Leonibus C, Marcoveccio ML, Chiarelli Update on statural growth and pubertal development in obese children. Pediatric Reports. 2012; 4: 119-123.
- Diamanti-Kandarakis Role of obesity and adiposity in polycystic ovary Syndrome. International Journal of Obesity. 2007; 31: S8-S13.
- Mihalko WM, Bergin PF, Kelly FB, et Obesity, Orthopaedics, and Outcomes. J Am Acad Orthop Surg. 2014; 22: 683-690.
- Dimitri P, Wales JK, Bishop Adipokines bone-derived factors and bone turnover in obese children evidence for altered fat- bone signalling resulting in reduced bone mass. Bone. 2011; 48: 189-196.
- Tsiros MD, Olds T, Buckley JD, et Coates AM Health-related quality of life in obese children and adolescents. Int J Obes. 2009; 33: 387-400.
- Colditz GA, Sellers TA, Trapido E. Epidemiology identifying the causes and preventability of cancer. Nat Rev Cancer. 2006; 6: 75-83.
- Maggio ABR, Martin XE, Saunders Gasser CS, et Medical and non-medical complications camong children and adolescents with excessive body weight. BMC Pediatrics. 2014; 14: 232-240.
- Stevens J, Cai J, Pamuk ER, et al. The effect of age on the association between body-mass index and mortality. N Engl J 1998; 338: 1-7.
- Must A, Jacques PF, Dallal GE, et al. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992; 327: 1350-
- Juonala M, Magnussen CG, Venn A, et al.The Muscatine Study. Influence of Age on Associations Between Childhood Risk Factors and Carotid Intima-Media Thickness in Adulthood The Cardiovascular Risk in Young Finns Study the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study and the Muscatine Study for the International Childhood Cardiovascular Cohort i3C Consortium. Circulation. 2010; 122: 2514-2520.
- Sourani M, Kakleas K, Critselis E, et Cross-sectional study on childhood obesity and central obesity on a rural Greek island. Acta Endocrinologica Buc. 2015; 11: 329-336.
- Rosenbloom AL, Joe JR, Young RS, et Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999; 22: 345-354.
- Sinha R, Fisch G, Teague B, et Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002; 346: 802-810.
- Propst M, Colvin C, Griffin RL, et al. Diabetes and prediabetes are significantly higher in morbidly obese children compared with obese Endocr Pract. 2015; 21: 1046-1053.
- Pires A, Sena C, Seiça Dyslipidemia and cardiovascular changes in children. Curr Opin Cardiol. 2016; 31: 95-100.
- Berenson GS, Srinivasan SR, BaoW, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J 1998; 338: 1650-1656.
- Herouvi D, Karanasios E, Karavanaki Cardiovascular diasease in childhood the role of obesity. European Journal of Pediatrics. 2013; 172: 721-732.
- Tracy RE, Newman WP, Wattigney WA, et al. Risk factors and atherosclerosis in youth autopsy findings of the Bogalusa Heart Am J Med Sci. 1995; 310: 37-41.
- Quante M, Hesse M, Döhnert M, et LIFE Child Study Investigators. The LIFE child study a life course approach to disease and health. BMC Public Health. 2012; 12: 1021.
- Yoshinaga M, Yuasa Y, Hatano H, et al. Effect of Total Adipose Weight and Systemic hypertension on Left Ventricular Mass in American Journal of Cardiology. 1995; 76: 785-787.
- Sakou Ι, Psaltopoulou Τ, Sergentanis ΤΝ, et Insulin resistance and cardiometabolic risk factors in obese children and adolescents A hierarchical approach. J Pediatr Endocrinol Metab. 2015; 28: 589-596.
- de Jonge L, van Osch-Gevers L, Willemsen SP, et al. Growth Obesity and Cardiac Structures in Early Childhood The Generation R Hypertension. 2011; 57: 934-940.
- Shah RV, Abbasi SA, Neilan TG, et Myocardial tissue remodeling in adolescent obesity. J Am Heart Assoc. 2013; 2: e000279.
- Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease a spectrum of clinical and pathological Gastroenterology. 1999; 116: 1413-1419.
- Aly FZ, Kleiner Update on fatty liver disease and steatohepatitis. Adv Anat Pathol. 2011; 18: 294-300.
- Scaglioni F, Ciccia S, Marino M, et ASH and NASH. Dig Dis. 2011; 29: 202-210.
- Schwimmer JB, Deutch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Paediatrics. 2006; 118: 1388-
- Xanthakos S, Miles L, Bucuvalas J, et al. Histologic spectrum of non-alcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol 2006; 4: 226-232.
- Chan DF, Li AM, Chu WC, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004; 28: 1257-1263.
- Tominaga K, Kurata JH, Chen YK, et al. Prevalence of fatty liver in Japanese children and relationship to An epidemiological ultrasonographic survey. Dig Dis Sci. 1995; 40: 2002-2009.
- Fu CC, Chen MC, Li YM, et al. The risk factors for ultrasound diagnosed non-alcoholic fatty liver disease among adolescents. Ann Acad Med 2009; 38: 15-17.
- Manco M, Marcellini M, Devito R, et al. Metabolic syndrome and liver histology in paediatric non-alcoholic Int J Obes. 2008; 32: 381-387.
- Sundaram SS, Zeitler P, Nadeau The metabolic syndrome and nonalcoholic fatty liver disease in children. Curr Opin Pediatr. 2009; 21: 529-535.
- Nobili V, Reale A, Alisi A, et Elevated serum ALT in children presenting to the emergency unit Relationship with NAFLD. Dig Liver Dis. 2009; 41: 749-752.
- Rodríguez G, Gallego S, Breidenassel C, et Is liver transaminases assessment an appropriate tool for the screening of non-alcoholic fatty liver disease in at risk obese children and adolescents. Nutr Hosp. 2010; 25: 712-717.
- Shannon A, Alkhouri N, Carter-Kent C, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With J Pediatr Gastroenterol Nutr. 2011; 53: 190-195.
- Marzuillo P, Miraglia del Giudice E, Santoro Pediatric fatty liver disease Role of ethnicity and genetics. World J Gastroenterol. 2014; 20: 7347-7355.
- Shannon A, Alkhouri N, Carter-Kent C, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With J Pediatr Gastroenterol Nutr. 2011; 53: 190-195.
- Schwenzer NF, Machann J, Schraml C, et Quantitative analysis of adipose tissue in single transverse slices for estimation of volumes of relevant fat tissue compartments a study in a large cohort of subjects at risk for type 2 diabetes by MRI with comparison to anthropometric data. Invest Radiol. 2010; 45: 788-794.
- Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease a spectrum of clinical and pathological Gastroenterology. 1999; 116: 1413-1419.
- Ko JS, Yoon JM, Yang HR, et Clinical and histological features of non-alcoholic fatty liver disease in children. Dig Dis Sci. 2009; 54: 2225-2230.
- Romeo S, Kozlitina J, Xing C, et Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008; 40: 1461-1465.
- Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012; 54: 700-713.
- Manco M, Bedogni G, Marcellini M, et al. Waist circumference correlates with liver fibrosis in children with non alcoholic Gut. 2008; 57: 1283-1287.
- Nobili V, Alisi A, Vania A, et al. The pediatric NAFLD fibrosis index a predictor of liver fibrosis in children with non-alcoholic fatty liver BMC Med. 2009; 7: 21.
- Nobili V, Svegliati-Baroni G, Alisi A, et A 360-degree overview of paediatric NAFLD recent insights. J Hepatol. 2013; 58: 1218-1229.
- Fan JG, Cao HX. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2013; 28: 81-87.
- Al Khater SA. Paediatric non-alcoholic fatty liver disease an Obes Rev. 2015; 16: 393-405.
- Rodriguez MA, Winkleby MA, Ahn D, et al. Identification of population subgroups of children and adolescents with high asthma prevalence findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2002; 156: 269-275.
- Quinto KB, Zuraw BL, Poon K-YT, et al. The association of obesity and asthma severity and control in children. Journal of Allergy and Clinical 2011; 128: 964-969.
- Taylor B, Mannino D, Brown C, et al. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008; 63: 14-20.
- Dias-Junior SA, Reis M, De Carvalho-Pinto RM, et Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014; 43: 1368-1377.
- Brüske I, Flexeder C, Heinrich J. Body mass index and the incidence of asthma in Curr Opin Allergy Clin Immunol. 2014; 14: 155-160.
- Beuther DA, Weiss ST, Sutherland Obesity and asthma. Am J Respir Crit Care. Med. 2006; 174: 112-119.
- Chinn Obesity and asthma evidence for and against a causal relation. Journal of Asthma. 2003; 40: 1-16.
- Jones RL, Nzekwu M-MU. The effects of body mass index on lung Chest journal. 2006; 130: 827–833.
- Gurkan F, Atamer Y, Ece A, et Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol. 2004; 93: 277-280.
- Baek HS, Kim YD, Shin JH, et al. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with Ann Allergy Asthma Immunol. 2011; 107: 14-21.
- Guler N, Kirerleri E, Ones U, et Leptin does it have any role in childhood asthma. Journal of Allergy and Clinical Immunology. 2004; 114: 254-259.
- Rastogi D, Canfield SM, Andrade A, et al. Obesity-associated asthma in children a distinct entity. CHEST Journal. 2012; 141: 895-905.
- Van Veen I, Ten Brinke A, Sterk P, et al. Airway inflammation in obese and nonobese patients with difficultâ?toâ?treat asthma. 2008; 63: 570-574.
- Ronmark E, Andersson C, Nystrom L, et al. Obesity increases the risk of incident asthma among adults. European Respiratory 2005; 25: 282-288.
- Assad N, Qualls C, Smith LJ, et Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women the longitudinal CARDIA study. American Journal of Respiratory and Critical Care Medicine. 2013; 188: 319-326.
- Thuesen BH, Husemoen LLN, Hersoug LG, et Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clinical and Experimental Allergy. 2009; 39: 700-707.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea a population health perspective. Am J Respir Crit Care 2002; 165: 1217-1239.
- Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008; 74: 834-838.
- Schwab RJ, Pasirstein M, Pierson R, et Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003; 168: 522-530.
- Spiegel K, Tasali E, Penev P, et al. Brief communication sleep curtailment in healthy young men is associated with decreased leptin levels elevated ghrelin levels, and increased hunger and Ann Intern Med. 2004; 141: 846-850.
- Olson A, Zwillich The obesity hypoventilation syndrome. Am J Med. 2005; 118: 948-956.
- Mokhlesi B. Obesity hypoventilation syndrome a state-of-the- art Respir Care. 2010; 55: 1347-1362.
- Chau E, Lam D, Wong J, et Obesity hypoventilation syndrome a review of epidemiology pathophysiology and perioperative considerations. Anesthesiology. 2012; 117: 188-205.
- Piper Obesity hypoventilation syndrome-the big and the breathless. Sleep Med Rev. 2011; 79-89.
- Zerah F, Harf A, Perlemuter L, et Effects of obesity on respiratory resistance. Chest. 1993; 103: 1470-1476.
- Ferretti A, Giampiccolo P, Cavalli A, et al. Expiratory flow limitation and orthopnea in massively obese Chest. 2001; 119: 1401-1408.
- Chlif M, Keochkerian D, Choquet D, et al. Effects of obesity on breathing pattern ventilator neural drive and mechanics. Respir Physiol 2009; 168: 198-202.
- Gunta SS, Mak Is obesity a risk factor for chronic kidney disease in children Pediatr Nephrol. 2013; 28: 1949-1956.
- Hsu CY, McCulloch CE, Iribarren C, et Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006; 144: 21-28.
- Filler G, Reimao SM, Kathiravelu A, et al. Pediatric nephrology patients are overweight 20 experience in a single Canadian tertiary pediatric nephrology clinic. Int Urol Nephrol. 2007; 39: 1235-1240.
- Sartori C, Scherrer U. Insulin nitric oxide and the sympathetic At the crossroads of metabolic and cardiovascular regulation. J Hypertens. 1999; 17: 1517-1525.
- Chagnac A, Weinstein T, Korzets A, et Glomerular haemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000; 278: 817-822.
- El-Ataf FA, Stas SN, McFarlane SI, et The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol. 2004; 15: 2816-2827.
- Kreisberg JI. Insulin requirement for contraction of cultured rat glomerular mesangial cells in response to angiotensin Possible role for insulin in modulating glomerular hemodynamics. Proc Nat Acad Sci USA. 1982; 79: 4190-4192.
- Facchini FS, Humphreys MH, DoNascimento CA, et Relation between insulin resistance and plasma concentrations of lipid hyperoxidases, carotenoidsand tocopherols. Am J Clin Nutr. 2000; 72: 776-779.
- Csernus K, Lanyi E, Erhardt E, et al. Effect of childhood obesity and obesity related cardiovascular risk factors on glomelural and tubular protein Eur J Pediatr. 2005; 164: 44-49.
- Ferris M, Hogan SL, Chin H, et al. Obesity albuminuria and urinalysis findings in US young adults from the Add HealthWave III Clin J Am Soc Nephrol. 2007; 2: 1207-1214.
- Goknar N, Oktem F, Ozgen IT, et al. Determination of early urinary renal injury markers in obese children. Pediatr Nephrol. 2015; 30: 139-144.
- Sahin C, Huddam B, Akbaba G, et Two brothers with Bardet- Biedl syndrome presenting with chronic renal failure. Case Rep Nephrol. 2015; 2015 :764973.
- Abitbol CL, Chandar J, Rodríguez MM, et Obesity and preterm birth additive risks in the progression of kidney disease in children. Pediatr Nephrol. 2009; 24: 1363-1370.
- De Carvalho JF, Pereira RMR, Shoenfeld Y. The mosaic of autoimmunity the role of environmental factors. Front Biosci Elite 2009; 1: 501-509.
- Munger KL, Chitnis T, Ascherio Body size and risk of MS in two cohorts of US women. Neurol. 2009; 73: 1543-1550.
- Lindegard B. Diseases associated with psoriasis in a general population of 159,200 middle-aged urban native Dermatologica. 1986; 172: 298-304.
- Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk a long term cohort study. Mult Scler J. 2013; 19: 1323-1329.
- Becker L, Tom WL, Eshagh K, et Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014; 150: 573-574.
- Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort Ann Rheum Dis. 2014; 73: 1914-1922.
- Laurberg P, Knudsen N, Andersen S, et Thyroid function and obesity. Eur Thyroid J. 2012; 1: 159-167.
- Ong KK, Kuh D, Pierce M, et al. Childhood weight gain thyroid autoimmunity at age 60-64 years. the 1946 British Birth Cohort J Clin Endocr Metabol. 2014; 98: 1435-1442.
- Cao Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014; 220: 47-59.
- Krysiak R, Handzlik-Orlik G, Okopien The role of adipokines in connective tissue diseases. Europ J Nutr. 2012; 51: 513-528.
- Li L, Wu Adiponectin and interleukin-6 in inflammation- associated disease. Vitam Horm Jan. 2012; 90: 375-395.
- Stofkova A, Resistin, regulators of insulin sensitivity inflammation and immunity. Endocr Regul. 2010; 44: 25-36.
- Brown K, DeCoffe D, Molcan E, et Diet induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012; 4: 1552-1553.
- Schoindre Y, Terrier B, Kahn JE, et Vitamin D and autoimmunity. First part fundamental aspects. La Rev Medicine Interne. 2012; 33: 80-86.
- Stofkova A. Leptin and adiponectin:from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009; 43: 157-168.
- Colditz GA, DeJong D, Hunter DJ, et al. Harvard report on cancer prevention. Volume I. Causes of human cancer. Cancer Causes 1996; 1-59.
- Calle EE, Kaaks Overweight obesity and cancer Epidimiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4: 579-591.
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight obesity and mortality from cancer in a prospectively studied cohort of US NEJM. 2003; 348: 1625-1638.
- Lee IM, Paffenbarger RS, Quetelets index and risk of colon cancer in college Journal of the National Cancer Institute. 1992; 84: 1326-1331.
- Le Marchand L, Wilkens LR, Mi MP. Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes 1992; 3: 349-354.
- Nimptsch K, Giovannucci E, Willett WC, et al. Body fatness during childhood and adolescence adult height and risk of colorectal adenoma in women. Cancer Prev Res. 2011; 4: 1710-
- Giovannucci E , Ascherio A, Rimm EB, et al. Physical activity obesity and risk for colon cancer and adenoma in men. Ann Intern 1995; 122: 327-334.
- Kitahara CM, Gamborg M, Berrington de González A, et al. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014; 74: 235-242.
- Cook MB, Freedman ND, Gamborg M, et Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer. 2015; 112: 60-67.
- Etemadi A, Golozar A, Kamangar F, et al. Large body size and sedentary lifestyle during childhood and early adulthood and esophageal squamous cell carcinoma in a high-risk population. Ann Oncol. 2012; 23: 1593-1600.
- Borovicka J, Krieger-Grübel C, van der Weg B, et al. Effect of morbid obesity gastric banding and gastric bypass on esophageal symptoms, mucosa and function. Surg Endosc. 2017; 31: 552-
- Calle EE, Thun Obesity and cancer. Oncogene. 2004; 23: 6365-6378.
- Whiteman MK, Hillis SD, Curtis KM, et al. Body mass and mortality after breast cancer Cancer Epidemiol Biomarkers Prev. 2005; 14: 2009-2114.
- Must A, Phillips SM, Naumova EN. Occurrence and timing of childhood overweight and mortality findings from the Third Harvard Growth J Pediatr. 2012; 160: 743-750.
- Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res 1982; 2: 5-73.
- Stoll Teenage obesity in relation to breast cancer risk. Int J Obes Relat Metab Disord. 1998; 22: 1035-1040.
- Michels KB, Willett Breast cancer early life matters. N Engl J Med. 2004; 351: 1679-1681.
- Baer JH, Tworoger SS, Hankinson SE, et al. Body fatness at young ages and risk of breast cancer throughout life. Amer J 2010; 171: 1183-1194.
- Whiteman MK, Hillis SD, Curtis KM, et al. Body Mass and Mortality After Breast Cancer Diagnosis. Obes Facts. 2009; 3: 179-186.
- Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity cancer J Nutr. 2014; 144: 109-113.
- Foltz CJ, Fox JG, Cahill R, et al. Spontaneous inflammatory bowel disease in multiple mutant mouse lines association with colonization by Helicobacter hepaticus. Helicobacter. 1998; 3: 69-78.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420: 860-867.
- Hursting SD, Dunlap Obesity metabolic dysregulation and cancer a growing concern and an inflammatory and microenvironmental issue. Ann N Y Acad Sci. 2012; 1271: 82- 87.
- Harvey AE, Lashinger LM, Hursting The growing challenge of obesity and cancer an inflammatory issue. Ann N Y Acad Sci. 2011; 1229: 45-52.
- Charriere G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003; 278: 9850-9855.
- Olefsky JM, Glass CK. Macrophages inflammation and insulin Annu Rev Physiol. 2010; 72: 219-246.
- Saiki A, Watanabe F, Murano T, et al. Hepatocyte growth factor secreted by cultured adipocytes promotes tube formation of vascular endothelial calls in vitro. Int J Obes. 2006; 30: 1676-
- Lolmede K, Durand De Saint Front V, Galitzky J, et al. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3t3-f442a Int J Obes Relat Metab Disord 2003; 27: 1187-1195.
- Omatzu-Kanbe M, Inoue K, Fujii Y, et al. Effect of ATP on preadipocyte migration and adipocyte differentiation by activating p2y receptors in 3t3-I1 cells. Biochem J. 2006; 393: 171-180.
- Subbaramaiah K, Howe LR, Bhardway P, et Obesity is associated with inflammation and elevated aromatase expression in themouse mammary gland. Cancer Prev. Res. 2011; 4: 329- 346.
- Kim DH, Kim JY, Yu BP, et al. The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008; 9: 33-47.
- Renehan AG, Roberts DL, Dive Obesity and cancer pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008; 114: 71-83.
- Harvey AE, Lashinger LM, Otto G, et al. Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth nuclear factor- kappaB activation, and inflammation related gene Mol Carcinog. 2012; 52: 997-1006.
- Olivo-Marston SE, Hursting SD, Lavigne J, et Genetic reduction of circulating insulin-like growth factor-1 inhibits azoxymethane- induced colon tumorigenesis in mice. Mol Carcinog. 2009; 48: 1071-1076.
- Ford NA, Nunez NP, Holcomb VB, et IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression epithelial-to-mesenchymal transition and chemokine expression. Endocr Relat Cancer. 2013; 20: 39-51.
- Lashinger LM, Malone LM, McArthur MJ, et Genetic reduction of insulin-like growth factor-1 mimics the anticancer effects of calorie restriction on cyclooxygenase-2-driven pancreatic neoplasia. Cancer Prev Res. 2011; 4: 1030-1040.
- O’Rourke Inflammation in obesity-related diseases. Surgery. 2009; 145: 255-259.
- Parker ED, Folsom Intentional weight loss and incidence of obesity-related cancers the Iowa Womens Health Study. Int J Obes Relat Metab Disord. 2003; 27: 1447-1452.
- Byers T, Sedjo Does intentional weight loss reduce cancer risk. Diabetes Obes and Metabolism. 2011; 13: 1063-1072.
- Douketis JD, Macie C, Thabane L, et Systematic review of long-term weight loss studies in obese adults clinical significance and applicability to clinical practice. Int J Obes. 2005; 29: 1153-1167.
- Weight National Task Force on the Prevention and Treatment of Obesity. JAMA. 1994; 272: 1196-1202.
- Thompson HJ, McTiernan Weight cycling and cancer weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res. 2011; 4: 1736-1742.
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood systematic review. International Journal of 2011; 35: 891-898.
- Bjorge T, Engeland A, Tverdal A, et al. Body mass index in adolescence in relation to cause specific mortality a follow-up of 230,000 Norweigan adolescents. Am J Epidemiol. 2008; 168: 30-37.
- Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity other cardiovascular risk factors and premature death. N Engl J 2010; 362: 485-493.
- Gunnell DJ, Frankel SJ, Nanchahal K, et al. Childhood obesity and adult cardiovascular mortality a 57-y follow-up study based on the Boyd Orr Am J Clin Nutr. 1998; 67: 1111-1118.
- DiPietro L, Mossberg HO, Stunkard A 40-year history of overweight children in Stockholm lifetime overweight morbidity and mortality. Int J Obes Relat Metab Disord. 1994; 18: 585-590.
- Whiteman MK, Hillis SD, Curtis KM, et al. Body mass and mortality after breast cancer Cancer Epidemiol Biomarkers Prev. 2005; 14: 2009-2114.
- Baer HJ, Tworoger SS, Hankinson SE, et al. Body fatness at young ages and risk of breast cancer throughout Am J Epidemiol. 2010; 171: 1183-1194.
- Baer HJ, Colditz GA, Rosner B, et Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women a prospective cohort study. Breast Cancer Res. 2005; 7: 14-25.
- Kuh D, Ben Shlomo Y, Gillman A life course approach to obesity In editors. A life course approach to chronic disease epidemiology. 2nd ed. Oxford University Press. 2004; 189-217.
- Hirko KA , Kantor ED, Cohen SS, et al. Body Mass Index in Young Adulthood Obesity Trajectory and Premature Mortality. Am J 2015; 182: 441-450.
- von Bonsdorff MB, Törmäkangas T, Rantanen T, et Early life body mass trajectories and mortality in older age findings from the Helsinki Birth Cohort Study. Ann Med. 2015; 47: 34-39.
- Wang Y. Epidemiology of childhood obesity methodological aspects and guidelines what is new Int J Obes Relat Metab 2004; 3: S21-S28.
- Zhao D, Zhang Y. Body mass index BMI predicts percent body fat better than body adiposity index BAI in school children. Anthropol 2015; 72: 257-262.
- Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body mass index and mortality among 1.46 million white adults. N Engl J 2010; 363: 2211-2219.
- Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of US. adults. N Engl J Med. 1999; 341: 1097-1105.
- Lifshitz Obesity in children. J Clin Res Pediatr Endocrinol. 2008; 1: 53-60.
- Troiano RP, Frongillo EA, Sobal J, et The relationship between body weight and mortality a uantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996; 20: 63-75.
- Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight overweight and obesity. JAMA. 2005; 293: 1861-1867.
- Flegal KM, Graubard BI, Williamson DF, et al. Cause-specific excess deaths associated with underweight overweight and JAMA. 2007; 298: 2028-2037.
- Olsshansky SJ, Passaro DJ, Hershow RC, et A potential decline in life expectancy in the United States in the 21st Century. N Engl J Med. 2005; 11: 1138-1145.
- Preston SH. Deadweight The influence of obesity on longevity. N Engl J 2005; 352: 1135-1137.
- Stettler N, Stallings VA, Troxel AB, et al. Strom BL Weight gain in the first week of life and overweight in adulthood a cohort study of European American subjects fed infant formula. 2005; 111: 1897-1903.
- Nader PR, Stone EJ, Lytle LA, et al. Three-year maintenance of improved diet and physical activity: the CATCH cohort. Child and Adolescent Trial for Cardiovascular Health. Arch Pediatr Adolesc Med. 1999; 153: 695-704.
- Epstein LH, Roemmich JN, Robinson JL, et al. A randomized trial of the effects of reducing television viewing and computer use on body mass index in young Arch Pediatr Adolesc Med. 2008; 162: 239-245.
- Kist C, Gier A, Tucker J, et al. Physical Activity in Clinical Pediatric Weight Management Programs Current Practices and Clin Pediatr Phila. 2016; 55: 1219-1229.