Clinical Implications of Persistence of Left Sided Superior Vena Cava
Author(s): Mandeep Kaur M.D1, Prema Bezwada M.D2, Aahana Gaur M.D1, Vishal Dhulipala M.D2, Cesar E Ayala Rodriguez M.D3 and Sarath Reddy M.D3
1Department of Internal Medicine, The Brooklyn Hospital Center, 121 DeKalb Avenue, Brooklyn, New York, USA.
2Department of Cardiology, Mount Sinai, Gustave L. Levy Place, New York, USA.
3Department of Cardiology, The Brooklyn Hospital Center, 121 DeKalb Avenue, Brooklyn, New York, USA.
*Correspondence:
Mandeep Kaur, MD, The Brooklyn Hospital Center, 121 DeKalb Avenue, Brooklyn, New York, USA, Tel: +1 623 703 6439.
Received: 04 October 2019; Accepted: 29 October 2019
Citation: Mandeep Kaur, Prema Bezwada, Aahana Gaur, et al. Clinical Implications of Persistence of Left Sided Superior Vena Cava. Cardiol Vasc Res. 2019; 3(5); 1-3.
Abstract
Persistent left superior vena cava (PLSVC) is a rare congenital vascular anomaly [1,2]. It is usually asymptomatic and found incidentally during imaging or identified while obtaining a vascular access for placing pacemakers [3,4]. Recently, it has been identified as a focus for Atrial fibrillation (AF) origin [5]. It is a congenital remnant of left sided pacemaker tissue, which can be visualized on a TTE have been identified to be the cause of refractory AF.
Keywords
Introduction
Association of Pulmonary vein as the source of ectopy of Atrial fibrillation is well known. However, other sources of ectopy, such as congenital remnant of left sided pacemaker tissue, which can be visualized on a TTE have been identified to be the cause of refractory AF. Early detection of Persistent Left Superior Vena Cava (PLSVC) on TTE can help to identify cause of refractory AF and guide the electrophysiological studies. This case report highlights the congenital anomaly PLSVC and associated clinical implications.
Case Presentation
A 43-year-old female with a past medical history of hypertension and pre-diabetes presented with acute onset of shortness of breath and palpitations for four weeks. Family history was significant for premature myocardial infarction in her brother and father. There was no significant smoking or illicit drug use history. Serial EKGs revealed Atrial fibrillation (AF) with rapid ventricular response at a rate of 140 beats per minute. She was hemodynamically stable. Labs revealed troponin < 0.01 ng/ml, Thyroid Stimulating Hormone (TSH) 4.41 mc IU/ml, BNP 18 pg/ml. D-dimer 0.7 mg/ mL. CT Angiography Chest was negative for pulmonary embolism but revealed a variant left sided Superior Vena Cava (Figure 1).
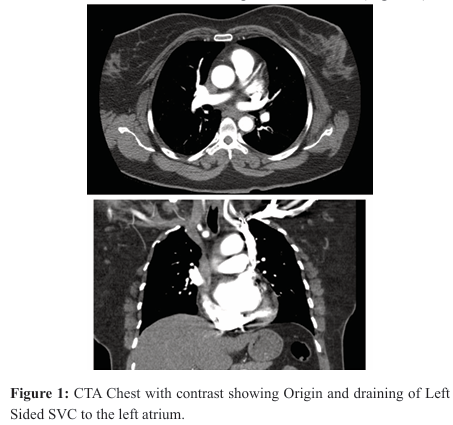
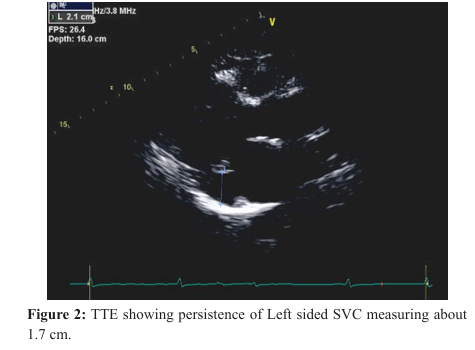
She received Metoprolol for rate control and IV Heparin for anticoagulation. A transthoracic Echo revealed left ventricular ejection fraction of 55-60% Normal right ventricular systolic function. Mildly dilated left atrium. Mild tricuspid regurgitation and incidental finding of presence of a persistent left superior vena cava (Figure 2). She was discharged with Metoprolol Succinate 100 mg BID after rate control and Rivaroxaban prescribed for anticoagulation.
Discussion
The description of a persistent left superior vena cava (PLSVC) in adults date back to 1787 [1,2]. Its prevalence is estimated to be 0.3% to 0.5% [2]. It is a remnant of a vessel that is present as a counterpart of a normal right-sided superior vena cava (SVC) in early embryological development which regresses to form the ligament of Marshall in an adult heart [3]. In the embryonic heart, bilateral pacemaking areas are present near the sinus horns and common cardinal veins. While the right side takes over cardiac pacemaking function as the sinoatrial node, persistence of the left common cardinal vein as the LSVC may be associated with a continued presence of pacemaker tissue and hence ectopic electrical activity in rare cases [3,4].
PLSVC had been associated with some arrhythmias in the past, but it was not until 2004, that it was recognized as source of ectopy, initiating Atrial Fibrillation (AF) [3]. Electroanatomic mapping has demonstrated the presence of electrical potentials within the LSVC. These ectopias were conducted through connections to the left atrium. Moreover, ablation of these connections resulted in electrical isolation and resolution of arrhythmias [3,4].
A multicenter retrospective study with drug refractory symptomatic AF and concomitant PLSVC was performed [5]. All patients were found to have spontaneous or isoproterenol induced triggers of AF. Electrical isolation of PLSVC was done without major complications and long-term freedom from AF in 75% of patients at 1-year follow-up.
Other clinical implications of PLSVC include, risk of paradoxical embolism because of accompanying lesions such as an atrial septal defect, unroofed coronary sinus and direct communication of the vein to the left atrium [1]. It can also complicate the insertion of a permanent pacemaker and implantable cardioverter defibrillator. Complications such as angina, arrhythmia, cardiogenic shock, and cardiac arrest have been reported when a guide wire or catheter is manipulated via a PLSVC [6,7].
Conclusion
PLSVC is a rare congenital vascular anomaly, known for decades [1,2]. It has been associated with various congenital heart diseases and arrhythmias [1,2]. Being aware of the association of PLSVC and Atrial fibrillation in the early stages of patient presentation can prompt to look for the characteristic features of this vascular anomaly on transthoracic echocardiography. Literature review supports the fact that in many patients with drug refractory AF, electrophysiological studies have identified PLSVC as a source of ectopy and the electrical isolation of the PLSVC has helped to maintain sinus rhythm [5].
Questions that remain unanswered are: It is unclear if only electrical isolation of PLSVC without PVI (Pulmonary Vein Isolation) can maintain sinus rhythm in the long run. Further studies and analysis are required to compare the clinical outcomes in patients who had 100% PLSVC triggers and received isolation versus those with PLSVC triggers who did not receive isolation [5,8]. In addition, more data is required regarding the long-term safety profile of the isolation of PLSVC [10].
References
- Tak T, Crouch E, Drake GB. Persistent left superior vena cava: incidence, significance and clinical correlates. Int J Cardiol. 2002; 82: 91-93.
- Sumi S, Debra P, Meiri R. Persistent left superior vena cava – considerations in fetal, pediatric and adult populations. Australas J Ultrasound Med. 2012; 15: 61-66.
- Chan KL, Abdulla A. Images in cardiology. Giant coronary sinus and absent right superior vena cava. Heart. 2000; 83: 704.
- Biffi M, Boriani G, Frabetti L, et al. Left Superior Vena Cava Persistence in Patients Undergoing Pacemaker or Cardioverter-Defibrillator Implantation. Chest. 2001; 120: 139-144.
- Li-Fern H, Pierre J, David K, et al. Atrial Fibrillation Originating From Persistent Left Superior Vena Cava. Circulation. 2004; 109: 828-832.
- Dong J, Zrenner B, Schmitt C. Existence of muscles surrounding the persistent left superior vena cava demonstrated by electroanatomic mapping. Heart. 2002; 88: 4.
- Turagam MK, Atoui M, Atkins D, et al. Persistent left superior vena cava as an arrhythmogenic source in atrial fibrillation: results from a multicenter experience. J Interv Card Electrophysiol. 2019; 54: 93-100.
- Doshi RN, Wu TJ, Yashima M, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999; 100: 876-883.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/ 2014; 130: 2071-2104 HRS guideline for the management of patients with atrial 10. Wissner E, Tilz R, Konstantinidou M, et al. Catheter ablation fibrillation: executive summary: a report of the American of atrial fibrillation in patients with persistent left superior vena College of Cardiology/American Heart Association Task cava is associated with major intraprocedural complications. Force on practice guidelines and the Heart Rhythm Society. Heart Rhythm. 2010; 7: 1755-1760.