Clinical Study to Determine Efficacy of Trigonella Foenum-Graecum Extract in Patients with Type 2 Diabetes
Author'(s): Gupta RS1, Grover AS2, Apurva Goel3* and Kiran Tiwari3
1Professor & Head, Department of Medicine, Gian Sagar Medical College & Hospital, Banur, Patiala, India.
2Professor and Head, Department of Surgery, Gian Sagar Medical College & Hospital, Banur, Patiala, India.
3Chemical Resources (CHERESO), R&D Department, Panchkula, Haryana, India.
*Correspondence:
Apurva Goel, Chemical Resources (CHERESO), R&D Department, Panchkula, Haryana, India, E-mail: apurva@chereso.net.
Received: 19 July 2018 Accepted: 13 August 2018
Citation: Gupta RS, Grover AS, Apurva Goel, et al. Clinical Study to Determine Efficacy of Trigonella Foenum-Graecum Extract in Patients with Type 2 Diabetes. Diabetes Complications. 2018; 2(3): 1-5.
Abstract
The anti-diabetic action of Fenugreek seeds is known to be effective by enhancing insulin sensitivity as well as reducing insulin resistance along with decreased glucose absorption. Trigonella foenum-graecum extract has been shown to exhibit potent antioxidant, hypoglycemic, nephroprotective, and cardiovascular activities. It serves as an excellent membrane stabilizer mainly because of its active ingredient, novel furostanolic saponins.
The aim of the study was to determine the efficacy of fenugreek seeds extract in Type 2 diabetic patients. An open labeled, two armed and single-centric study on 104 Type 2 diabetic patients was conducted using a novel fenugreek seed extract for a period of 12 weeks to determine its efficacy in diabetic patients in management of glucose metabolism.
The results showed that Trigonella foenum-graecum extract caused significant reduction in fasting glucose, Post Prandial glucose levels as well as in HbA1C levels. Fasting blood glucose levels were decreased in 95.2% of the study population.
As observed on completion of the treatment with Trigonella foenum-graecum extract, 88.10% of study population was shown to have decrease in PP glucose levels. The HbA1c levels came to normal range (Good control range - 4.5-6.3%) in almost all of the study population whereas these were still abnormal (Poor control levels – 7.6%) in on-going anti-diabetic therapy treated population after 12 weeks of treatment.
No significant changes were observed in liver function tests, urea and creatinine levels and hematological parameters.
Keywords
Introduction
Type II diabetes is a fast spreading metabolic disease worldwide. It has been known to affect 415 million people from which, 193 million people are known to have undiagnosed diabetes. From all the patients diagnosed with diabetes, 90% are those affected specifically with type 2 diabetes. Thus, type I diabetes is found to affect only 10% patients [1].
Diabetes is the outcome of disturbed body metabolism. The blood sugar sugar levels are raised only under two situations i.e. either the insulin production is limited by the β-cells of pancreas or the cells of the body become unable to detect insulin to use it to convert blood sugar into energy. In both cases, sugar present in blood unable to enter the cells resulting in raised blood sugar levels. Type II diabetes originates in those cases, when cells become unable to respond to insulin properly, which is known as ‘insulin resistance’. This happens because the insulin binds to its receptor normally, but the signal is not sent into the cell and the cells do not take up glucose. This causes rise in blood sugar levels. There are many risk factors for causing insulin resistance inside body, which contributes in development as well as progression of type II diabetes such as [2,3],
Obesity Dyslipidemia Oxidative stress
Non-alcoholic fatty liver disease
Stress
Genetical and lifestyle factors Sleep apnea
Smoking Overweight and many more.
Insulin resistance is responsible for developing type II diabetes, which further gives rise to many complications, ranging from microvascular to macrovascular complications. It has been observed that type I diabetes is associated with much lesser complications than type II diabetes. The microvascular complications associated with type II diabetes include nephropathy, retinopathy, neuropathy, etc and macrovascular complications include renal diseases, cardiac diseases, cerebrovascular diseases, peripheral vascular diseases, etc [4].
Development of these complications with diabetes weakens the body to a much higher extent. These complications also interfere with the treatment of diabetes. The number of people with diabetic complications is increasing day-by-day. According to the year 2013 data, 381.8 million adults from 219 countries were suffering from type II diabetes and there are estimates that this number will raise to 591.9 million adults till 2035.
The lifestyle choices of diabetic patients and complications interfering in the treatment of diabetes are major reasons behind this prevalence. It has been observed that countries with large number of adult population are associated with high number of people with diabetes. Among this list, India is on 2nd number with 65.1 million people with diabetes (among top 10 countries most prevalent with diabetes) [5].
Besides lifestyle changes, medications are prescribed by doctors to slow down or treat the progression of type II diabetes. There are a large number of medications available for managing type II diabetes but the disease still develops in the population of every age group and every region of the world. It has been observed that these medications cannot cure diabetes but may slow down its development and help in the management. The reason behind this might be the incomplete effectiveness of the medications
Investigational Product- Fenfurotm
Numerous herbs are reported to possess anti-diabetic activity but a significant amount of research and traditional usage suggest that Fenugreek seeds are among the best in terms of safety and efficacy. The anti-diabetic action of Fenugreek is known to be mediated by improving insulin sensitivity and decreasing insulin resistance along with reduced glucose absorption [6,7].
FENFURO is a natural product developed innovatively from Fenugreek seeds extract. It contains many bioactive components from that help to lower the blood glucose levels. FENFURO contains furostanolic saponins extracted from the seeds of fenugreek, which exert hypoglycaemic effect in type II diabetic patients. It has been suggested that saponins and fiber component (32% insoluble & 13% soluble) present in fenugreek seeds help to lower blood sugar levels. These bioactive components of fenugreek seeds delay the gastric emptying and cause the inhibition of glucose transport (by inhibiting lipid and carbohydrate-hydrolyzing enzymes).
Fenugreek seeds are reported to affect insulin resistance. It has been observed in animal studies that bioactive components of fenugreek seeds increase the sensitivity of tissues to available insulin by increasing insulin receptor sites. Increase in insulin receptor sites helps to send the signals from insulin to the tissues more effectively, making the tissues sensitive to the insulin levels. Thereby, more glucose starts to enter the tissues from blood to be converted to energy, decreasing the blood glucose levels [8].
All these mechanisms are responsible for the anti-diabetic action of FENFURO.
Such anti-diabetic action has also been observed in the previous clinical as well as preclinical studies on FENFURO.
Aims and Objectives
The aim of the study was to evaluate the efficacy of FENFURO in patients suffering from Type 2 diabetes.
Primary objective
To determine the decrease in plasma glucose levels due to FENFURO in type 2 diabetic patients
Secondary objective
To determine the safety of FENFURO in type 2 diabetic patients.
Material and Methods
This was an open labeled and two-armed study, which was carried out on Indian population suffering from type 2 diabetes. The study was carried out at Gian Sagar.
Medical College & Hospital, Patiala- 140601.
There were two groups, i.e. Group A & Group B, in this study in which, 50 patients each were enrolled. Group A with type 2 diabetes patients were planned to consume investigational product (FENFURO) along with their on-going anti-diabetic therapy. Group B with type 2 diabetes patients were planned to be on their on-going anti-diabetic therapy only. Key inclusion and exclusion criteria were as follows:
Inclusion Criteria
- Agreed to written informed consent.
- Fasting plasma glucose level <180 mg/dL.
- HbA1c level more than 7.5%.
- Not received any steroids
- Patient on anti-diabetic therapy
Exclusion Criteria
- Uncooperative Subjects
- Diabetes other than type-2 diabetes
- Evidence of renal & liver disease.
- History of any hemoglobinopathy that may affect determination of HbA1c
- Lactating and Pregnant or planning to conceive females.
- Physically/ mentally unwell as certified by physician-in- charge.
- Participation in any other clinical trial within the last 30
- Subjects with allergy to investigational product.
The subjects were followed up after 4 weeks, 8 weeks and 12 weeks. Safety was assessed at each follow-up visit. Subjects complaining of significant symptoms following administration of investigational product were planned to be evaluated for objective parameters of adverse drug reactions. The investigational product Fenugreek Seed Extract (FENFURO) 500mg was given on twice daily basis and was allotted after screening and enrolment of the study subject.
The efficacy of investigational product (FENFURO) in type 2 diabetes patients was evaluated by the laboratory investigations. The following investigations were done at baseline and at the end of the study (12 weeks):
- Blood pressure
- Body weight
- BMI
- Waist circumference
- Fasting glucose
- Post-prandial (PP) glucose
- HbA1c
- C-peptide levels
- TSH
Results
There were two groups in the study population i.e. Group A & Group B. Group A patients were treated with investigational product- FENFURO along with their on-going anti-diabetic therapy and Group B patients were treated only with their on- going anti-diabetic therapy. Both male and female patients of type II diabetes were enrolled in the study. The study population consisted of 46.9% male patients and 53.1% female patients. In FENFURO-treated group, average age of the study population was 52.45 years, with minimum age of 28 years and maximum age of 64 years. In on-going anti-diabetic therapy-treated group, average age of the study population was 50.69 years with minimum age of 28 years and maximum age of 65 years.
Mean systolic blood pressure of study population of Group A was 129.04 mmHg and Group B was 125.64 mmHg. Mean diastolic blood pressure of study population of Group A was 84.9 mmHg and Group B was 77.43 mmHg.
Efficacy Conclusions
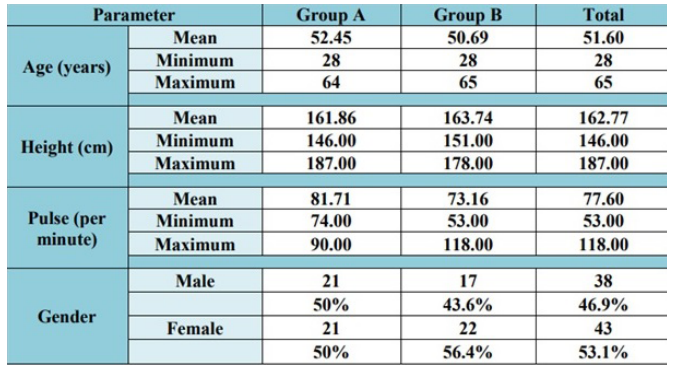
Fasting glucose (mg/dl)
In FENFURO treated group, the mean fasting glucose levels significantly (p = 0.0001**) decreased from baseline value of 159.97 mg/dl to 98.76 mg/dl on completion of the treatment whereas in on-going anti-diabetic therapy-treated group, these levels were significantly (p = 0.023*) increased from baseline levels of 147.12 mg/dl to 173.76 g/dl on completion of the treatment.
In FENFURO-treated group, 95.20% study population showed decrease in fasting blood glucose levels on completion of the treatment. In on-going anti-diabetic therapy-treated group, 60.50% study population showed increase in fasting blood sugar levels on completion of the treatment.
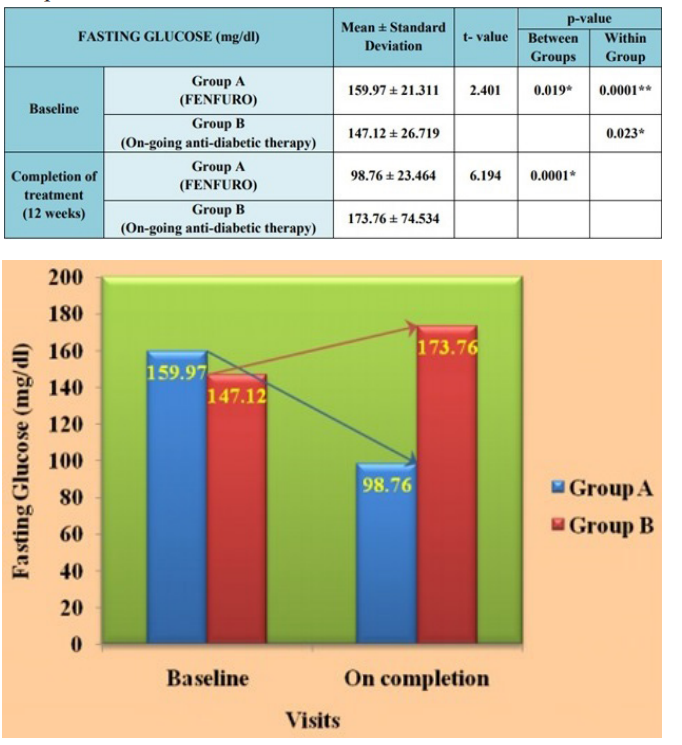
Post-prandial (PP) glucose (mg/dl)
In FENFURO-treated study population, the mean PP glucose levels decreased significantly (p = 0.0001**) from baseline levels of 254.33 mg/dl to 142.30 mg/dl on completion of the treatment whereas in on-going anti-diabetic therapy-treated study population, they were non-significantly (p = 0.839) changed from baseline levels of 219 mg/dl to 222 mg/dl on completion of the treatment. In FENFURO-treated group, 44.04% decrease in the PP glucose levels was observed in the study population on completion of the treatment whereas in on-going anti-diabetic therapy-treated group, 1.71% decrease in PP glucose levels was observed on completion of the treatment.
In FENFURO-treated group, 88.10% study population showed decrease in PP glucose levels on completion of the treatment. In on-going anti-diabetic therapy-treated group, 47.40% study population showed increase in PP sugar levels on completion of the treatment.
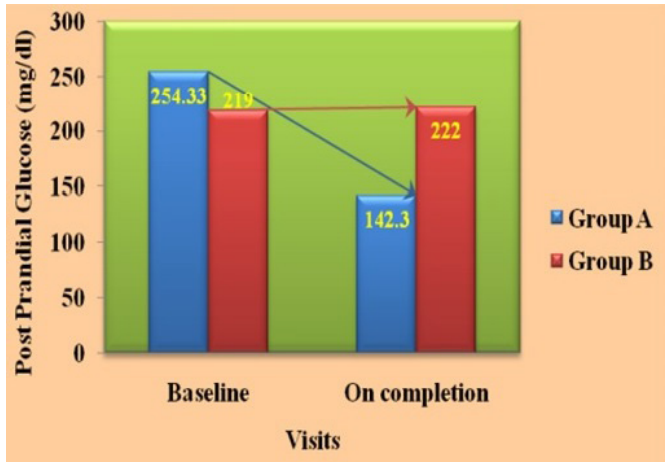
Glycated hemoglobin (HbA1c) (%)
The mean HbA1c levels decreased significantly (p = 0.0001**) in FENFURO-treated group from baseline levels of 9.37% to 6.11% on completion of the treatment and these levels also decreased significantly (p = 0.0001**) in on-going anti-diabetic therapy-treated group from baseline levels of 9.78% to 7.65% on completion of the treatment.
Mean HbA1c levels of the FENFURO-treated group decreased in the whole study population (100%) of the group whereas they were decreased in 86.8% of the study population of on-going anti- diabetic therapy-treated group on completion of the treatment. Mean HbA1c levels decreased up to 34.7% in FENFURO-treated group and up to 21.51% in on-going anti-diabetic therapy-treated group.
Safety Conclusions
On completion of the study, following safety conclusions were made:
- No significant change in the liver function tests (serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, alkaline phosphates activities and serum bilirubin levels) was observed on completion of the treatment.
- No significant change in the serum urea levels and creatinine levels was observed on completion of the treatment.
- No significant change in the hematological parameters was observed on completion of the treatment.
Discussion
Type II diabetes is a disease involving increase in blood sugar (glucose) levels due to the inability of cells to respond properly to insulin. Response towards insulin is a very critical step in balancing blood sugar levels, as this response will transfer the sugar dissolved in blood towards tissues to convert it to energy.
The risk for diabetes is mostly at the age after 35 years but diabetes is not limited to this age group as diabetes cases have been observed in adoloscent population also. Diabetes has been observed to be mostly prevalent in urban population as compared to rural population due to the unhealthy lifestyle. Doctors have also reported the contribution of unhealthy eating pattern and limited physical activity in the development of diabetes. Beside this, genetical factors also contribute in developing diabetes.
Mostly, doctors suggest lifestyle changes and exercise as first remedy for diabetes. Then, medications are prescribed, if diabetes is not reverted to normal. People are turning towards nutraceuticals for treating any kind of disease as they are effective to treat disease and they are devoid of any adverse effects. One such natural product is FENFURO, which was evaluated in the present study for its efficacy and safety in type II diabetic patients.
In the present study, two groups were made from which Group A included type II diabetic patients to be administered with FENFURO along with their on-going anti- diabetic therapy. The effect of FENFURO alone was evaluated by comparing Group A with Group B, which included those patients who were administered only with on-going anti-diabetic therapy. Thus, the anti-diabetic effect of FENFURO was evaluated by comparing results of both the groups.
Fasting Glucose Levels
Fasting glucose level is the most affected parameter under diabetic condition, which is raised above 110 mg/dl during diabetes. It is the primary test to be recommended to ensure the presence of diabetes. Fasting blood glucose is suggested because during fasting condition, no food is eaten and the body requires energy, which is supplied through glucagon hormone. During fasting condition, the pancreatic cells secrete glucagon, which stimulates the conversion of glycogen to glucose which results in the increase in blood sugar (glucose) levels. Thus, during diabetic condition, increase in fasting sugar levels contributes to worsening diabetes.
In the present study, FENFURO caused decrease in fasting glucose levels up to 38.26% on completion of the treatment. This means that FENFURO has a role in inhibiting the production of glucose by glucagon or inhibit the production of glucagon itself, which resulted in decrease in fasting glucose levels.
Post-Prandial (PP) Glucose Levels
PP glucose is another laboratory test recommended during diabetes. It is recommended because food contains carbohydrates and thus, the blood glucose levels always rise after meals. PP glucose levels measure the amount of blood glucose levels raised after eating food. As the blood glucose levels rise after eating, doctors always restrict the carbohydrate intake during diabetes.
In the present study, FENFURO was successfully able to decrease the PP glucose levels in blood. They were decreased up to 44.04% in the FENFURO-treated study population. But PP glucose levels significantly increased in on-going anti-diabetic therapy-treated study population.
HbA1c levels
HbA1c or Glycated hemoglobin is originated when hemoglobin (protein within RBCs carrying oxygen in whole body) attaches itself to blood glucose. Thus, more the available glucose; more will be the chances of attaching hemoglobin to it, raising HbA1c levels. In other words, the amount of glucose that combines with hemoglobin is directly proportional to the total amount of sugar in the human body at that time. Thus, under diabetic condition, HbA1c levels will also become high. It has been suggested that if HbA1c is improved by even 1% in people with type II diabetes, it cuts the risk of micro vascular complications of type II diabetes by 25%.
In the present study, FENFURO was able to lower the HbA1c levels up to 34.70% in the type II diabetic study population on completion of the treatment, meaning it is able to lower the glucose levels in blood resulting in reduction in HbA1c levels. This indicates that FENFURO is able to lower the risk of development of microvascular complications of type II diabetes.
Safety
As the safety parameters of the study are considered, FENFURO did not alter the liver profile or renal profile or haematological parameters of the study population proving the safety of FENFURO in human beings.
Conclusion
Efficacy and safety data of the present clinical study in type II diabetic patients clearly indicate that the addition of FENFURO to on-going anti-diabetic therapy is more effective than the anti- diabetic therapy alone in the patients. FENFURO is able to safely lower the fasting glucose levels, PP glucose levels and HbA1c levels of type II diabetic patients. FENFURO is also proved to be completely safe in type II diabetic patients.
Funding
The study was funded by Chemical Resources, Panchkula, Haryana, India.
Consent To Publish
All authors have read, consented and approved the final manuscript for publication. This manuscript doesn't contain any individual person's data.
References
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. The 2017; 389: 2239-2251.
- Kahn SE, Cooper ME, Prato Pathophysiology and treatment of type 2 diabetes Perspectives on the past, present and future. Lancet. 2014; 383: 1068-1083.
- Samuel VT, Shulman The pathogenesis of insulin resistance integrating signalling pathways and substrate flux. J Clin Invest. 2016; 126: 12-22.
- Dart AB, Martens PJ, Rigatto C, et Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014; 37: 436-443.
- Guariguata L, Whiting DR, Hambleton I, et Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014; 103: 137-149.
- Gaddam A, Galla C, Thummisetti S, et al. role of fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. Journal of Diabetes and Metabolic 2015; 14: 74.
- Fuller S, Stephens JM. Diosgenin, 4-hydroxyisoleucine and fiber from fenugreek: Mechanism of actions and potential effects on metabolic Adv Nutr. 2015; 6: 189-197.