Concomitant Cardiac Carcinoid and Amyloid in a Patient with a Caecal Neuro-Endocrine Tumour with Metastases
Author'(s): Gordon Pate*, Evan Mannion and John Greally
The Galway Clinic, Doughiska, Galway.
*Correspondence:
Gordon Pate, the Galway Clinic, Doughiska, Galway
Received: 14 Apr 2023; Accepted: 22 May 2023; Published: 26 May 2023
Citation: : Pate G, Mannion E, Greally J. Concomitant Cardiac Carcinoid and Amyloid in a Patient with a Caecal Neuro-Endocrine Tumour with Metastases. Cardiol Vasc Res. 2023; 7(3): 1-3.
Keywords
Case
A seventy-two year old man presented with increasing dyspnoea, on a background of known coronary artery disease and four stents to his right coronary artery six months previously. His main complaint on admission was of bilateral leg swelling and abdominal discomfort and bloating. He also had some diarrhoea with some blood and increasing shortness of breath.
On examination his blood pressure was 90/60 and he had giant v-waves in his jugular venous system consistent with marked tricuspid valve regurgitation. He had bibasal pleural effusions, right greater than left and a palpable liver edge with some ascites. He had no pedal oedema. His ECG was of low voltage but was otherwise normal. He was anaemic.
Echocardiogram showed severe tricuspid regurgitation with failure of leaflet coaptation due to shortening of the chordae tendineae. He also had marked concentric left ventricular hypertrophy, 15mm, with speckling suggestive of cardiac amyloid.
CT scan showed a tumour in the caecum, which was causing sub- acute obstruction, a large (5cm) adrenal deposit, and substantial liver metastases. Biopsy confirmed the tumour to be neuroendocrine in nature with appearance consistent with carcinoid tumor. The same tissue sample was also found to have kappa light chain amyloid deposits.
Twenty-four hour urine for 5HIAA was elevated at 113 µmol. Urinalysis for Bence-Jones proteins and serum electrophoresis revealed a monoclonal IgG kappa band with a serum paraprotein measurement of 7.9G/L. Incidentally, his TSH came back at 75mIU/L, T4 2.0pmol/L and he was commenced on thyroxine. His iron levels also came back low at 4.0umol/L.
Palliative right hemicolectomy was undertaken. Recovery from this was extremely slow. His albumin decreased to 14g/L likely due to a combination of liver dysfunction due to right heart failure and metastatic replacement, enteropathy, diarrhoea and poor intake. He developed anasarca. He continued to deteriorate and subsequently died several weeks later without post mortem examination.
Discussion
Although cardiac involvement in either carcinoid syndrome or amyloidosis is well-recognised [1-12], the association of pathological findings consistent with both serotonergic syndrome and cardiac amyloidosis have only been reported once previously [13]. At that time, only M-mode ultrasound was available. Echocardiogram in this case showed severe tricuspid regurgitation with failure of leaflet coaptation due to shortening of the chordae tendineae, which is almost pathognomonic of carcinoid syndrome. He also had the marked concentric left ventricular hypertrophy with speckling that is typical of cardiac amyloid deposition.
Biochemistry revealed only a modest rise in serotonin of 113 ng/mLs, which is not particularly high for carcinoid syndrome resulting in the typical clinical and echocardiographic features of cardiac serotonergic syndrome (more often seen to be in the thousands); this is likely due to respiratory monoamine oxidase activity reducing measured venous levels [14]. Though thyroid carcinoma has been associated with carcinoid and amyloid syndromes, as well as with multiple endocrine neoplasia [15- 17], he had no clinical findings to suggest carcinoma and thyroid biopsy was not performed.
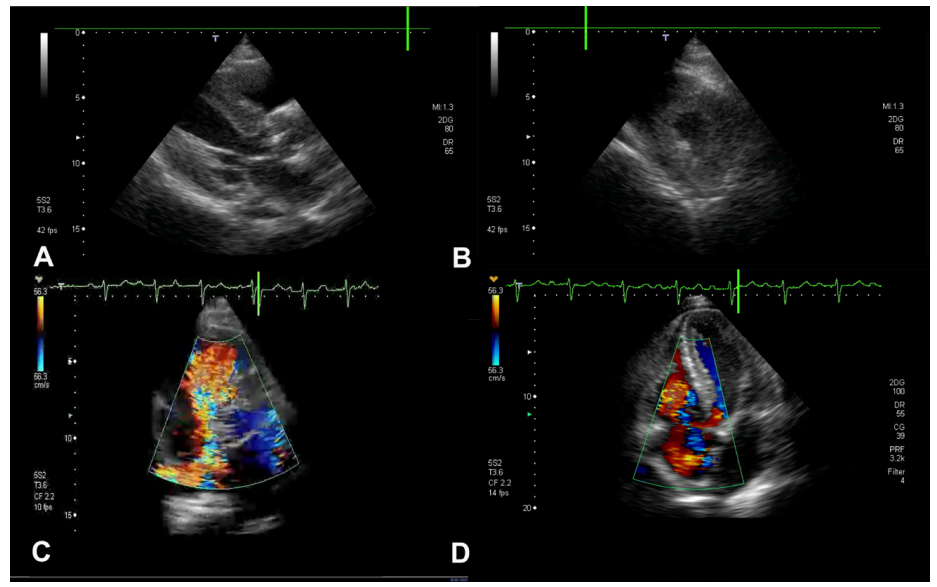
Figure 1: Echocardiographic views demonstrating findings of both amyloid deposition and serotonin syndrome.
(A) Parasternal long axis view in diastole showing significant concentric left ventricular hypertrophy. (B) Parasternal short axis view in diastole showing significant concentric left ventricular hypertrophy. (C) Apical four-chamber view in systole showing significant tricuspid regurgitation. (D) Parasternal short axis view in systole showing significant tricuspid regurgitation.
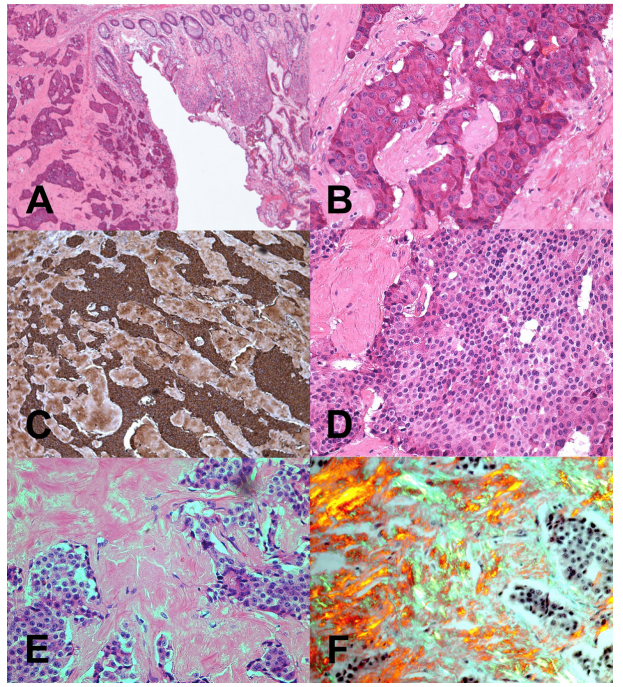
Figure 2: (A) Low power view of colonic mucosa (right) and one of two patterns of invasive carcinoid tumour islands (left). (B) High power view of one of two patterns of tumour showing broad cords of tumour cells separated by connective tissue. Prominent nuclei with small condensations of nuclear chromatin are present and the nucleus is surrounded by eosinophilic cytoplasm. No mitotic activity is seen and no amyloid was detected in this section of the tumour. (C) This shows positive Chromagranin A staining in the section of tumour shown in A and B. The dark brown stain represents the presence of secretory granules in neuroendocrine cells. (D) The second tumour pattern is illustrated here i.e. closely packed tumour cells of uniform size with a dense nucleus and limited cytoplasm. This pattern is also seen in carcinoid tumours. (E) Using light microscopy, the second tumour pattern stains positively with Congo red stain, showing deposition of pink material i.e. amyloid. (F) Under polarized light, Congo red stain shows light green birefringence, characteristic of amyloid. No mitotic activity is identified.
The colonic biopsy identified a chromogranin positive neuroendocrine tumor with closely packed tumor cells of uniform size constant with a carcinoid tumor. There was localised amyloid deposition in the same tumor, which was found to be composed of kappa light chain. Colorectal tissue is a common site of deposition in systemic amyloidosis [18] and while amyloidosis is a recognised (but unusual) feature of neuro-endocrine tumours [19,20], it is more commonly due to increased production of amyloid pre- cursor protein or islet amyloid polypeptide [21,22].
Unfortunately, by the time this man presented he already had widely disseminated disease, as is usually the case with carcinoid syndrome, and this information was of little assistance in his management. Had a diagnosis been made earlier it is possible that tumour removal and plasmapheresis or somatomedian analog therapy [23-25] might have helped to retard the deposition of cardiac amyloid and tricuspid regurgitation but it is unlikely that cardiac surgery would have made any difference to his outcome [26,27]. Bone marrow biopsy was not performed.
References
- Stollberger C, Finsterer J. Extracardiac medical and neuromuscular implications in restrictive cardiomyopathy. Clin Cardiol. 2007; 30: 375-380.
- Artz G, Wynne J. Restrictive Cardiomyopathy. Curr Treat Options Cardiovasc Med. 2000; 2: 431-438.
- Click RL, Olson LJ, Edwards WD, et al. Echocardiography and systemic diseases. J Am Soc Echocardiogr. 1994; 7: 201-216.
- Schoen FJ, Berger BM, Guerina NG. Cardiac effects of noncardiac neoplasms. Cardiol Clin. 1984; 2: 657-670.
- Wheeler R, Abelmann WH. Cardiomyopathy associated with systemic diseases. Cardiovasc Clin. 1972; 4: 283-303.
- Garibaldi B, Zaas D. An unusual case of cardiac amyloidosis. J Gen Intern Med. 2007; 22: 1047-1052.
- de Diego C, Marcos Alberca P, Cabrera JA, et al. Pathognomonic echocardiographic features of carcinoid syndrome. Clin Cardiol. 2006; 29: 134.
- Pearlman AS. Carcinoid heart disease: echocardiographic recognition and differential diagnosis. Am Heart Hosp J. 2005; 3: 132-135.
- Wonnink-De JW, Knibbeler-Van RC, et al. Echocardiographic diagnosis in carcinoid heart disease. Neth J Med. 2002; 60: 181-185.
- Comenzo RL. Primary systemic amyloidosis. Curr Treat Options Oncol. 2000; 1: 83-89.
- Plockinger U, Gustafsson B, Ivan D, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: echocardiography. Neuroendocrinology. 2009; 90: 190-193.
- Dero I, De Pauw M, Borbath I, et al. Carcinoid heart disease-a hidden complication of neuroendocrine tumours. Acta Gastroenterol Belg. 2009; 72: 34-38.
- Jacobovitz D, Dustin P, Jr. Right heart valvular amyloidosis in a patient with duodenal carcinoid tumours. J Pathol. 1974; 112: 195-197.
- Wells S, Buford M, Porter V, et al. Role of the Serotonergic System in Reduced Pulmonary Function after Exposure to Methamphetamine. American Journal of Respiratory Cell and Molecular Biology. 2010; 42: 537-544.
- Marchevsky AM, Dikman SH. Mediastinal carcinoid with an incomplete Sipple's syndrome. Cancer. 1979; 43: 2497-2501.
- Harach HR, Bergholm U. Medullary carcinoma of the thyroid with carcinoid-like features. J Clin Pathol. 1993; 46: 113-117.
- Stridsberg M. The use of chromogranin, synaptophysin and islet amyloid polypeptide as markers for neuroendocrine tumours. Ups J Med Sci. 1995; 100: 169-199.
- Freudenthaler S, Hegenbart U, Schönland S, et al. Amyloid in biopsies of the gastrointestinal tract—a retrospective observational study on 542 patients. Virchows Archiv. 2016; 468: 569-577.
- Wilander E. Diagnostic pathology of gastrointestinal and pancreatic neuroendocrine tumours. Acta Oncol. 1989; 28: 363-369.
- Perfetti V, Garini P, Vignarelli MC, et al. Diagnostic approach to and follow-up of difficult cases of AL amyloidosis. Haematologica. 1995; 80: 409-415.
- Arvidsson Y, Andersson E, Bergstrom A, et al. Amyloid precursor-like protein 1 is differentially upregulated in neuroendocrine tumours of the gastrointestinal tract. Endocr Relat Cancer. 2008; 15: 569-581.
- Stridsberg M, Eriksson B, Lundqvist G, et al. Islet amyloid polypeptide (IAPP) in patients with neuroendocrine tumours. Regul Pept. 1995; 55: 119-131.
- Bhattacharyya S, Gujral DM, Toumpanakis C, et al. A stepwise approach to the management of metastatic midgut carcinoid tumor. Nat Rev Clin Oncol. 2009; 6: 429-433.
- Gustafsson BI, Hauso O, Drozdov I, et al. Carcinoid heart disease. Int J Cardiol. 2008; 129: 318-324.
- Fiasse R, Pauwels S, Rahier J, et al. Use of octreotide in the treatment of digestive neuroendocrine tumours. Seven year experience in 20 cases including 9 cases of metastatic midgut carcinoid and 5 cases of metastatic gastrinoma. Acta Gastroenterol Belg. 1993; 56: 279-291.
- Castillo JG, Filsoufi F, Rahmanian PB, et al. Early and late results of valvular surgery for carcinoid heart disease. J Am Coll Cardiol. 2008; 51: 1507-1509.
- Connolly HM, Pellikka PA. Carcinoid heart disease. Curr Cardiol Rep. 2006; 8: 96-101.