Correlation of Diabetes Mellitus and COVID-19: A Review
Author(s): Mehrie Harshad Patel, MBBS1,3*, Arjola Agolli, MD, MBA1, Mariana da Costa Rocha1,2, Lucas Riquieri Nunes1,2, Hansal Girish Mistry, MBBS3, Zeel Hemant Patel, MBBS3, Mitali Adhvaryu, BSc4, Olsi Agolli, MD1, Ilmaben Sikandarbhai Vahora, MBBS1,3, Kinal Paresh Bhatt, MD, MPH1, Eugenio Angueira, MD5, Carlo A Candelario Rodriguez, MD5, and Jose Cardona-Guzman, MD5
1Division of Clinical & Translational Research, Larkin Health System, South Miami, FL, USA.
2Universidade Federal de Uberlandia, Minas Gerais, Brazil.
3Pramukhswami Medical College, Gujarat, India.
4Department of Radiation Oncology, University of Toronto, Ontario, Canada.
5Larkin Community Hospital, Hialeah, FL, USA.
*Correspondence:
Dr. Mehrie H Patel, Division of Clinical & Translational Research, Larkin Health System, 7000 SW 62nd Ave, Suite 601, South Miami, FL 33143, USA, Tel: +1 305-284-7608.
Received: 27 March 2021 Accepted: 14 April 2021
Citation: Patel MH, Agolli A, Rocha MC, et al. Correlation of Diabetes Mellitus and COVID-19: A Review. Diabetes Complications. 2021; 5(1); 1-8.
Abstract
In the early pandemic, it was brought to attention that individuals with Diabetes Mellitus (DM) are prone to a more severe form of Coronavirus Disease 2019 (COVID-19). With this in mind, healthcare professionals need to be vigilant about their patients’ medical history in these challenging times as this could change the course of treatment and follow-ups for someone with COVID-19 and DM. Moreover, this is of utmost importance as the coronavirus is deemed to thrive in the elevated blood glucose environment. Though there is little known about the best medications for glycemic control in COVID-19, however, there are multiple other factors that can be beneficial in exploring for management in DM patients who are infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and some of these factors are as follow adequate glycemic control, medication dosages adjustment, diet, physical guidelines, thromboembolism prophylaxis, and empirical treatments for the possible co-infections. We conducted a literature review of publicly available information to summarize knowledge about the correlation between Diabetes Mellitus and the COVID-19 infections. The main objective of this manuscript is to provide a brief overview of the potential pathophysiologic correlation of DM and COVID-19, optimal management and prevention.
Keywords
Introduction
Coronavirus is a large, enveloped, single-stranded RNA virus that gained importance in the early 2010s, both in the medical and in the veterinary field, because of the acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), which were caused by newly emerged coronaviruses at the time. In 2019, a new coronavirus was identified as the cause of a disease outbreak that originated in Wuhan, China which has now come to be known as Coronavirus Disease 2019 (COVID-19). At the molecular level, they have a complex gene expression [1].
Recently emerged SARS-CoV-2 has been the causative agent of the third large-scale pandemic within the past two decades. It was discovered in December 2019 in Wuhan City, Hubei province in China, when patients started to present with symptoms of fever, dry cough, dyspnea, and bilateral lung infiltrates on imaging. The World Health Organization (WHO) named the disease COVID-19 by February 2020 [2] and no later than March 11, 2020, WHO declared the COVID-19 outbreak a pandemic [3].
Until this day, the complete clinical manifestation of the disease remains unclear as symptoms may vary from mild to severe, with some cases resulting in death. Mild symptoms present with complete recovery after 1 week, while severe cases can progress to respiratory failure due to alveolar damage from the virus, which may even lead to death. Deadly cases are reported to occur primarily in middle-aged and elderly patients with pre-existing comorbidities (tumour surgery, cirrhosis, hypertension, coronary heart disease, diabetes and Parkinson’s disease) [4].
Studies have shown that underlying Cardiovascular Disease (CVD) and Diabetes Mellitus (DM) are common health conditions among those patients who need ICU admission [5,6]. Besides, some evidence suggests an association between ACE2 and glucose regulation. For instance, ACE2-knockout mice were discovered to be more susceptible to high-fat-diet-induced pancreatic β-cell dysfunction than wild-type mice. Indeed, infection with SARS- CoV-2 can cause hyperglycemia even in people without a baseline DM. With all of these findings considered, plus the localization of ACE2 expression in the endocrine pancreas, it can be deduced that coronavirus might have a role in islet cell damage, potentially leading to hyperglycemia. Nonetheless, hyperglycemia was reported to be persistent for three years after recovery from SARS, which might indicate long-term damage to islet cells. All of the above proposes that the ACE2 might represent a key role in the association between COVID-19 and Diabetes Mellitus [7].
This article aims to provide a complete review, summarize and clarify previous studies that correlate Diabetes Mellitus with COVID-19. This article also aims to provide an overview of DM management in COVID-19. It is unique and of great importance in present-day, because of the magnitude of the COVID-19 pandemic and the high prevalence of DM worldwide.
Diabetes and susceptibility to COVID-19
Diabetes is a chronic medical condition characterized by glucose intolerance and insulin insensitivity or reduced insulin production. Coronavirus binds to the epithelial cells of lungs, intestine, kidney and blood vessels via an enzyme called Angiotensin Converting Enzyme-2 (ACE2). This ACE-2 enzyme is a key factor in linking Diabetes Mellitus (DM) and Coronavirus Disease-19 (COVID-19). Patients with DM taking Pioglitazone, ACEI or ARB are vulnerable to COVID due to increased expression of ACE-2 by these drugs. Coronavirus uses ACE-2 as an intermediate to bind epithelial cells of the body’s various organ systems [10]. Patients with diabetes have a compromised immune system making it harder to fight the virus and leading to a longer recovery period. Moreover, the virus may thrive in an environment of elevated blood glucose [10].
Various epidemiological studies have shown that DM is more frequent in patients with severe COVID-19. One retrospective study showed that patients with DM had more severe pneumonia, a higher concentration of Lactate dehydrogenase (LDH), Alanine transaminase (ALT), Gamma-glutamyltransferase (GGT) and fewer lymphocytes with a higher neutrophil count. One prospective cohort study conducted in New York City showed that the prevalence of DM was higher in individuals admitted to hospital than those not admitted. Many other studies like meta- analysis have been conducted concluding higher mortality in patients with DM [11].
Also, hyperglycemia at hospital admission was the best predictor of worst chest radiographic imaging results. This is because ACE2 receptors are highly expressed in pancreatic tissue and beta cells in particular. Thus, an acute loss of insulin secretory capacity along with stress condition and cytokine deterioration results in the development of Diabetic Ketoacidosis (DKA) and Hyperosmolar Hyperglycemic State (HHS). HHS additionally increases the risk of thrombosis that already characterizes severe COVID-19 [12,13].
It has been found that patients infected with the COVID-19 virus have high chances of developing acute DM, due to SARS-CoV-2 tropism for beta cells leading to acute impairment of insulin secretion or destruction of beta cells [14]. New-onset DM is characterized by FBS>7 MMOL/L and HbA1C>48 MMOL/MOL, carrying a greater risk of mortality than those with hyperglycaemia or DM [12].
Patients with DM infected with COVID-19 have worsening metabolic control due to beta-cell damage and cytokine storm, having: inflammation, coagulation, DKA/HHS, older age, HTN/CVD, renal disease, obesity are at higher chances of poor outcome [12, 15]. Furthermore, viral infection can also increase inflammation or internal swelling in people with DM, and that inflammation can contribute to more severe complications [16].
Type 1, Type 2 Diabetes Mellitus (DM) and COVID-19!
The increasing prevalence of DM is a concerning comorbid factor in many diseases including in SARS-CoV-2. The patients with Diabetes are susceptible to getting more severe forms of COVID-19 infection, have increased mortality and increased Hospital stay versus non-DM patients diagnosed with COVID-19 [17, 19, 20]. A whole population study conducted in England by Barron et al. (2020) showed that during the 72 days period during which the study was conducted, a total of 23,698 in-hospital COVID-19-related deaths occurred. One third occurred in patients having diabetes: 7434 (31.4%) in patients with Type 2 Diabetes (T2DM), 364 (1.5%) in those with Type 1 Diabetes Mellitus (T1DM), and 69 (0.3%) in people with other types of diabetes. Unadjusted mortality rates per 100 000 people over the 72-day period were 27 (95% CI 27–28) for those without diabetes, 138 (124–153) for those with T1DM, and 260 (254–265) for those with type 2 diabetes. Adjusted for age, sex, deprivation, ethnicity, and geographical region, compared with people without diabetes, the odds ratios (ORs) for in-hospital COVID-19-related death were 3.51 (95% CI 3.16–3.90) in people with T1DM and 2.03 (1.97– 2.09) in people with type 2 diabetes. These effects were attenuated to ORs of 2.86 (2.58–3.18) for T1DM and 1.80 (1.75–1.86) for T2DM when also adjusted for previous hospital admissions with coronary heart disease, cerebrovascular disease, or heart failure [18].
Glycemic control also plays a major role in the prognosis of COVID-19 infection and mortality. Previously, it has been observed that uncontrolled Diabetes (glycated Hb [HbA1c] >9%) has been associated with an increased risk of pneumonia-related hospitalization in bacterial infection [17]. Also, in SARS-CoV-19 infection with DM studies has shown to increase the risk of severe pneumonia, the release of tissue injuryâ?related enzymes, excessive uncontrolled inflammatory responses and hypercoagulable state in association with dysregulation of glucose metabolism [13]. Well- controlled blood glucose (upper limit ≤ 10 mmol/L) patient has a better prognosis of COVID-19 infection compared with poorly controlled blood glucose (Upper limit > 10 mmol/L), a study conducted in China by Zhu et al. (2020) indicates that patients from the well-controlled blood glucose group had significantly lower incidences of lymphopenia, lower rates of leukocytosis and neutrophilia, elevated CRP and procalcitonin, the same pattern was observed for aspartate transaminase and D-dimer. Also, the in-hospital death rate was significantly lower in the well- controlled group relative to the poorly controlled group [21]. Another study conducted in the United States with hospitalized COVID-19 patients where patients with Diabetes defined as A1c ≥6.5% and uncontrolled hyperglycemia was defined as ≥2 blood glucose (BGs) > 180 mg/dL within any 24-hour period. A total of 451 patients with diabetes and/or uncontrolled hyperglycemia spent 37.8% of patient days having a mean blood glucose > 180 mg/dL. Among 570 patients who died or were discharged, the mortality rate was 28.8% in 184 diabetes and/or uncontrolled hyperglycemia patients versus 6.2% of 386 patients without diabetes or hyperglycemia (P < .001). Among the 184 patients with diabetes and/or hyperglycemia who died or were discharged, 40 of 96 uncontrolled hyperglycemia patients (41.7%) died compared with 13 of 88 patients with diabetes (14.8%, P < .001). Among 493 discharged survivors, the median length of stay (LOS) was longer in 184 patients with diabetes and/or uncontrolled hyperglycemia versus 386 patients without diabetes or hyperglycemia (5.7 vs 4.3 days, P < .001) [19].
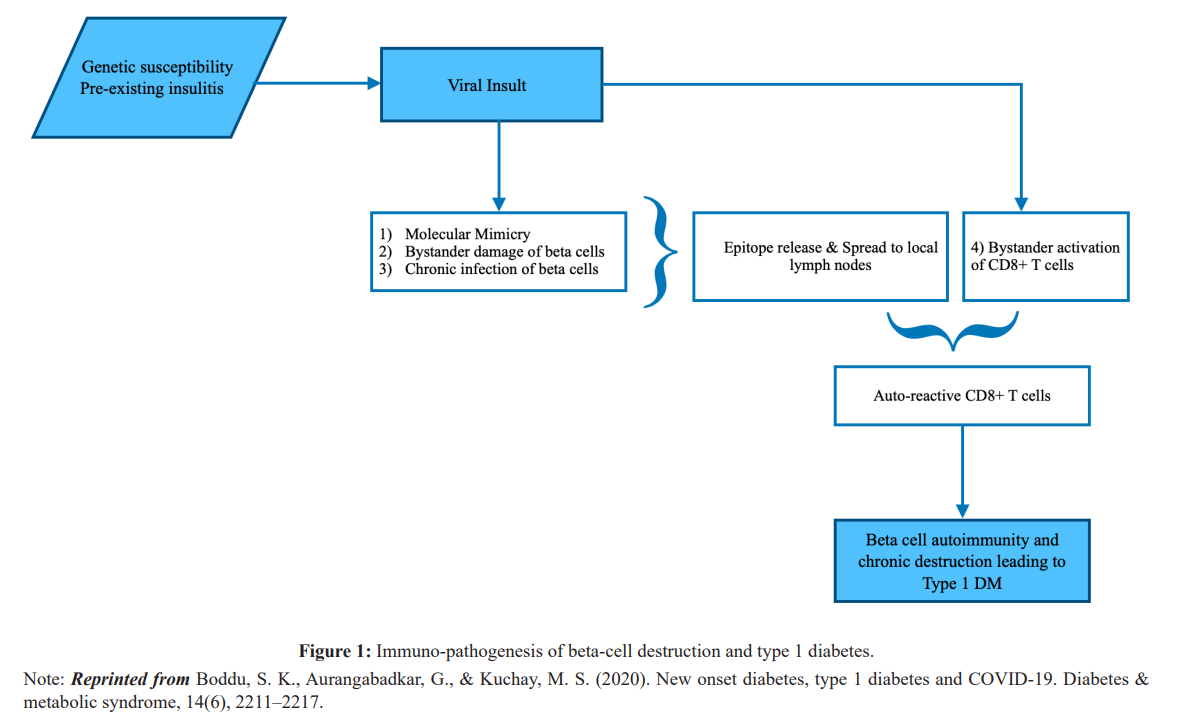
It has been speculated that COVID-19 infection is associated with a new onset of T1DM. In theory, virus-induced B-cell damage is due to either direct lytic effects of viral replication and/or host inflammatory response mediated damage by autoreactive CD+ T cells. This will lead to an autoimmune response. Immuno- pathogenesis of beta-cell destruction and T1DM is displayed in figure 1. The first evidence of viral-induced cases of fulminant T1DM reported almost in Japan and predominantly in adults, preceded by minor URIs or GI infections, HHV6, Coxsackie B3, B4, HSV, Mumps, Influenza B, Parainfluenza and Hepatitis A. The fulminant T1DM is characterized by acute onset of hyperglycemic ketoacidosis, very short duration of symptoms, absent of islet- related autoantibodies, extremely low C-peptide levels, elevated serum pancreatic enzyme levels and HbA1c less than 8.5 during the 1st examination [22].
Previous studies on SARS CoV-1 shows that the virus binds with the ACE2 receptor which converts Angiotensin II to Angiotensin I to IV was found to be a functional receptor for virus entry. ACE2 immunostaining was found in the lung, kidney, heart, and islets of the pancreas [23,24]. Therefore the interaction between SARS CoV-1 and more recent CoV-2 with the RAAS system through ACE2 enzyme, which counters RAAS activation and has potential involvement of the SARS virus infection [25]. In a recent study by Wang et al. on 52 patients with COVID-19 pneumonia, 17 % of patients experienced pancreatic injury (abnormality in amylase or lipase). However, the patients did not experience any symptoms of severe pancreatitis. Further, the study shows that the ACE2 receptor is also highly expressed in pancreatic islet cells, therefore theoretically SARS-CoV-2 infection can cause islet damage resulting in acute diabetes. Of the nine patients in the study with pancreatic injury, six had abnormal blood glucose levels [14].
Pancreatic islet injury directly entry of SARS-CoV-2 into pancreatic islet cells or downregulation of ACE2 after viral entry can lead to unopposed angiotensin II, which may impede insulin secretion. Which can be present as Diabetic ketoacidosis (DKA) in patients with type 1, type 2 or even as a new onset of diabetes. Usually, DKA is a complication of Type 1 diabetes but it can also present in a patient with type 2 in physiologically stressful condition like trauma, surgery or infection as in COVID-19 can trigger severe DKA which can further increase in complication and mortality among these patients [22,26,27]. A further note on the treatment of DKA can be complicated by excess RAAS activation with the interaction between COVID-19 and ACE2 enzyme. The rise in Angiotensin II level increases pulmonary vascular permeability and as part of treatment excess fluid resuscitation can further damage lung parenchyma. Also, with RAAS activation, there is a rise in aldosterone level which can potentiate the risk of hypokalemia, these factors should be considered while treating patients with covid-19 and diabetes [22].
In this pandemic time treatment of Diabetes poses great challenges as many of the states were in lockdown, fear in patients of getting infected while visiting primary care can hamper management of diabetes [8]. Further due to the unpredictability of the disease as well as social immobility there is an increased rise in stress, fear, increase in high sugar diet, decrease in exercise which can complicate the glycemic control [28]. Diabetics are considered as high risk with COVID-19 pandemic and to prevent getting COVID-19 in these individuals routine clinical visits should be reduced [29] and telemedicine should be considered for routine management of Diabetes an important approach to improve access, efficacy, efficiency, and cost-effectiveness of medical care for people with diabetes [30].
DM and COVID – Management
Diabetic patients require a continuous and present care chain in their lives to guarantee them adequate glycemic control, medication adjustment, nutritional and physical guidelines, and access to prescribed drugs. However, in the face of pandemics, confinement drives risk for unhealthy diets, diminishing physical activity, mental health issues, and delayed care-seeking due to fear of contracting COVID-19 [31].
Hyperglycemia is commonly seen in hospitalized patients, especially in inflammatory conditions, including viral infections. Critical illnesses, vasopressors, enteral feeding, steroids, inflammatory cytokines are classically associated with insulin resistance and suppressed insulin secretion from beta cells [32]. It is known that patients with hyperglycemia, whether diabetic or not, have a higher risk of developing severe COVID, ARDS, acute heart or kidney injury, septic shock and DIC [21].
Currently, there is little evidence in patients infected with SARS CoV-2 to assist in choosing the best glycemic target. A retrospective study in patients with type 2 DM from Hubei Province, China, observed a decrease in mortality in those patients with controlled blood glucose (between 70-180mg / dL, mean blood glucose 115 mg / dL, 7.3% HbA1c) compared with those whose blood glucose exceeds 180 mg / dL. (HR 0.13, 95% CI 0.04–0.44, p < 0.001, following adjustment for age, gender, severity of COVID-19 comorbidities, and site effect) [21].
Treatment of empirical infections Several entities recommend in favour oftheempiricaluseofinfluenza therapy for hospitalized patients with suspected or confirmed COVID-19 in places where the influenza virus is circulating. On the other hand, empirical treatment for bacterial pneumonia for patients with COVID is not routinely recommended, unless there is clinical or laboratory evidence that points to a bacterial infection (new feverish peak after temperature stabilization, new image consolidation), since bacterial superinfections do not appear to be common in COVID [33]. Prophylaxis for thromboembolism in hospitalized patients with COVID19 [34] is recommended which is further described later in the paper.
NSAIDs
There is little data on the risks of using NSAIDs in patients with COVID-19. Acetaminophen should be used as an antipyretic agent initially, and if it is necessary to use NSAIDs, this should be done with the lowest possible dose, which is in line with the general approach to fever reduction in adults [35]. For patients who are already using NSAIDs chronically for other conditions, as in diabetics undergoing prophylaxis with ASA, or in other patients with chronic inflammatory diseases, there is no evidence to discontinue the medication, as long as there are no new reasons to stop them (kidney injury, gastrointestinal bleeding, etc.) [36].
ACEi/ARBs
Patients using ACEi or ARBs should continue treatment with these medications if there are no new reasons for discontinuation (such as acute kidney injury or hypotension). As many diabetics use these drugs for various reasons, it is important to emphasize that evidence increasingly shows that there is no association between the use of these drugs and more severe disease [37].
Statins
It is recommended to continue the use of statins in patients hospitalized with COVID-19 who were already using them. A large proportion of patients in ICUs with COVID-19 have underlying cardiovascular disease, diabetes mellitus, and it is known that acute cardiac injury is a common complication of COVID-19, justifying the maintenance of the medication. Despite the theoretical fear of hepatotoxicity by statins, particularly since the virus commonly increases transaminase, most evidence indicates that this is an unusual side effect of the drug 9 [38].
Corticosteroids
As exposed in the RECOVERY study, the use of dexamethasone at a dose of 6 mg daily for 10 days or until discharge, is recommended for patients using oxygen, being able to reduce mortality, mainly in critically ill patients [39].
The use of glucocorticoids should be done with attention to the possible adverse effects, such as hyperglycemia, an increased risk of infections (bacterial, fungal and Strongyloides infections), hyperglycaemic hyperosmolar, and, mainly in diabetic patients, life- threatening diabetic ketoacidosis. Knowing that dexamethasone/ glucocorticoids can complicate glycemic control, it is reasonable to individualize the choice of use of these drugs in diabetic patients [40]. For a safer choice, look at this data about glucocorticoids use in COVID-19 patients from the RECOVERY study:
- Patients on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) at baseline – 36 per cent relative reduction (29.3 versus 4 per cent, RR 0.64, 95% CI 0.51- 0.81)
- Patients on noninvasive oxygen therapy (including noninvasive ventilation) at baseline – 18 per cent relative reduction (23.3 versus 2 per cent, RR 0.82, 95% CI 0.72-0.94).
- Benefit not seen among patients who did not require either oxygen or ventilatory support; there was a non-statistically significant trend towards higher mortality (17.8 versus 14 per cent, RR 1.19, 95% CI 0.91-1.55).
Remdesivir
The use of Remdesivir in hospitalized patients with severe COVID-19 is recommended since its ability to reduce hospital stay has already been observed. Priority should be given to its use for patients requiring low-flow supplemental oxygen, because it may also reduce mortality in this population [41].
The pharmacokinetics of Remdesivir in patients with renal injury is uncertain, and it is prepared in a cyclodextrin vehicle that may be toxic; So, Remdesivir should not be recommended in cases with a low estimated glomerular filtration rate (eGFR) [42], which is often seen in diabetic patients due to kidney complications from the disease.
According to the SOLIDARITY study, which evaluated the benefit of 4 treatment options for COVID-19, the use of Remdesivir had little or no clinical benefit [43]. In another study - ACTT-1 - a multinational, randomized, placebo-controlled trial of Remdesivir revealed the reduced time to recovery was only statistically significant among patients who were on low-flow oxygen at baseline ACTT-1 [44].
Glycemic control-related drugs
Insulin
Insulin’s ability to improve infection in both clinical and nonclinical studies is also well known [45]. With the current evidence, there is no reason to believe that insulin should not be our first treatment option for glycemic control in patients with COVID.
DPP4- inhibitors
Some concerns about the interactions between DPP-IV inhibitors and the immune system have been raised. Studies have already shown a higher incidence of nasopharyngitis and upper respiratory tract infections associated with the intake of DPP-IV inhibitors. However, according to new reports, the likelihood of a significant effect of DPP-IV inhibitor use on altering the course of a SARS- CoV-2 infection is low. Given its clinical safety, DPP-IV inhibitors, together with insulin, remain the recommended glycemic control drugs for use in patients hospitalized with COVID-19 [7].
GLP-1 receptor agonists
The GLP-1 receptor agonists have a broad anti-inflammatory action in patients with DM2 and obesity, reducing markers of systemic inflammation, pulmonary inflammation in humans and preserving lung function in experimental studies [46]. Although GLP-1R agonists safely reduce blood glucose in intubated patients, there are no studies that recommend its use in patients with COVID-19 [47].
SGLT2 inhibitors
SARS-COV-2 infections are associated with anorexia, dehydration and rapid clinical deterioration, which can lead patients using SGLT2 inhibitors to volume depletion and euglycemic ketoacidosis. Thus, studies already recommend reassessing the need or discontinuing these agents in unstable patients, either on an outpatient basis or in hospitalized patients [48].
Sulfonylureas
Sulfonylureas increase the risk of hypoglycemia and should be avoided in hospitalized patients with severe medical illness [47].
Metformin
It is known that metformin has immunomodulatory effects, with some reports studying antibody titres in a small number of individuals suggesting that immune responses to influenza vaccination are modestly impaired; however, it is not possible to know its clinical significance against the coronavirus [49].
Metformin should be used with caution in unstable hospitalized patients and should be discontinued in people with concomitant sepsis or severe impairment of hepatic and renal function [50].
Preventive Care in Diabetics with COVID-19
As preventive measures for diabetic patients, a healthy balanced diet with a good amount of protein, fibre and low saturated fat and regularity to maintain good glycemic control. Consider home-based exercise. With regular follow up on telephonic talk, patients can be educated on the importance of regular anti-diabetic medication and foot care. The patient should also be educated on seeking medical care in emergencies like vomiting, drowsiness, shortness of breath, chest pain, weakness of limbs, altered sensorium etc [8].
Prophylaxis for thromboembolism
Several entities recommend in favour of prophylactic use of drugs against thromboembolism in patients hospitalized with COVID-19. This recommendation should be kept for diabetic patients since DM2 also increases the risk of thromboembolic [34].
Conclusion
The pancreas is one of the many organs affected by COVID-19. And this manuscript serves as a brief overview of the potential pathophysiologic correlation of DM and COVID-19, optimal management and prevention. As it has been reported, patients with COVID-19 infection are prone to developing acute-new-onset- DM. This is because of SARS-CoV-2 tropism for beta cells leading to acute impairment of insulin secretion or destruction of beta cells. Furthermore, DM patients infected with COVID-19 tend to have more severe outcomes as they have worsening metabolic control due to beta-cell damage and cytokine storm. As the next wave of COVID-19 cases, there may be more concerns in Diabetic patients. Glycemic control medications like Insulin, DPP4-inhibitors, GLP1 receptor agonists, SGLT2 inhibitors, Sulfonylureas, and Metformin are ideal, however, Insulin with DPP-4 inhibitors is suggested to be used in hospitalized COVID-19 patients with DM. GLP-1 receptor agonists safely reduce blood glucose in intubated patients, there are no studies that recommend its use in patients with COVID-19. Studies recommend reassessing the need or discontinuation of SGLT2 inhibitors in unstable patients, either on an outpatient basis or in hospitalized patients. Sulfonylureas are not to be used in hospitalized patients as it has shown to increase the risk of hypoglycemia. Metformin’s clinical significance against coronavirus remains unknown, however, Metformin should be used with caution in unstable hospitalized patients. Also, Metformin should be discontinued in people with concomitant sepsis or severe impairment of hepatic and renal function. Regular telephonic follow-up with regular home-based exercises, balanced diet, education about alarming symptoms should be all taken care of in Diabetic patients for early care. Moreover, because DM and COVID-19 both increase the risk of thromboembolism, prophylaxis for the same should be considered in these patients. Given the correlation and close association of COVID-19 and Diabetes Mellitus (DM), the clinicians may need to be more vigilant of their patients’ ongoing DM management, empirical management of the infections and prophylaxis/preventive care in Diabetics, especially in conjunction with COVID-19.
Acknowledgments
A big thank you to Dr. Jack Michel, Dr. Marcos Sanchez-Gonzales, and the Research Department for the Team 11 initiative. Also a big thank you to Dr. Jack Michel, Dr. Marcos Sanchez-Gonzales,and the Research Department for the Team 11 initiative. Also, a heartfelt thanks to the Institution, Larkin Health System.
References
- Masters The molecular biology of coronaviruses. Advances in virus research. 2006; 66: 193-292.
- Lauxmann MA, Santucci NE, Autrán-Gómez The SARS- CoV-2 coronavirus and the COVID-19 outbreak. International braz j urol. 2020; 46: 6-18.
- Silva DAR, Pimentel RFW, Merces MC. Covid-19 and the pandemic of fear: reflections on mental health. Revista de Saúde Pública. 2020: 54.
- Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping Infectious diseases of poverty. 2020; 9: 29.
- Myers LC, Parodi SM, Escobar GJ, et al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in california. 2020; 323: 2195-2198.
- Xiong S, Liu L, Lin F, et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational BMC Infect Dis. 2020; 20, 787.
- Lim S, Bae JH, Kwon HS, et al. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nature Reviews Endocrinology. 2021; 17: 11-30.
- Singh AK, Gupta R, Ghosh A, et al. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical Diabetes & metabolic syndrome. 2020; 14: 303-310.
- Chen Y, Gong X, Wang L, et al. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis.
- https://idf.org/aboutdiabetes/what-is-diabetes/covid-19-and-diabetes/1-covid-19-and-diabetes.html
- Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta- Diabetes & metabolic syndrome. 2020; 14: 535-545.
- https://www.thelancet.com/journals/landia/article/PIIS2213-8587(20)30315-6/fulltext
- Guo W, Li M, Dong Y, et Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/ metabolism research and reviews. 2020; 36: e3319.
- Wang F, Wang H, Fan J, et Pancreatic Injury Patterns in Patients with Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020; 159: 367-370.
- Fang L, Karakiulakis G, Roth Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet. Respiratory medicine. 2020; 8: e21.
- https://www.diabetes.org/coronavirus-covid-19/how-coronavirus-impacts-people-with-diabetes
- Singh AK, Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: A narrative Diabetes research and clinical practice. 2020; 165: 108266.
- Barron E, Bakhai C, Kar P, et Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The lancet. Diabetes & endocrinology. 2020; 8: 813-822.
- Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United Journal of diabetes science and technology. 2020; 14: 813-821.
- Tadic M, Cuspidi C, Sala COVID-19 and diabetes: Is there enough evidence? Journal of clinical hypertension (Greenwich, Conn.). 2020; 22: 943-948.
- Zhu L, She ZG, Cheng X, et Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre- existing Type 2 Diabetes. Cell metabolism. 2020; 31: 1068- 1077.
- Boddu SK, Aurangabadkar G, Kuchay New onset diabetes, type 1 diabetes and COVID-19. Diabetes & metabolic syndrome. 2020; 14: 2211-2217.
- Yang JK, Lin SS, Ji XJ, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta 2010; 47: 193-199.
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. 2003; 426: 450-454.
- Vaduganathan M, Vardeny O, Michel T, et Renin- Angiotensin-Aldosterone System Inhibitors in Patients with Covid- 19. The New England journal of medicine. 2020; 382: 1653-1659.
- Chee YJ, Ng S, Yeoh Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes research and clinical practice. 2020; 164: 108166.
- Croft A, Bucca A, Jansen JH, et First-time Diabetic Ketoacidosis in Type 2 Diabetics With Covid-19 Infection: A Novel Case Series. The Journal of emergency medicine. 2020; 59: e193-e197.
- Ruiz-Roso MB, Knott-Torcal C, Matilla-Escalante DC, et al. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Nutrients. 2020; 12: 2327.
- Katulanda P, Dissanayake HA, Ranathunga I, et Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020; 63: 1440- 1452.
- Garg SK, Rodbard D, Hirsch IB, et al. Managing New-Onset Type 1 Diabetes during the COVID-19 Pandemic: Challenges and Diabetes technology & therapeutics. 2020; 22: 431-439.
- Beran D, Perone SA, Perolini MC, et al. Beyond the virus: Ensuring continuity of care for people with diabetes during COVID-19. Primary Care 2020; 15: 16-17.
- Donath MY, Størling J, Berchtold LA, et Cytokines and beta-cell biology: from concept to clinical translation. Endocrine Reviews. 2008; 29: 334-350.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, JAMA. 2020; 323:1061-1069.
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. Journal of thrombosis and haemostasis: JTH. 2020; 18: 1023-
- High KP, Bradley SF, Gravenstein S, et Infectious Diseases Society of America. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-termcare facilities: 2008 update by the Infectious Diseases Society of America. Journal of the American Geriatrics Society. 2009; 57: 375-394.
- https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19
- https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician.
- Zhang XJ, Qin JJ, Cheng X, et In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell metabolism. 2020; 32: 176-187.
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. The New England journal of medicine. 2021; 384: 693-704.
- https://wwdiabetes.org.uk/resources-s3/public/2020-06/COvID_Dex_v1.4.pdf
- Siemieniuk R, Rochwerg B, Agoritsas T, et A living WHO guideline on drugs for covid-19. BMJ (Clinical research ed.). 2020; 370: m3379.
- Bhimraj A, Morgan RL, Shumaker AH, et Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020; ciaa478.
- Pan H, Peto R, Henao-Restrepo AM, et Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. The New England journal of medicine. 2021; 384: 497-511.
- Beigel JH, Tomashek KM, Dodd LE, et Remdesivir for the Treatment of Covid-19 - Final Report. The New England journal of medicine. 2020; 383: 1813-1826.
- Andersen SK, Gjedsted J, Christiansen C, et al. The roles of insulin and hyperglycemia in sepsis pathogenesis. Journal of leukocyte biology. 2004; 75: 413-421.
- Drucker Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metabolism. 2018; 27: 740-756.
- Drucker DJ. Coronavirus Infections and Type 2 Diabetes- Shared Pathways with Therapeutic Implications. Endocrine 2020; 41: 457-470.
- Hamblin PS, Wong R, Ekinci EI, et al. SGLT2 Inhibitors Increase the Risk of Diabetic Ketoacidosis Developing in the Community and During Hospital The Journal of clinical endocrinology and metabolism. 2019; 104: 3077-3087.
- Agarwal D, Schmader KE, Kossenkov AV, et al. Immune response to influenza vaccination in the elderly is altered by chronic medication use. Immunity & 2018; 15: 19.
- Korytkowski M, Antinori-Lent K, Drincic A, et A Pragmatic Approach to Inpatient Diabetes Management during the COVID-19 Pandemic. The Journal of clinical endocrinology and metabolism. 2020; 105: 3076-3087.