Defining Myocardial Injury in COVID-19: Is Troponin Enough? The COVID-19 Disease and Cardiac Events (Covicare) Study
Author(s): Claire Glen1, Kirsty McDowell1, Calum Milne1, Kathryn Kirkpatrick1, Jennifer Lochrie1, Paul Welsh2 and Robin AP Weir1*
1 University Hospital Hairmyres, NHS Lanarkshire, Scotland, UK.
2University of Glasgow, BHF Glasgow Cardiovascular Research Centre, Glasgow, Scotland, UK.
*Correspondence:
Robin A.P. Weir, Cardiology Department, University Hospital Hairmyres, Eaglesham Road, Glasgow G75 8RG, Scotland, UK, Tel: +441355585000.
Received: 01 Jun 2023; Accepted: 03 Aug 2023; Published: 10 Aug 2023
Citation: : Glen C, McDowell K, Milne C, et al. Defining Myocardial Injury in COVID-19: Is Troponin Enough? The COVID-19 Disease and Cardiac Events (Covicare) Study. Cardiol Vasc Res. 2023; 7(4): 1-6.
Abstract
Background: Myocardial injury is a complication of COVID-19 infection and is associated with adverse outcome, but the definition is variable and based on troponin elevation alone. We utilised a revised definition of myocardial injury using biomarkers, electrocardiography (ECG) and echocardiography and assessed its predictive efficacy.
Methods and Results: 100 patients (age 64.8 ± 13.2yr, 53% female) with COVID-19 had admission highsensitivity c-troponin-T (hs-cTnT), N-terminal-pro-B-type-natriuretic-peptide (NTproBNP), interleukins (IL), tumour necrosis factor-α (TNFα), ECG and echocardiography. 51(53%) of patients had hs-cTnT >14ng/L; 10(20%) died vs 3(7%) without hs-cTnT elevation (p=0.059). 30-day major adverse cardiac events (MACE) increased with increasing hs-cTnT (HR 1.69 [1.0-2.86]; p=0.05). IL-6, IL-8 and TNFα were associated with 30-day MACE and were higher in those with hs-cTnT >14ng/L. 36(38%) of patients had an abnormal ECG, which was associated with higher NTproBNP (589 [211–1696] vs 146 [79–390]ng/L, p=0.001). 55 (71%) of 78 patients with interpretable echocardiograms were abnormal; NTproBNP was higher in those with abnormal echocardiography (390 [131-1118] vs 129 [96-460]ng/L, p=0.036). Combining hs-cTnT elevation with ECG and echocardiographic abnormalities identified a group with markedly elevated NTproBNP (1163 [565-3156] vs 147[78-404]ng/L, p<0.001).
Conclusions: A refined definition of myocardial injury using ECG/echocardiography and biomarkers may identify higher risk patients with COVID-19.
Keywords
Introduction
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) can directly affect myocardial tissue resulting in myocardial injury, inflammation, impairment of contractile function, arrhythmia and death [1-4]. Elevation of the cardiac biomarker troponin in patients hospitalised with coronavirus disease-2019 (COVID-19) is associated with increased severity of disease, invasive ventilation and death [5-7]. Such “myocardial injury” is defined by elevated troponin alone in several studies, with limited data on structural and functional cardiac abnormalities as assessed by cardiac imaging [6,8-10]. We assessed for myocardial injury using a multimodality approach including multiple biomarkers, electrocardiographic (ECG) and echocardiographic data in patients hospitalised with COVID-19, and evaluated clinical outcomes up to 30 days to determine whether ECG and echocardiography enhance the definition of myocardial injury.
Methods
COVID-19 disease and CARdiac Events (COVICARE) is a prospective, non-randomised, single-centre study characterising early cardiac involvement in patients hospitalised with COVID-19. 100 consecutive patients were enrolled at admission if they were ≥18 years old, able to provide written, informed consent, and were not known to have left ventricular (LV) impairment. Ethical approval was obtained from the North of Scotland Research Ethics Committee (clinicaltrials.gov NCT04438993). Data collected from electronic health records included patient demographics, admission ECG and routine haematology and biochemistry results. High sensitivity cardiac troponin-T (hs-cTnT), N-terminal pro- B-type natriuretic peptide (NTproBNP), angiotensin-converting enzyme-2 (ACE2) and cytokines relevant to the ‘cytokine storm’ phenomenon recognized in COVID-19 (tumour necrosis factor [TNF]α, interleukin [IL]-1β, IL-6 and IL-8) were measured [11]. Transthoracic echocardiography was performed by the study team as early as possible after enrolment. Subjects were monitored during admission up until discharge or death. Clinical outcomes were in-hospital mortality, admission to high dependency/ intensive care units (HDU/ICU), and 30-day major adverse cardiac events (MACE).
Baseline characteristics were defined according to those with or without myocardial injury (hs-cTnT >14ng/L). Differences in baseline characteristics and results according to myocardial injury were calculated using Pearsons chi-squared test for categorical variables. For comparisons of parametric data the student T-test was used and for nonparametric data the Mann-Whitney-U test. Kaplan-Meier survival curves were constructed to show in-patient (IP) survival of patients and cumulative probability for requirement of HDU/ICU care stratified by presence of (1) elevated hs-cTnT (>14ng/L) and (2) hs-cTnT >14ng/L in addition to ECG and echocardiographic abnormalities. The prognostic value for determining likelihood of IP death or high-level care was evaluated using the Cox proportional hazards model. Multivariate analysis was performed adjusting for variables, which were significantly different between those who died as an IP and those who survived. Non-parametric continuous data were appropriately log-transformed. All analyses were conducted using Stata version 16.1(College Station, TX, USA); p-value <0.05 was considered statistically significant.
Results
100 patients with COVID-19 admitted to hospital were enrolled in the study; biomarker data were not obtained in 3 subjects. All had positive reverse transcriptase PCR for SARS-CoV-2 on nasopharyngeal swabs. The mean age was 64.8 ± 13.2 years and 53% were female. The mean body mass index was 31.9 ± 8.1; 21% had a history of diabetes mellitus, 44% hypertension and 10% ischaemic heart disease (IHD). Myocardial injury was initially defined as hs-cTnT >14ng/L; subsequent to this ECG and echocardiographic abnormalities were included in the definition.
Myocardial injury defined as hs-cTnT >14ng/L identified 51 patients (53%) with mean value 22.7 ± 37.6ng/L (Table 1). Patients with elevated baseline hs-cTnT were older, more frequently male and had a higher prevalence of diabetes, hypertension, and IHD. A higher proportion of patients with an elevated hs-cTnT died as an inpatient (10 [20%] vs 3 [7%], p=0.059). The univariate hazard ratio (HR) of 30 day MACE approached significance at 1.69 (1.0- 2.86) per log unit increase in hs-cTnT (p=0.05); HR for in-patient death (1.17 [0.55-2.51], p=0.67) and HDU/ICU care (0.91 [0.56-1.47], p=0.75) were non-significant. NTproBNP concentrations were higher in those with hs-cTnT >14ng/L than in those without hs-cTnT elevation (751 [281 – 1839] vs 122 [54 – 185]ng/L, p<0.001).
Data presented as numbers (proportions), medians (interquartile range) and means (standard deviation) ACEi, angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker; ARNI angiotensin receptor blocker neprilysin inhibitor; BB beta blocker; BMI body mass index; CRP C-reactive protein; eGFR estimated glomerular filtration rate; HDU high dependency unit; ICU intensive care unit; IHD Ischaemic heart disease; SGLT2i sodium glucose transporter 2 inhibitor.
38% of patients had an abnormal admission ECG (atrial fibrillation [AF] n=9 [9%], atrial flutter n=3 [3%], supraventricular tachycardia n=1 [1%], bundle branch block [BBB] n=5 [5%] or ST-T wave changes n=22 [22%]). An abnormal ECG was associated with higher NTproBNP concentrations than a normal ECG (589 [211- 1696] vs 146 [79-390]ng/L, p=0.001), although an abnormal ECG in isolation was not associated with higher in-patient mortality (HR 0.49 [0.13 – 1.8] p=0.283), HDU/ICU care (HR 1.67 [0.74-3.71 p=0.21) or 30-day MACE (HR 0.54 [0.15 – 1.99] p=0.35).
55 (71%) of 78 patients with interpretable echocardiograms were abnormal – LV dilatation (5.1%), RV dilatation (17.9%), LV systolic impairment (28.2%), or RV systolic impairment (25.6%). The most common finding was LV diastolic impairment (42.3%). An abnormal echocardiogram was associated with significantly higher NTproBNP concentrations than normal echocardiography (390 [131-1118] vs 129 [96-460]ng/L, p=0.036). Patients with abnormal echocardiograms did not suffer higher in-patient mortality (HR 0.98 [0.25 – 3.81] p=0.98), HDU/ICU care (HR 0.78 [0.33 – 1.83] p=0.56) or 30-day MACE (HR 0.99 [0.25 – 3.84] p=0.99).
When defining myocardial injury as abnormality in all 3 domains (hs-cTnT, ECG and echocardiography), 14 patients were identified. These patients were older (73 ± 6.9 vs 61.9 ± 14.1 years, p<0.01) and had a higher prevalence of hypertension and diabetes. Baseline renal function was significantly reduced (eGFR 49.7 ± 12.7 vs 57.9 ± 5.5 mL/min/1.73m2, p<0.001) and NTproBNP significantly higher (1163[565-3156] vs 147[78-404]ng/L, p<0.001) in those with abnormalities in all 3 domains. A similar proportion of patients with myocardial injury using the above definition died as an inpatient (2 [14.2%] v 7 [11.7%], p=0.70) or required escalation to HDU/ICU (4 [28.6%] v 17 [28.3%], p=0.99) compared to
Table 1: Baseline demographics according to high-sensitivity cardiac troponin T (hs-cTnT).
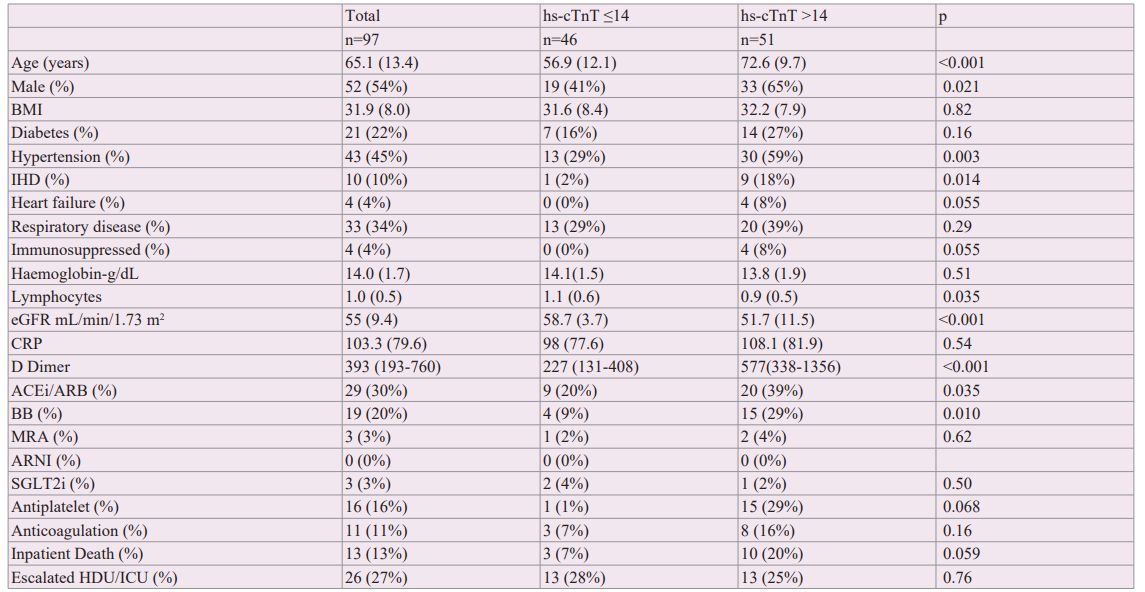
Table 2: Table demonstrates univariate and multivariate hazard ratios for outcomes according to biomarkers when considered as a categorical variable (above median, below is reference) and continuous variable (per log unit increase) Abbreviations ACE2 angiotensin converting enzyme 2, CI confidence intervals, HDU high dependency unit, HR hazard ratio, ICU intensive care unit. IL1B interleukin 1 beta, IL interleukin, MACE major adverse cardiac event, TNFa tumour necrosis factor alpha.
*Median (IQR) IL-Iβ 0.428 (0.28-0.76) IL-6 12.3 (5.7-40.5), IL-8 30.6 (18.3-51), TNFα 13.9 (11.5-18.3). ACE2 0.9 (0.4-5.8)
Myocardial injury defined by hs-cTnT alone. The adjusted risk of pre-defined outcomes was not significantly associated with the presence of myocardial injury as defined by an abnormality in all 3 domains (Figure 1).
IL-6, IL-8 and TNFα concentrations above median were associated with increased risk of 30-day MACE (HR 10.9 [1.39-85.1]; 5.15 [1.12-23.9]; 4.83 [1.04-22.4] respectively, p<0.05) but not IP death or escalation to higher-level care (Table 2). Higher ACE2 concentrations were associated with increased requirement for HDU/ICU care (HR 3.35 [1.22-9.2], p=0.019). Patients with hs- cTnT >14ng/L had higher levels of IL-6, IL-8 and TNFα (Figure 2).
Discussion
Admission hs-cTnT was frequently elevated and was associated with higher NTproBNP levels and poorer outcomes in keeping with previous studies [12-14]. IL-6, IL-8 and TNFα were higher in those with elevated hs-cTnT. Abnormalities were frequently detected on ECG and echocardiography and were associated with elevated NTproBNP levels.
38% of our cohort had abnormal ECGs. The definition of an abnormal ECG varies between COVID studies and some include sinus tachycardia. In a cohort of COVID-19 patients in Jordan, 83% of 176 patients had an abnormal ECG with AF and ST-T wave

Figure 1: Cumulative incidence of (A) in-patient death, (B) High Dependency Unit (HDU)/intensive care unit (ICU) care and (3) 30-day major adverse cardiac events (MACE) according to presence of myocardial injury (defined as abnormal ECG and echocardiogram in addition to high-sensitivity cardiac troponin T [hs-cTnT] >14ng/L).
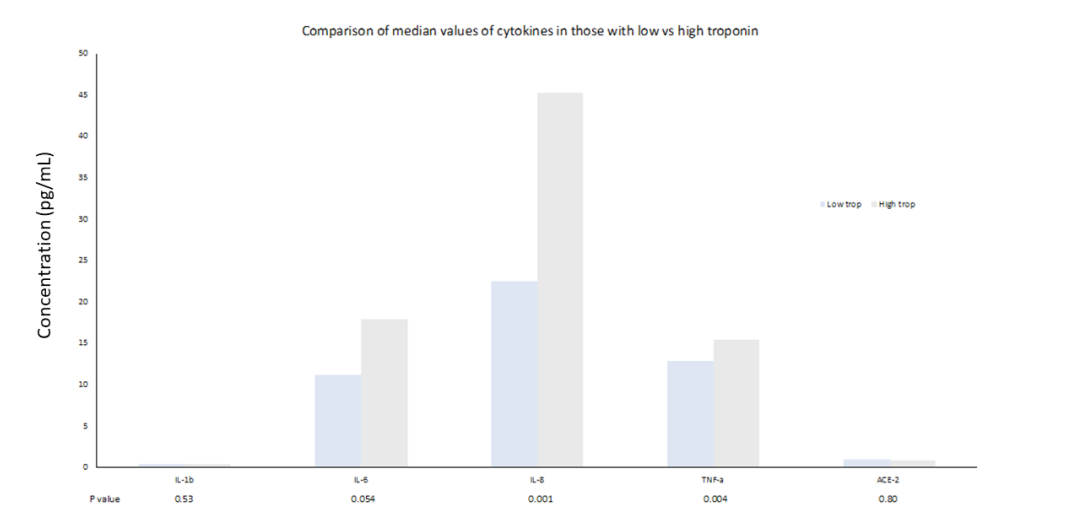
Figure 2: Comparison of cytokine values according to troponin low (≤14ng/L) or high (>14ng/L).
Changes seen in 18% and 30% respectively [15]. Mortality was higher in those with either AF or premature ventricular complexes, while sinus tachycardia or AF were associated with mortality and requirement for HDU/ICU care. A retrospective analysis of 431 COVID-19 patients who died or required mechanical ventilation revealed an ECG abnormality in 93% on admission, with ECG signs of RV pressure overload (defined as S1Q3T3 pattern, incomplete or complete right BBB) being the commonest (~30%) [16]. Both studies included sinus tachycardia in the definition of ECG abnormality which explains the higher incidence of abnormal ECGs than in our cohort. Left BBB and axis deviation on admission ECG were found to be predictive of in-hospital mortality among 275 patients hospitalized with COVID-19 in a French cohort [17]. Our study is the first, we believe, to demonstrate a higher NTproBNP in those with an abnormal ECG on admission. That we did not demonstrate an effect on outcomes relates most likely to the sample size and duration of follow-up.
An abnormal echocardiogram was recorded in over 70% of our cohort. LV diastolic dysfunction was the commonest abnormality (presumably pre-dating admission and prevalent in a predominantly overweight hypertensive population), occurring in just over 40%, with LV systolic dysfunction in 30%. While there was a numerical trend towards higher in-patient mortality and requirement for HDU/ICU in those with an abnormal echocardiogram, this was non-significant although the event rate was low. In a large international survey of over 1200 patients with COVID-19, an abnormal echocardiogram was recorded in 55% and influenced patient management – through change in medical therapy, invasive procedures including pericardiocentesis and end of life decisions – in one-third [18]. In a similar-sized cohort to our study, 32 of 100 patients had an abnormal echocardiogram on day 1 of admission with COVID-19 [19]. The commonest finding was RV dilatation/dysfunction (39 v 25.6% in our study). Consistent with our findings, LV systolic function was no different in those with elevated troponin compared to those without troponin elevation. In contrast to hs-cTnT, we found that NT-proBNP was significantly higher in those with an echocardiographic abnormality suggesting that echocardiography alone might identify patients at risk of adverse outcome.
IL-6, IL-8 and TNFα were higher in those with myocardial injury in our cohort and were associated with 30-day outcomes. IL-6, a potent mediator of the cytokine storm phenomenon recognised in COVID-19 disease, has been shown to be higher in non-survivors in ICU patients and dynamic changes in IL-6 correlate with disease severity [20,21]. IL-8 independently predicts mortality in ICU patients with COVID-19 [22]. TNFα is associated with increased mortality in ICU patients and, together with IL-6, is predictive of post-COVID sequelae including the symptom group now termed ‘long COVID’ [20,23]. Our data suggest a single sample of these cytokines on admission in mild/moderate COVID-19 might help the early risk stratification of such patients.
Using a revised definition of myocardial injury to encompass elevated hs-cTnT together with an abnormality on ECG and echocardiography identified a small group of patients with markedly higher NTproBNP levels, which merits further investigation, given the powerful prognostic effect of natriuretic peptides in a wide variety of cardiac and non-cardiac conditions. IL-6, IL-8 and TNFα were higher in those with myocardial injury and were associated with 30-day outcomes. Additional biomarkers may be of use – adding copeptin (the C-terminal component of vasopressin) to hs-cTnI independently predicted 28-day mortality and ICU admission in a cohort of 213 hospitalized COVID-19 patients [24]. These data suggest that a more robust definition of myocardial injury – incorporating multiple biomarkers – may have a role in highlighting a higher-risk group of patients at the point of admission with mild-moderate COVID-19.
Our trial is subject to certain limitations including those inherent to single centre studies. The event rate was low. Echocardiography was challenging in some subjects (body habitus, patient positioning, personal protective equipment). Outcomes are limited to in-hospital death and 30-day MACE; longer-term follow-up would be of use particularly given the subgroups identified by elevated natriuretic peptide levels.
Conclusions
Myocardial injury is common in hospitalized COVID-19 patients and portends adverse outcome; the definition of myocardial injury may be augmented by the addition of biomarkers, ECG and echocardiographic abnormalities as we attempt to identify high- risk patients, and merits further study as we strive to ameliorate outcome in this group.
References
- Chung MK, Zidar DA, Bristow MR, et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ Res. 2021; 128: 1214-1236.
- Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020; 116: 1666-1687.
- Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020; 106: 1132-1141.
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323: 1061-1069.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020; 395: 1054-1062.
- Sandoval Y, Januzzi JL, Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19. Journal of the American College of Cardiology. 2020; 76: 1244-1258.
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020; 5: 802-810
- Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020; 5: 811-818.
- Shi S, Qin M, Cai Y, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020; 41: 2070-2079.
- Giustino G, Croft LB, Stefanini GG, et al. Characterization of Myocardial Injury in Patients With COVID-19. Journal of the American College of Cardiology. 2020; 76: 2043-2055.
- Alam M, Choudhury R, Lamers RJ. An adaptable in vitro cytokine release assay (CRA): Susceptibility to cytokine storm in COVID-19 as a model. Curr Res Immunol. 2022; 3: 239-243.
- Izquierdo A, Mojón D, Bardají A, et al. Myocardial Injury as a Prognostic Factor in Mid- and Long-Term Follow-Up of COVID-19 Survivors. J Clin Med. 2021; 10: 5900.
- Melillo F, Napolano A, Loffi M, et al. Myocardial injury in patients with SARS-CoV-2 pneumonia: Pivotal role of inflammation in COVID-19. Eur J Clin Invest. 2022; 52: e13703.
- De Michieli L, Ola O, Knott JD, et al. High-Sensitivity Cardiac Troponin T for the Detection of Myocardial Injury and Risk Stratification in COVID-19. Clin Chem. 2021; 67: 1080-1089.
- Alrawashdeh S, Alrabadi N, Obeidat O, et al. The Value and Applicability of the Electrocardiography in Revealing the Cardiac Involvement of COVID-19 Patients. Acta Inform Med. 2021; 29: 253-259.
- Bertini M, Ferrari R, Guardigli G, et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: an insight into the mechanisms of cardiac involvement. Europace. 2020; 22: 1848-1854.
- De Carvalho H, Leonard-Pons L, Segard J, et al. Electrocardiographic abnormalities in COVID-19 patients visiting the emergency department: a multicenter retrospective study. BMC Emerg Med. 2021; 21: 141.
- Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020; 21: 949-958.
- Szekely Y, Lichter Y, Taieb P, et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation. 2020; 142: 342-353.
- Li J, Rong L, Cui R, et al. Dynamic changes in serum IL-6, IL- 8, and IL-10 predict the outcome of ICU patients with severe COVID-19. Ann Palliat Med. 2021; 10: 3706-3714.
- Chang Y, Bai M, You Q. Associations between Serum Interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis. Biomed Res Int. 2022; 2022: 2755246.
- Li H, Zhang J, Fang C, et al. The prognostic value of IL-8 for the death of severe or critical patients with COVID-19. Medicine (Baltimore). 2021; 100: e23656.
- Schultheiß C, Willscher E, Paschold L, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022; 3: 100663.
- Kaufmann CC, Ahmed A, Kassem M, et al. Improvement of outcome prediction of hospitalized patients with COVID-19 by a dual marker strategy using high-sensitive cardiac troponin I and copeptin. Clin Res Cardiol. 2022; 111: 343-354.