Digestives Hemorrhagae During Cirrhosis In Bangui
Author'(s): Camengo Police Serges Magloire1*, Youssouf Oumarou, Elowa Benoît1, Mofini Eveline1, Bessanguem Bernard1, Puemeu Edna Nadège1, Boua-Akelelo Nathalie Philomène1, Service Georges2, and Koffi Boniface3
1Department of hepato-gastroenterology and Internal Medicine of « Amitié Sino-Centrafraine » University Hospital Center, Bangui, Central African Republic.
2Department of Internal Medicine « Maman Elisabeth Domitien » University Hospital Center, Bimbo, Central African Republic.
3Department Pathological Anatomy National Laboratory of Clinical Body and Public Health, Bangui, Central African Republic.
*Correspondence:
Pr Ag Camengo Police Serges Magloire, Department of hepatogastroenterology and Internal Medicine, of « Amitié Sino-Centrafraine » University Hospital Center Bangui, Central African Republic, Tel: +236 75 50 59 65.
Received: 27 November 2020 Accepted: 18 December 2020
Citation: Magloire CPS, Oumarou Y, Benoît E, et al. Digestives Hemorrhagae During Cirrhosis In Bangui. Gastroint Hepatol Dig Dis. 2020; 3(2): 1-5.
Abstract
Objective: To determine the epidemiological, clinical and endoscopic characteristics of gastrointestinal bleeding in cirrhosis.
Patients and Methods: This was a retrospective analytical study lasting 51 months conducted in the Department of Hepato-gastroenterology and Internal Medicine of « l'Amitié Sino-centrafricaine » University Hospital Center Bangui, in Bangui. Included were patients of both sexes with cirrhosis in whom esogastroduodenal endoscopy was performed.
Results: A total of 2383 patients were hospitalized during the study period, including 250 (10.49%) for cirrhosis. One hundred and seven patients (4.5%) met our criteria. These were 58 cases (54.2%) of cirrhosis complicated by gastrointestinal bleeding and 49 cases (45.8%) of cirrhosis uncomplicated with bleeding. There were 73 men (68.2%) and 34 women (31.8%). The sex ratio was 2.15. The average age was 40 ± 13 years. The most common mode of expression of gastrointestinal bleeding was haematemesis and melena (79.3%). The Discontinuation of bêta-blocker therapy (p = 0.0004) and the use of nonsteroidal anti-inflammatory drugs (p = 0.0000) were risk factors for gastrointestinal bleeding. Cirrhosis was caused by the hepatitis B virus in 84 cases (78.5%). Hypovolemic shock was observed in 43 patients (40.2%) who presented with HD. The main endoscopic lesions responsible for the bleeding were VO (p = 0.001) and gastroduodenal erosions (p = 0.035). The hemorrhagic risk was linked to the size of the esophageal varices (EV) and the presence of red signs (p = 0.00005). Death was observed in 7 patients (6.5%).
Conclusion: Digestive hemorrhage (DH) is one of the frequent complications of cirrhosis, the primary cause of which is the rupture of the EV. Upper digestive endoscopy is essential in the event of suspicion of cirrhosis, in search of EV in order to begin primary prevention of their rupture according to the stage of severity.
Keywords
Introduction
Cirrhosis is a serious liver disease characterized by mutilating fibrosis that destroys the normal architecture of the liver and isolates abnormally structured hepatocyte nodules [1]. It constitutes a real public health problem [2-5]. In France [2], the prevalence of cirrhosis is estimated between 2,000 to 3,300 cases per million inhabitants, with an annual incidence of 150 to 200 cases per million inhabitants. In Africa, its hospital frequency is variable. In Bangui, it is 19.7% [6], and 5.8% in Burkina Faso [7].
Cirrhosis is a major concern for practitioners in Africa because of the dreadful complications represented by gastrointestinal bleeding complicating portal hypertension; edema-ascitic syndrome and hepatocellular carcinoma [4,7]. Of all of these complications, gastrointestinal bleeding can be serious, life-threatening [8-11]. It is more serious in patients with cirrhosis [9,12]. DH in cirrhosis may be due to ruptured esophageal and / or cardiotuberosal varices, erosions, ulcers or peptic ulcers [13]. The frequency of digestive haemorrhages from ruptured esophageal varices is 12% in Europe [14]. In Africa, the frequency of gastrointestinal bleeding from ruptured esophageal varices is variable [9,10,15]. Some authors estimate that 70% of digestive haemorrhages during cirrhosis are related to the rupture of esophageal varices with a mortality rate of 30 to 50% during the first episodes [8]. In Bangui, the frequency of gastrointestinal bleeding during cirrhosis ranged from 35% [6] and 51% [16], but factors related to DH were not studied [16-18]. The aim of the present study was to contribute to the improvement of the management of gastrointestinal bleeding during cirrhosis.
Patients and Methods
Our study took place in the Department of hepato-gastroenterology and Internal Medicine Of « Amitié Sino-Centrafraine » University Hospital Center Bangui. This was a retrospective analytical study lasting 51 months from April 1, 2010 to July 31, 2014. Patients of both sexes in whom the diagnosis of cirrhosis was made and who had performed upper gastrointestinal endoscopy (UGE) were included consecutively without sorting. The diagnostic arguments for cirrhosis were clinical [clinical signs of hepatocellular insufficiency (HCI), clinical signs of portal hypertension (PHT), liver characteristics (large hard liver, painless, regular surface, thin or sharp lower border) in case of hypertrophic liver]; biological (signs of IHC); morphological (ultrasound signs of PH, the appearance of liver liver on ultrasound, endoscopic signs of PHT). The patients were divided into two groups (first group consisting of patients with complicated bleeding cirrhosis and the second group of patients with uncomplicated bleeding cirrhosis). The hemorrhagic complication of cirrhosis could be hematemesis, melena, or hematochesia. Our sample size was convenient, covering all cases meeting the inclusion criteria. Data collection was carried out using an individual survey sheet with direct administration. Information was collected from patients, from patient record, hospitalization and digestive endoscopy registers. The parameters studied were epidemiological [Sociodemographic characteristics (age, sex, profession); HD risk factors, in particular the severity of cirrhosis, assessed by the Child Pugh classification [19], thrombocytopenia, the notion of taking gastrotoxic drugs (Aspirin and NSAIDs), the consumption of traditional herbal teas or foods solids, breaking the beta blocker treatment, alcohol consumption (daily quantity, duration of exposure in years)]; clinical (Signs of severity of DH including pulse, blood pressure, respiratory rate, General signs (temperature, asthenia), physical signs (HCI syndrome, PHT syndrome, liver characteristics); biological [blood count, serum creatinemia, bilirubinemia, serum cholesterol, gamma-glutamyl transpeptidase, aspartate amino-transferase, alanine amino- transferase, electrophoresis of serum proteins, prothrombin level, serum iron, hepatitis B virus (HBV) serology, hepatitis C virus (HCV), human immunodeficiency virus (HIV)]; endoscopic (signs of portal hypertension such as esophageal varices (EV), mosaic gastropathy, gastroduodenal erosions, antral vascular ectasia [20], and gastric and / or duodenal ulcers classified according to Forrest [21]. EV is classified into three stages: stage, small esophageal varices, are flattened by insufflation; stage 2, esophageal varices persist under insufflation, but are separated by intervals of healthy mucosa; stage 3: esophageal varices persist under insufflation and are confluent; the presence of red signs which are hematocystic spots, cherry red macules, red welts and diffuse redness [20]; ultrasound (appearance of the liver, signs of portal hypertension including thickening of the gallbladder wall, splenomegaly, increase in the size of the portal vein ≥ 12 mm and of the splenic vein ≥ 7 mm, repermeabilization of the umbilical vein); prognosis (signs of shock, degree of hepatocellular insufficiency by the Child-Pugh classification, the presence or not of red signs. Data entry and analysis were done using Epi-info 3.5.1 software. The Chi2 statistical test was used for comparison with a significance level p <0.05.
Results
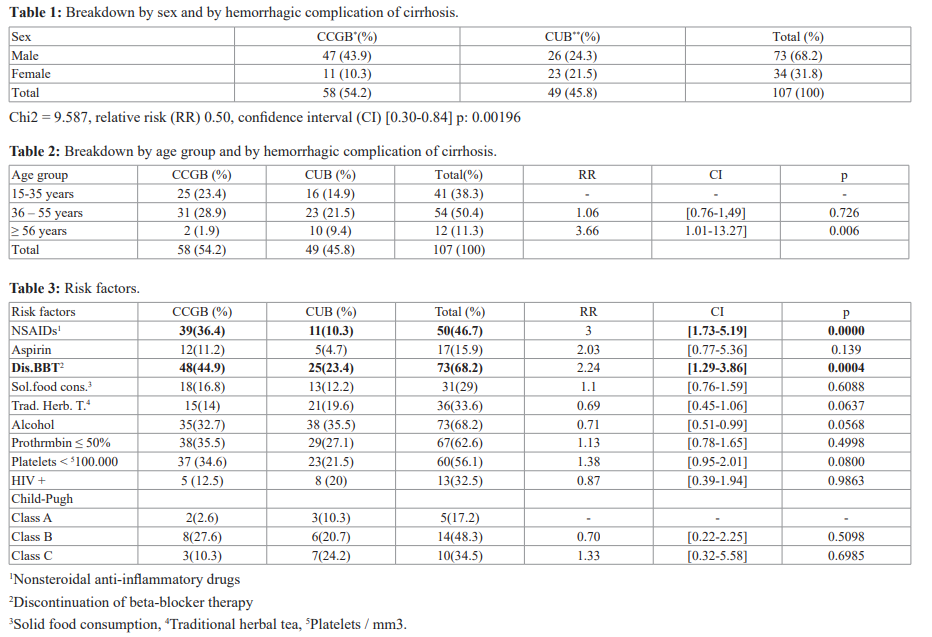
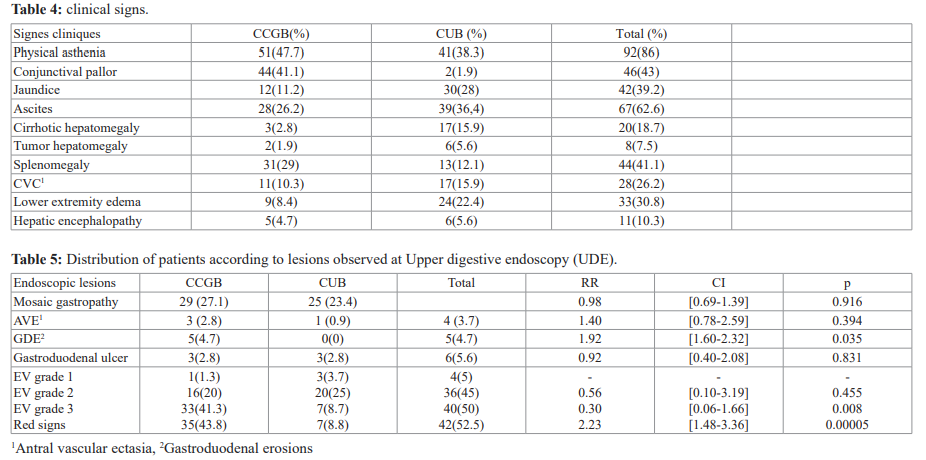
During the study period, 2383 patients were hospitalized, including 250 (10.49%) for cirrhosis. Among the 250 patients with cirrhosis, we included 107 (4.5%). These were 58 cases (54.2%) of cirrhosis complicated by gastrointestinal bleeding (CCGB) and 49 cases (45.8%) of cirrhosis uncomplicated with bleeding (CUB). We noted an average of 5 cases of complicated DH cirrhosis per month. Among the 107 patients with cirrhosis, there were 73 men (68.2%) and 34 women (31.8%). The sex ratio was 2.15. Table I presents the distribution by sex and by hemorrhagic complication of cirrhosis. The mean age of the cirrhotic patients was 40 ± 13 with extremes of 15 and 70 years. Table II shows the distribution by age group and by hemorrhagic complication of cirrhosis. The risk factors for hemorrhage in patients with cirrhosis are presented in Table III. Child Pugh's classification was only made in 29 patients who were able to perform all laboratory tests. HIV status was only known in 40 patients (37.4%). HIV serology was positive in 12 patients (11.2%) with cirrhosis complicated by bleeding and in 5 patients (4.6%) with uncomplicated bleeding cirrhosis. The mode of expression of gastrointestinal bleeding in the 58 patients (54.2%) was hematemesis in 8 cases (13.8%), hematemesis and
melena in 46 cases (79.31%), rectal bleeding in 4 cases (6.9%).
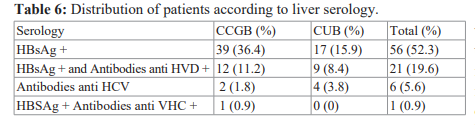
Thirty patients (51.7%) were in their 1st bleeding episode, 17 (29.3%) were in the 2nd episode, 8 (13.8%) in the 3rd episode and 3 (5.2%) at least 4th episode . The clinical signs found in the patients are presented in Table IV. Hypovolemic shock requiring blood transfusion was observed in 43 patients (74.1%) who had gastrointestinal bleeding. The average 450ml blood bag administered was 5 with extremes of 1 and 9 bags. There was no indication of blood transfusion in the second group. UDE had found EV in 80 cases (74.8%) including 50 (46.7%) in the DH group. The endoscopic lesions responsible for gastrointestinal bleeding are presented in Table V. The etiologies of cirrhosis are reported in Table VI.
Seven patients (6.5%) had died, of which 5 (4.7%) were admitted for DH. Deaths observed in the DU group were due to hypovolemic shock in 4 cases and hepatorenal syndrome in 1 case. Hepatic encephalopathy was the cause of 2 deaths in the uncomplicated HD cirrhosis group.
Discussion
We did not perform liver biopsy punctures for histological analysis, nor non-invasive fibrosis tests, to confirm the diagnosis of cirrhosis. The fact that the patients had decompensated cirrhosis made the diagnosis easy. The Haute Autorité de Santé considers that in the presence of obvious epidemiological, clinical and morphological arguments, the diagnosis of cirrhosis can be made without having to resort to liver biopsy [2]. The diagnosis of cirrhosis was made in 250 patients hospitalized in our department, but only 107 patients were included in our study. More patients should be more interesting. The other patients were not taken into account because they had not performed certain additional examinations due to the cost which was not accessible to them. The prevalence of gastrointestinal bleeding during cirrhosis was observed in 54.2% of cases in our series. It is similar to that already observed in the service which was 50.1% [16]. On the other hand, our prevalence is higher than that observed during another study carried out in the department on the complications of cirrhosis (35%) [6]. This shows that, in the same population, the frequency of gastrointestinal hemorrhage is variable and would probably depend on hemorrhagic risk factors. The mean age of our patients was 40 ± 13 years. It is comparable to that already observed in the department in Bangui [6,16], in Gabon (41 years) [22]. It is higher than that reported by the authors in Bamako (37 years old) [23] and lower than that reported by other authors in Madagascar [9] and Morocco [24] who found 45 and 47 years old, respectively. In the United States [25] and in Asia [26], the authors found a mean age which was respectively 60 years and 63.24 ± 8.72. These results show that cirrhosis is more common in young adults in sub-Saharan Africa. It could be linked to life expectancy, which is shorter in Africa. Subjects 56 years of age or older were more exposed to DH (p = 0.0006). The sex ratio of 2.15 in our series is similar to that found by the authors in Bamako in Mali [4], in Libreville in Gabon [22] and in Dakar in Senegal [27], which respectively reported 2.6 ; 2.78 and 2.5. In another study in Bamako, the authors reported a sex ratio of 6.1 [23]. Cirrhosis is the most common liver disease in men. Women are said to be protected by estrogen, which has anti-fibrotic properties. All patients with hypovolemic shock were transfused with an average of 5 blood bags of 450 ml, while the number of RBC bags transfused was 4 in Mali [23] and 3.37 in Lebanon [26]. This explains why DH in cirrhosis is severe. The most common mode of expression of gastrointestinal bleeding in our series was hematemesis and melena (79.31%). The latter were also reported by the authors in Yaoundé in Cameroon (42.36%) [28] and in Brazzaville in Congo (50%) [29]. Aspirin and NSAIDs were found in our study in 20.7% and 67.2%, respectively. The use of NSAIDs was reported by the authors in 45.45% of cases in Benin [15], 34.3% of cases in Brazzaville [29], 17% of cases in Togo [30]. Other authors had reported taking NSAIDs and / or aspirin in 45.8% [26]. The intake of gastrotoxic drugs has not been observed by some authors [12,22,28]. Patients with cirrhosis should be made aware of the harmful effects of taking NSAIDs and / or aspirin which may lead to gastrointestinal bleeding and the development of hepatorenal syndrome. The lesions responsible for DH were identified in 70.1% of cases in our series. On the other hand, some authors in Cotonou [15], in Libreville [22] and in Lebanon [26] found lesions in 65.45%, 62.5% and 67.7% of cases respectively. The delay in performing the endoscopic examination could explain why some lesions which would have bled could not be identified. In our study, the lesions most frequently responsible for DH are esophageal varices and gastroduodenal erosions associated with the use of NSAIDs. The hemorrhages are linked to the size of the EV and the presence of red signs. The rupture of the EV and gastroduodenal erosions are at the origin of the hypovolemic shock, responsible for 4 deaths. In Bamako, the rupture of the EV was responsible for death in 48% of cases. Some authors [10,26] have described that the death rate from EV rupture is high. This shows that gastrointestinal bleeding is more severe in patients with cirrhosis [12,25] and may be a predictor of mortality [9].
Conclusion
This study shows that HD in cirrhosis is common and severe. She is responsible for death from hypovolemic shock. The main factors promoting DH are taking nonsteroidal anti-inflammatory drugs and stopping beta blocker therapy. Potentially hemorrhagic abnormalities in patients with cirrhosis are esophageal varices and gastroduodenal erosions secondary to NSAIDs. Gastrointestinal bleeding from ruptured esophageal varices is related to the large size of esophageal varices and the presence of red signs. The primary etiology of cirrhosis is HBV.
Upper gastrointestinal endoscopy should be performed in all patients with suspected cirrhosis in order to begin primary prevention of their rupture early. Cirrhotics with EV should be made aware of the risk factors for DH.
References
- Anthony PP, Ishak KG, Nayak NC, et The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978; 31: 395-414.
- https://www.has-sante.fr/upload/docs/application/pdf/fs_pdf
- Boursier J, Asfar P, Joly-Guillou M L, et al. Infection and variceal bleeding in cirrhosis. Gastroentérol Clin Biol. 2007; 31: 27-38.
- Maïga MY, Dembélé M, Diallo F, et al. Upper digestive endoscopy in the diagnosis of cirrhosis. Acta Endoscopica. 2002; 32: 211-215.
- Cales Epidémiologie. pronostique de la cirrhose. Concours Médical. 1995; 117: 2707-2711.
- Camengo Police SM, Koffi B, Boua-AKelelo N, et al. Complications of the cirrhosis to the university hospital “Amitié” of Bangui. Méd Afr Noire. 2014; 61: 537-542.
- Serme AK, Ilboudo PD, Bougouma A, et al. La cirrhose au Centre National Hospitalier de Yalgado Ouédraogo (CNHYO). Méd Afr Noire. 2002; 49: 481-486.
- Oberti F. Prognosis of portal hypertension: gastrointestinal bleeding from ruptured esophageal varices. Hépato-Gastro. 1998; 5: 371-377.
- Razafimahefa SH, Rabenjanahary TH, Rakotozafindraibe R, et al. Upper gastrointestinal bleeding: clinical, endoscopic features and outcome. Analysis of 62 patients from Madagascar. Rev Méd Madagascar. 2011; 1: 6-10.
- Ben Chaabane N, Ben Youssef H, Ghedira A, et al. Upper gastrointestinal bleeding epidemiology in Tunisia. Acta Endoscopica. 2010; 40: 176-182.
- Borsari G. Rupture of esophageal varices: myth or reality? Acta Endoscopica. 1977; 7: 279-284.
- Van Leerdam M E. Epidemiology of upper gastro intestinal Best Practice & Research. Clinical Gastroenterology. 2008; 22: 209-224.
- Sassenou I, Aourarh A, Hachim M, et al. Contribution of endoscopy in upper digestive haemorrhages. Experience of the medical department A of the Mohamed V Military Hospital Médecine du Maghreb. 2004; 117: 13-20.
- Debongnie JC. Endoscopy and prognosis in upper gastrointestinal bleeding. Gastroentérol Clin Biol. 1989; 13: 890-898.
- Kodjoh N, Hountondji A, Addra B. Hémorragies digestives hautes et pathologie oeso- gastroduodénale dans un service de médecine interne en milieu Méd Afr Noire. 1992; 39: 25-30.
- Camengo Police SM, Mbeko Simaleko M, Boua Akelelo NP, et al. The cirrhosis and her complications to the academic hospital of the friendship of Bangui: survey of the financial cost of the hold in charge. J Afr Hépatol Gastroentérol. 2013; 7: 78-81.
- Ouavéné JO, Koffi B, Mobima T, et al. Cirrhose de foie à l’hôpital de l’Amitié de Bangui : aspects épidémiologiques, cliniques, échographiques et problèmes de diagnostic. J Afr Image Méd. 2013; 5: 1.
- Bekondi C, MobimaT, Ouavéné JO, et al. Etiopathological factors of hepatocellular carcinoma in Bangui, Central African Republic: clinical, biological characteristics and virological aspects of patients. Pathol Biol. 2010; 58: 152-155.
- Pugh RNH, Murray-Lyon IM, Dawson JL, et al. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973; 60: 646-654.
- Peron J M. Quand et comment évaluer les risques d’une première hémorragie digestive ? Gastroentérol Clin Biol. 2004; 28: 35-43.
- Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974; 17: 394-397.
- Abdou Raouf O, Mistoul I, Obamengwa C, et al. Upper digestive hemorrhages: Epidemiological aspects and value of emergency endoscopy Méd Afr Noire. 2002; 49: 395-398.
- Konaté A, Diarra MT, Souckho A, et al. Hémorragies digestives par rupture des varices oesophagiennes. Mali Médical. 2008; 23: 32-35.
- Elmekkaoui A, Touyar A, Mellouki I, et al. Les hémorragies digestives hautes au CHU de Fès: Etude épidémiologique. Revue d’Epidémiologie et de Santé 2009; 57: S3-S59.
- Lecleire S, Difiore F, Merle V, et al. Acute upper gastro- intestinal bleeding in patients with liver cirrhosis and in non cirrhosis patients: Epidemiology and predictive factors of mortality in a prospective Multicenter population- Based study. J Clin Gastroenterol. 2005; 39: 321-327.
- Slim R, Yaghi C, Khalil H, et Hémorragie digestive haute: Identification de facteurs pronostiques. J Méd Libanais. 2005; 53: 143-150.
- Bassene ML, Diouf ML, Dia D, et al. Ligation of esophageal varices in the digestive endoscopy center of Universitary Hospital Aristide-Le- Dantec of Dakar: preliminary study of 60 cases. J Afr Hépatol Gastroentérol. 2010; 4: 194-198.
- Ndjitoyap Ndam EC, Koki Ndombo PO, Fouda OA, et al. Upper digestive system hemorrhages in Cameroon: apropos of 172 cases examined via endoscopy. Méd Trop. 1990; 50: 181-184.
- Ibara JR, Massamba Miabaou D, Gassaye D, et al. Les hémorragies digestives hautes du sujet âgé : A propos de 64 cas. Ann Gastroentérol Hépatol. 1998; 34: 233-235.
- Bagny A, Bouglouga O, Djibril MA, et etiologic profile of therapeutic upper gastrointestinal bleeding of the adult in the CHU-campus of LOME(Togo). J Afr Hépatol Gastroentérol. 2012; 6: 38-42.