Drugs with Inhibitory Potentials on Polyol Pathways of Diabetic Neuropathy: An Overview
Author'(s): Saganuwan Alhaji Saganuwan*
Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Federal University of Agriculture, P.M.B. 2373, Makurdi, Benue State, Nigeria.
*Correspondence:
Saganuwan Alhaji Saganuwan, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Federal University of Agriculture, P.M.B. 2373, Makurdi, Benue State, Nigeria, Tel: +2347039309400.
Received: 25 July 2020 Accepted: 29 August 2020
Citation: Saganuwan Alhaji Saganuwan. Drugs with Inhibitory Potentials on Polyol Pathways of Diabetic Neuropathy: An Overview. Diabetes Complications. 2020; 4(3); 1-7.
Abstract
About one third of diabetic patients develop diabetic foot problems that may originate from peripheral neuropathy and or peripheral neurovascular disease. Because of chronic and difficult to treat nature of diabetic foot ulcers, there is need for literature search with intent to understanding neuropathic and neuroischaemic nature of diabetes in order to control, prevent and treat diabetic foot ulcers. Findings have shown that inhibitors of aldose reductase, sorbitol dehydrogenase, activation of protein kinase C, activators of nitric oxide synthase, myo-inositol bisphosphatase, and other enzymes that play different roles in polyol pathway of diabetic neuropathy are the key points to prevention, control, management and treatment of diabetic foot ulcers. The use of central nervous system acting drugs, potent antimicrobials and traditional antidiabetics are complementary to holistic permanent cure of the disease. More potent antidiabetic ulcers could be synthesized from varieties of heterocyclic compounds reported in many journals of organic, pharmaceutical and medicinal chemistry. Therefore, successful treatment of diabetic foot ulcers is multimodal, but the major key success is constant adequate control of blood sugar.
Keywords
Introduction
Diabetes is a metabolic disorder characterized by damage of β-cells called islet of langahern, which secrete insulin that control blood sugar level. Chronic diabetes could lead to diabetic foot disease which affects 25% of diabetic patients. Ankles are lost to diabetes every 30 seconds across the globe, accounting for 30% of hospital admission and over 85% of lower limb amputations are preceded by diabetes. Diabetic foot ulcers are cost-effective and diabetes causes charcot neuroarthropathy that could be prevented [1-7]. Over 60% of diabetic foot ulcers are connected with neuropathy [8,9]. Fifty percent of diabetic foot ulcers occur on the plantar surface and the remaining 50% occur on other areas of the foot [10]. In the US more than 80,000 legs were amputated, average healing cost of diabetic foot is $8,000, infected ulcer ($17,000) and major amputation ($45,000). About 50% of amputees develop ulcerations and infections in the contralateral limb in 18 months, 58% would have a contralateral amputation 3-5 years after the 1st amputation, and mortality rate of 20-50% in 3 years has been estimated, with the continuous trend for 30 years [11].
Glucose from gluconeogenesis, diet or glycogenolysis is either oxidized or incorporated into glycogen. However some glucose is converted to fructose in the eye lens, pancrease, testes and brain. Aldose reductase converts glucose to sorbitol by reduced nicotinamide adenine dinucleotide phosphate (NADPH) and polyol dehydrogenase oxidizes sorbitol to fructose by oxidized nicotinamide adenine dinucleotide (NAD+) which is the main fuel for sperm cell. In individuals with diabetes increased sorbitol causes precipitation of protein [12], abnormal collagen [13], cataract, retinopathy and ulcer [14]. Sugar alcohols (polyols) reduce caloric intake due to their metabolic differences as compared to other carbohydrates and may have 1 to many sugar base(s) [15]. They are of straight chain (glycitols) and cyclic (inositol). Xylitol, a polyol resembles sweeteners may be related to ketose [16]. Short chain polyols e.g. ethanediol, butanediol, and propanediol are comparable with corn-based glucose, suggesting that polyols could be produced from lignocellulose [17]. Hence sorbitol, mannitol, xylitol, isomalt, erythritol and hydrogenated starch occur naturally and affect sugar level less than that of sucrose [18], indicating that fructose-based polyurethane could be beneficial in treatment of diabetic foot ulcers [19]. Polyols are characteristic of translocable carbon that play a role in stress tolerance by recycling excess NADPH. They are low molecular-weight, highly soluble and non-reducing carbohydrates [20].
Diabetic Foot Neuropathy
Diabetic foot neuropathy is manifested (Figure 1) via motor, autonomic and sensory nervous system [8,9]. Peripheral arterial disease contributes to foot ulcers in 50% of cases. It affects tibial and peroneal arteries in calf. However, smoking, hypertension and hyperlipidemia contribute to peripheral artery disease [21]. Callus formation, insensitivity and high foot pressure lead to clawing of the affected toes [22,23]. Measurement of the Ankle Brachial Index (ABI) is used to assess extent of vascular disease. ABI is defined as ratio of systolic blood pressures in the ankles (dorsalis pedis and posterior tibial arteries) and arms (brachial artery) using a handheld doppler. The ratio is calculated. Ratio below 0.91 suggests obstruction of poorly compressible vessels and aortoiliac stenosis could complicate the result. But vascular imaging and peripheral arterial angiogram may reveal arterial disease [24]. About 215 drugs have been implicated in induction of neuropathy [25]. Toxic neuropathy is observed in largest and longest nerve fibers. It occurs in persons with neurodegenerative diseases of the nervous system, uremia, nutritional deficiency and diabetes [26]. Semmes-Weinstem 10-g monofilament and vibratory sensation with a 128-Hz tunning fork and cold-warm discrimination are used for neurological evaluation [10].
Diabetic Peripheral Neuropathy
Peripheral nerve injury affects nerve cells, satellite cells and myelin cells. Neural inducing drugs include but not limited to paclitaxel, bortezomib, Ixabepiline and oxaliplatin [27]. Drugs that trigger peripheral neuropathy include some antibiotics, infliximab, etanercept, leflunomide, linezolid, statins, dichloroacetate [28-30]. Peripheral sensitization occurs in response to neural tissue injury [31]. Reduced response threshold in nociceptive small C-fibre sensory neurons lower ectopic impulse activity generated along Aβ sensory neurons and expression of substance C have been responsible for generation of neuropathic pain [27]. A cut on axon induces influx of calcium, that disrupts ionic balance and initiates transport of intracellular and extracellular chemical to the nerve cell body in the dorsal root ganglion humiliating nociceptive and non-nocileptive neurons [32].
Neurotrauma As the Cause of Diabetic Foot Ulcers
Microorganisms found in acute diabetic foot infection are Staphylococcus aureus, β-haemolytic Streptococcus (A, B, C and G), whereas microorganisms found in chronic infection are Staphylococcus spp, Streptococcus spp, Enterococcus spp, Emterobacter spp, Escherichia coli, Proteus spp, Klebsiela spp, Pseudomonas aeruginosa, Peptococcus spp, Peptostreptococcus spp, Clostridium spp, Fusobacterium spp and Bacterioides spp. Infection of the wounds take place after neurotrauma that could lead to abnormal extensor and flexor tendons leading to extension of ligaments, joint distension, microfractures and dislocation of the bones (German theory). Whereas neurovascular damage of autonomic neuron results in hyperemia. Arteriorvenous shunts cause increased vascular flow leading to osteopenia and bone resorption (French theory). None of the theories accounts for unilateral presentation of diabetic foot ulcer [32].
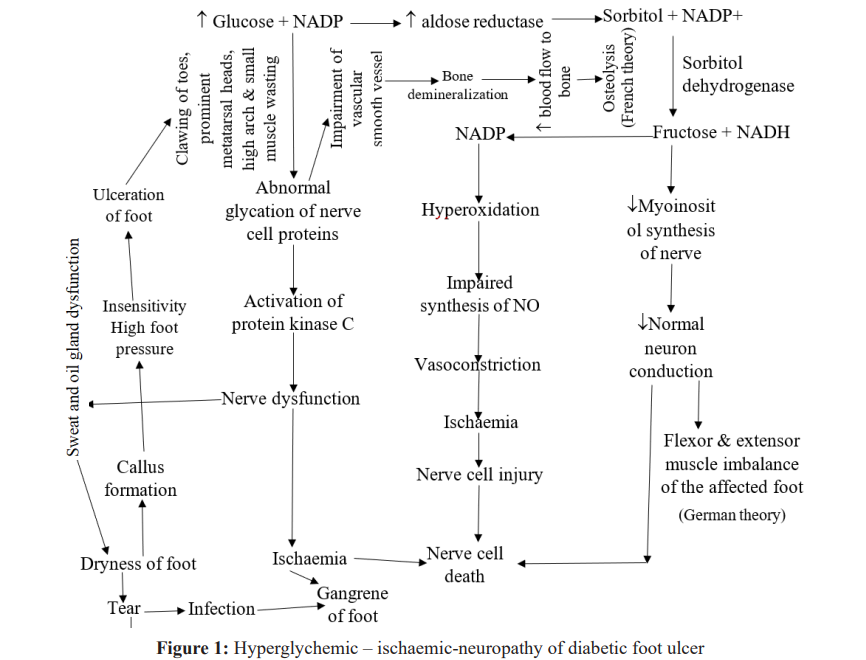
Drugs with Inhibitory Potentials on Polyol Pathways of Diabetic Neuropathy
The polyol pathways consume about 30 % glucose , suggesting that nicotinamide riboside could be used to restore redox balance [33]. Also increased aldose reductase activity and sorbitol content in glomeruli leads to glomerulonephritis that could be prevented by sorbinil [34]. Polymorphisms of the aldose reductase genes are associated with susceptibility or progression of diabetes complications [35]. Absence of water in the body generates oxide and hydroxide radicals [36] that could damage tissue. Hence changing lifestyle and dietary pattern could prevent, delay and treat diabetes-related complications [37]. Whole grains, fruits, vegetables, low-fat milk, (low glycemic index food), dietary fibre, sucrose and sucrose-containing food, protein, reduced food diet, vitamins and minerals from natural source, reduced alcohol intake, antihypertensive and antidiabetic drugs are highly beneficial [38].
Linear polyether polyol (PEP) made of glycidol units could provide multiple anchorage points for different functional groups with antidiabetic potentials [39]. Vegetable oils with two-steps polyol synthesis is safer than those with one-step polyol synthesis [40]. Polyol fatty acid polyesters particularly sucrose polyester via tranesterification is less toxic [41]. Quinazoline acetic acids and related analogs inhibit aldose reductase [42,43]. Alrestatin and quercetin also inhibit the enzyme [44].
All aldose reductase inhibitors, inhibit aldehyde reductase [45]. Spirosuccinamide/hydantoins and carboxylic acids are two chemical classes of inhibitors of aldose reductase that convert hyperglycaemia into glucose toxicity in neural and glial cells in the retina [46]. Sorbitol dehydrogenase inhibitor such as 2-methyl-4-[NN-dimethylsulfamoyl-piperazino]-pyrimidine (S-0773) prevents vascular and neural dysfunction in diabetes [47]. Fructose-1, and 6-bisphosphatase inhibitors are potential antidiabetics [48]. Fructose bypasses glycolysis, glucokinase and phosphofructokinase in liver.
Fructose is metabolized by fructokinase that has no negative feedback system and adenosine triphosphate (ATP) is used for phosphorylation which leads to depletion of intracellular phosphate and rapid generation of uric acid owing to activation of adenosine monophosphate (AMP) deaminase. Uric acid causes endothelial dysfunction, insulin resistance and hypertension [49]. Myo-inositol, the precursor of phosphoinostide mediates multiple cellular events. Therefore, treatment of myo-inositol depletion improves β-cells and prevents diabetes [50]. Gliclazide activates nicotinamide adenine dinucleotide phosphate (NADP) oxidase by advanced glycation and ends oxidative stress [51]. Adiponectin induces endothelial nitric oxide synthase activation and nitric oxide production, thereby causing vasodilation leading to enough oxygen supply and survival of nerve cells [52]. Azetidione like quinolones have hypolipidemic activity. Tricyclic derivatives with cyclo-condensed pyrido-pyrizine and pyrido-diazepine have antinoxiceptive, muscle-relaxing and spontaneous motor activity with narcotic effect. The most promising analgesic and neuroleptic pyrido [2,3-e pyrrolol] 1,2-a pyrazine could be used [53]. Some synthetic heterocyclics synthesized from acetamide and α-haloketone have potent analgesic properties. Some imidazole compounds that mediate their actions via opioid and serotonin receptors may relieve pain centrally. Pyrrolo benzothiazoles could act via glutamate in neurological conditions. Chloro and methoxy- derivatives of benzylidene-imidazothiazoline have potent analgesic, anticonvulsant and antidepressant activities [54].
Triazole compound with 5C has anti-inflammatory, analgesic, sedative and anticonvulsant activities [54] and could be of high benefit in treatment of diabetic foot ulcers. Since benzimidazole, albendazole promotes anti-TNF mediated induction of regulatory macrophages, it could act as anti-inflammatory mediator. Substituted benzimidazole and analogues used in treatment of neurodegenerative disorders could be used. Benzimidazole and imidazole compounds may have antimicrobial activity against S. aureus and E. coli found in diabetic ulcers in addition to analgesic effects [54]. Piroxicam and its metabolites have cholinergic and GABAergic effect by causing stimulation and depression of central nervous system with resultant potent analgesic effect. Hence, it could be used for modulation of motor-sensory pathway of diabetic ulcers [55,56]. A number of central nervous system acting drugs used in the management of diabetic ulcers are chiral and all of them are toxic. Therefore, their use should be based on principle of individualized medicine [57]. Polymeric materials could be used for CNS modifying drugs against diabetic foot ulcers to increase duration of action, efficiency and reduce toxicity [58]. But hyperglycaemia and hypoglycaemia must be controlled to avoid depression of brainstem that may lead to cardiopulmonary failure and death [59]. Therefore, physicochemical and structural – activity properties of the central nervous system acting drugs used to manage diabetic foot ulcers should be adequately put into consideration to avoid aggravation of the condition [60]. A number of medicinal plants used in management of diabetes and hypertension have been reported. They are highly beneficial in control and management of diabetes and hypertension that may result in diabetic foot ulcers [61].
Chemotherapy of Peripheral Nerve Injury in Diabetic Foot Ulcers
Treatment of peripheral nerve injury is by nerve repair or nerve grafting. Axon’s capacity to regrow is always incomplete; the regeneration misses the original tissue target. But multimodal analgesics, anticonvulsants, botulinum toxin, peripheral nerve electrical stimulation, spinal cord stimulation and stem cells implant are quite beneficial in the treatment of peripheral neuropathy. Prolonged treatment with NK-1 receptor antagonist and progesterone has long-term beneficial effects [27]. However, changes in functional chemistry and structures of neurons (neural plasticity) cause neuropathic pain. Opioids, anticonvulsants, antidepressants, lidocaine patch, capsaicin, ketamine, pregabalin, gabapentine, intrathecal drug administration and neurosurgical intervention are highly useful [62]. Dose-limiting paclitaxel induced peripheral neuropathy could be prevented by minocycline [63], suggesting that minocycline has peripheral anti-neuropathic activity. Voltage proton and ligand-gated ion channels modulators are highly beneficial in the treatment of neuropathic pain [64]. Diabetic foot ulcers have been classified as neuropathic, ischaemic and neuroischaemic depending on complications of peripheral neropathy and arterial disease [65].
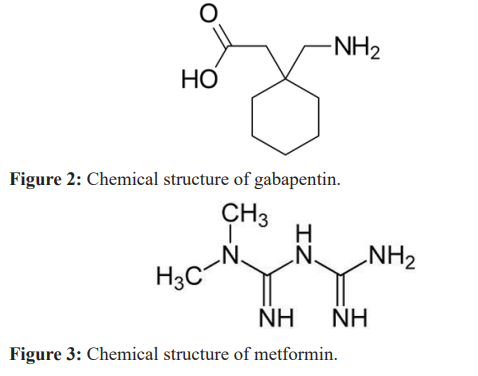
Inflammatory mediators such as cytokines, growth factors, kinins, purines, amines, ions, prostanoids and protons play a great role on neurogenesis of pain. Mexiletine and dextrophan have limited value whereas amitriptyline and gabapentine are of high clinical value. Therapeutic agents that could modify vanilloid receptors, tetrado- toxin-resistant sodium channels, calcium channels, N-methyl-D- aspartate (NMDA) receptors, cannabinoid receptors are very vital to control, prevention, management and treatment of diabetic foot ulcers [66]. Recreational drugs such as sedatives, stimulants, hallucinogens, organic solvents and athletic performance enhancer are highly beneficial, but with neurological consequences [67]. Suppression of neuropathic pain by cannabinoids is via α3 glycine receptors [68]. Neuropathic pain causes enduring prefrontal cortex dysfunction not corrected by gabapentin (Figure 2) but by metformin (Figure 3) [69]. But nerve injury could be successfully managed [70]. Hence, gabapentin should be considered an important drug in the management of neuropathic pain syndromes [71] in diabetic foot ulcer.
Neuropathy, neuropathic pain and painful peripheral neuropathy could be treated holistically but require careful interdisciplinary monitoring and follow-up [72]. ABI of 0.91-1.3 (normal), 0.7-0.9 (mild obstruction), 0.4 – 0.69 (moderate obstruction), <0.4 (severe obstruction) and > 1.3 (poorly compressible vessel) are used to assess arterial occlusion [73]. Becaplermin though useful could cause cancer [74].
Adjunt Therapy of Diabetic Foot Ulcers
Diabetes impairs peripheral circulation, alters leukocyte function, causes imbalance of cytokines and proteases. Levels of matrix metallo-proteinases (MMP-1) could predict likelihood of wound healing. High level of MMP-1 is essential to wound healing [75,76]. The neurophathic foot heals completely using Total Contact Cast (TCC) where pressure is mitigated and the device is irremovable thereby enforcing therapeutic compliance [75].
Anaerobes and aerobes colonize the ulcer and they can be eliminated using clindamycin and augmentin. Ciprofloxacin, flucloxacillin, ampicillin and metronidazole could be used initially and linezolid is recommended for Mathicillin-Resistant Staphylococcus Aureus (MRSA) [76]. Antibiotics could be given for a period of 12 weeks [77]. Platelete growth factor, becaplermin could be used as adjunct therapy [78]. Diabetic bone infection is difficult to treat and often surgery is done. In fact, all infected foot lesions other othan primary cellulitis require surgical intervention.
Antibiotics such as cephalexin, ampicillin/sulbactam, clindamy- cin/ciprofloxacin, imipenem/cilastatin, clindamycin/tobramycin/ ampicillin, levofloxacin/clindamycin, trimethoprim-sulfamethox- azole, piperacillin/sulbactam, clindamycin/ceftazidime, and van- comycin/ aztreonam/metronidazole are used in the treatment of diabetic foot infections with 80-90% success in mild to moderate infections and 50-60% for deeper and more extensive infection [79].
Mild infections should be treated with oral antibiotics while severe infections should be treated initially with broad spectrum antibiotics parenterally, following oral antibiotics [80]. The highlighted pathway of development of diabetic foot ulcers outlines prevention, chemotherapeutic, endovascular and revascularization strategies aimed at controlling or treating the wound [81].
Hyperbaric oxygen, negative pressure wound therapy, bioengineered skin substitute in 8 larval therapy should be tried, specialized foot wear with padded hosiery, injectable silicone and patient education are complementary interventions against diabetic foot ulcers. Revascularization to restore arterial blood flow is also helpful [21]. Biophosphonates are used against osteoclast activation whereas pamidronate reduces disease activity in acute charcot neuroarthropathy [82]. Reversibility of neuropathic pain is determined by inflammatory mediators’ actions [83].
Conclusion
Holistic permanent cure for diabetic foot ulcers is the inhibition of polyol pathway. Diabetes is adequately controlled and trauma is not induced. The CNS effects are caused by both stimulants and depressants for sensitization of the nerve to avoid nerve damage, and depression of nerve to reduce pain, and control inflammatory mediators’ activities.
ORCID ID
0000-0002-0963-5569
Acknowledgements
I sincerely thank Kehinde Ola Emmanuel of National Open University and Williams Yusuf of Federal University of Agriculture Makurdi Nigeria for typing the work.
References
- Assal Patient education in diabetes. In Recent Trends in Diabetic Research, Stockholm. Almquist and Wiksel International. 1982; 276-289.
- Thomson FJ, Veves A, Ashe H, et al. A team approach to diabetic foot care: the Manchester experience. Foot. 1991; 1: 75-82.
- Singh N, Armstrong DG, Lipsky Preventing foot ulcer in patients with diabetes. JAMA. 2005; 293: 217-228.
- Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005; 366: 1719-1724.
- Ragnarson-Tennvall G, Apelqvist J. Prevention of diabetes- related foot ulcers and amputations: a cost-utility analysis based on Markov model Diabetologia. 2014; 44: 2077-2087.
- Boulton The diabetic foot from art to science the 18th Camillo Golgi lecture. Diabetologia, 2004; 47: 1343-1353.
- Williams R, Airey The Size of the Problem: Epidemiological and Economic Aspects of Foot Problems in Diabetes. Wiley. 2000; 3-17.
- Bowering CK. Diabetic foot ulcers: pathophysiology assessment and Can. Fam. Phys. 2001; 47: 1007- 1016.
- Dyck PJ, Davis JL, David M. Wilson, et al. Risk factors for severity of diabetic polyneuropathy. Diabet Care. 1999; 22: 1479-1486.
- Mavrogenis AF, Megaloikonomos PD, Antoniaddou T, et al. Current concepts for the evaluation and management of diabetic foot ulcers. EOR. 2018; 3: 513-525.
- Kruse I, Edelman Evaluation and treatment of diabetic foot ulcers. Clin. Diabet. 2006; 24: 91-93.
- Horton HR, Moran LA, Scriimgeour KG, et Principles of Biochemistry 4th ed., Pearson Prentice Hall. New Jersey. 2006; 852.
- Mclennan S, Yue DK, Fisher E, et al. Deficiency of ascorbic acid in experimental diabetes: relationship with collagen and polyol pathway abnormalities. Diabetes. 1988; 37: 359-361.
- Mathebula SD. Polyol pathway: a possible mechanism of diabetes complications in the eye. Afr. Vision Eye Healt. 2015; 74: 1-5.
- Rapaille A, Heume M. Sugar alcohols. In: Encyclopedia of Food Science Nutrition, 2nd ed. Elsevier. 2003; 1-11.
- Bieleski RL. Sugar alcohols. In Encyclopedia of Plant Physiology. 13: 158-192.
- Fang ZH, Zhang J, Lu QM, et al. Process development of short chain polyols synthesis from corn stover by combination of enzymatic hydrolysis and catalytic hydrogenolysis. Biotechnol. Rep. 2014; 3: 15-20.
- Godswill Sugar alcohols:chemistry, production health concerns and nutritional importance of mannitol, sorbitol,xylitol, and erythritol. Int J Adv Acad. Res. 2017; 3: 31-35.
- Lu MY, Suranyi A, Viskolcz B, et al. Molecular design of sugar-based polyurethanes. Croat. Chem. 2018; 91: 1-9.
- Loescher WH. Physiology and metabolism of sugar alcohols in higher plants. Physiol. Plantat. 1987; 70: 553-557.
- Najpur Management of diabetic foot. Med Update. 2012; 22: 287-293.
- Boulton AJM, Armstrong DG, Albert SF, et Comprehensive foot examination and risk assessment. Diabetes, Translating Research into Practice, Fonseca VA. Elsevier. 2006; 179-195.
- Boulton AJM, Richard IG, Flyvbjerg A, et al. Foot problems in patients with diabetes In: Textbook of Diabetes, 4th ed. Wiley-Blackwell. 2010; 727-742.
- American Diabetes Association ADA. Peripheral arterial disease in people with Diabet Care. 2003; 26: 3333- 3341.
- Guo A, Racz R, Hur J, et al. Ontology based collection, representation and analysis of drug-associated neuropathy adverse events. J. Biom. Semant. 2016; 7: 1-12.
- Sterman AB. Toxic neuropathy. Mayo Clin. Proc. 1985; 60: 59-63.
- Henry JL. Mechanisms of peripheral nerve injury-what to treat when to treat. Intech Open. London. 2014; 1-23.
- Kambayashi Y, Hosokawa T, Okamoto K, et al. Statistical identification of predictors for peripheral neuropathy associated with administration of bortezomib, taxanes, oxaliplatin or vincristine using ordered logistic regression analysis. Anticancer. Drug. 2010; 21: 877-881.
- Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer CSP-HOR O2 study. Supp Care Cancer. 2009; 17: 1071-1080.
- Weimer LH, Sachdev N. Update on medication-induced peripheral Curr Neurol. Neurosci. Report. 2009; 9: 69-75.
- Deror M. Ectopic discharge in Aβ afferents as a source of neuropathic pain. Exp. Brain Res. 2009; 196: 115-128.
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to Ann. Rev. Neurosci. 2009; 32: 1-32.
- Yan LJ. Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Model Exp. Med. 2018; 1: 7-13.
- Beyer-Mears A, Ku L, Cohen MP. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Dibetes. 1984; 33: 604-607.
- Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and Exp. Diabet Res. 2007, ID 61038.
- Poul L, Sammer S, Jouin N, et al. Synthesis of inorganic compounds, metal oxide and hydroxide in polyol medium: a reversible route related to the sol-gel process. J. Sol-Gel Sci. 2003; 26: 261-265.
- Asif M. The prevention and control of the type 2 diabetes by changing lifestyle and dietary J Educ. Health Promot. 2014; 3: 1-13.
- American Diabetes Association Nutritional principles and recommendations in diabetes. Diabet Care. 2004; 27: 36-46.
- Li Z, Chau Synthesis of linear polyether polyol derivatives as new materials for bioconjugation. Bioconjug. Chem. 2009; 4: 780-789.
- Anya AU, Isa MT, Musa SH, et al. A review of process used in polyol synthesis from vegetable oils. Sch. J. Biosci. 2014; 2: 141-143.
- Riggi GP, Taylor HM. Synthesis of higher polyol fatty acid polyesters. USA Patent. 1976; 1-9.
- Shapiro J, Koshimune D, Moellmer R. Diabetic foot ulcers- treatments and prevention. Intech Open. London. 2013; 269-
- Malamas MS, Millen J. Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J. Med. Chem. 1991; 34: 1492-1503.
- Kador PE, Robinson WG, Kinishith JH. The pharmacology of aldose reductase Ann. Rev. Pharmacol. Toxicol. 1985; 25: 691-714.
- Sato S, Kador Inhibition of aldehyde reductase by aldose reductase inhibitors. Biochem. Pharmacol. 1990; 40: 1033- 1042.
- Sun W, Oates PJ, Coutcher JB, et al. A selective aldose reductase inhibitor of a new structural class prevents or reverse early retinal abnormalities in experimatal diabetic retinopathy. 2006; 55: 2757-2762.
- Tilton RG, Chang K, Nyengaard JR, et Inhibition of sorbitol dehydrogenase: effects on vascular and neural dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1995; 44: 234-242.
- Kaur R, Dahiya L, Kuma M. Fructose-1, 6-bisphosphatase- inhibitors: a new valid approach for management of type-2 diabetes mellitus. Eur. J. Med. Chem. 2017; 141: 473-505.
- Khitan Z, Kim Fructose: a key factor in the development of metabolic syndrome and hypertension. J. Nutr. Metab. 2013: 682673.
- Li SYT, Cheng STW, Zhang D, et al. Identification and functional implications of sodium/myo-inositol cortransporter in pancreatic β-cells and type 2 diabetes. Diabtes. 2017; 66: 1258-1271.
- Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate oxidase reduced form by advanced glycation end-products links oxidative stress to alter retinal vascular endothelial growth factor expression. Metabolism. 2006; 55: 1516-1523.
- Cheng KKY, Lam KSL, Wang et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPLi in endothelial cells. Diabetes. 2007; 56: 1387-1394.
- Saganuwan SA. Functional chemical groups that may likely become a source for the synthesis of novel central nervous system cns acting Cent. Nerv. Syst. Agents Med. Chem. 2017; 17(3): 178-186.
- Saganuwan, Conversion of benzimidazoles, imidazothiazoles and imidazoles into more potent central nervous system acting drugs. Cent. Nerv. Syst. Agents Med. Chem. 2020; 20(1): 3-12.
- Saganuwan SA. Piroxicam: source for synthesis of central nervous system cns acting drugs. Cent. Nerv. Syst. Agents Med. Chem. 2017; 17(1): 172-177.
- Saganuwan SA. In vivo piroxicam metabolites: possible source for synthesis of central nervous system cns acting depressants. Cent. Nerv. Syst. Agents Med. Chem. 2017; 17(3): 172-177.
- Saganuwan Chirality of central nervous system cns acting drugs: a formidable therapeutic hurdle against cns diseases. Cent. Nerv. Syst. Agents Med. Chem. 2019; 19(3): 171-179.
- Saganuwan SA. Biomedical application of polymers: a case study of non-cns drugs becoming cns acting drugs. Cent. Nerv. Syst. Agents Med. Chem. 2018; 18(1): 32-38.
- Saganuwan SA. Chemistry and effects of brainstem acting drugs. Cent. Nerv. Syst. Agents Med. Chem. 2019; 19(3): 180-186.
- Saganuwan SA. Physico-chemical and structure-activity properties of piroxicam: a mini-review. Comp. Clin. Pathol. 2016; 25: 941-945.
- Saganuwan SA. Tropical plants with antihypertensive, anti- asthmatic and anti-diabetic J. Herbs Spices Med. Plant. 2009; 15(1): 24-44.
- Vranken JH. Mechanisms and treatment of neuropathic pain. Cent. Nerv. Syst. Agents Med. Chem. 2009; 9: 71-78.
- Masocha Paclitaxel-induced hyposensitivity to nociceptive chemical stimulation in mice can be prevented by treatment with minocycline. Sci. Report. 2014; 4: 1-5.
- Colombo E, Francisconi S, Faravelli L, et al. Ion channel blockers for the treatment of neuropathic pain. Futur. Med. Chem. 2010; 2: 803-842.
- Cavanagh PR, Lipsky BA, Bradbury AW, et Treatment for diabetic foot ulcers. Lancet. 2005; 366: 1725-1735.
- Banos JE, Sanchez G, Berrendero F, et al. Neuropathic pain: some clues for future drug Min. Rev. Med. Chem. 2003; 3: 719-727.
- Enevoldson Recreational drugs and their neurological consequences. J. Neurol. Neurosurg. Psychiat. 2004; 75: 9-15.
- Xiong W, Cui T, Cheng K, et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 2012; 209: 1121-1134.
- Shiers S, Pradham G, Mwirigi J, et al. Neuropathic pain creates an enduring prefrontal cortex dysfuction corrected by the type II diabetic drug metformin but not by gabapentin. J. Neurosci. 2018; 38: 7337-7350.
- Adetoye AO, Aaron OI, Orimolade EA, et Management of neuropathic pain following traumatic brachial plexus injury with neurolysis and oral gabapentin: a case report. Nig. J. Clin Pract. 2019; 22: 1301-1303.
- Rose MA, Kam PCA. Gabapentin pharmacology and its use in pain management. Anaesthesia. 2002; 57: 451-462.
- Christelis Neuropathy, neuropathic pain and painful neuropathy. www.neuromodulation.com.2017; 1-6.
- Mishra SC, Chchatbar KC, Kashikar A, et al. Diabetic foot. BMJ. 2017; 359: 1-14.
- Alexiaduou K, Doupis Management of diabetic foot ulcers. Diabet. Ther. 2012; 3: 1-15.
- Sibbald RG, Wio KY. The biology of chronic foot ulcers in person with Diabet. Metab. Res. Rev. 2008; 24: 25- 30.
- Muller M, Trocme C, Lardy B, et Matrix metalloproteinases and diabetic foot ulcers. The ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet. Med. 2008; 25: 419-426.
- Lipsky BA. New developments in diagnosing and treating diabetic foot infections. Diabet. Metab. Res. Rev. 2008; 24: 66-71.
- Game FL, Jeffloate WJ. Primarily non-surgical management of osteomyelitis of the foot in diabetes. Diabetologia. 2008;51: 962-967.
- Lipsky Medical treatment of diabetic foot infections. CID. 2004; 39: 104-114.
- Chahine EB, Harris S, Williams Diabetic foot infections: an update on treatment. US Pharm. 2013; 35: 23-26.
- Kalish J, Hamdan Management of diabetic foot problems.
- Vasc. Surg. 2010; 51: 476-486.
- Jude EB, Selby PL, Birgess J, et Bisph0sphonates in the treatment of Charcot neuroarthropathy a double-blind randomized controlled trial. Diabetologia. 2001; 44: 2032-2037.
- Junior JOD, Junior CSAP, Cohen Inflammatory mediators of neuropathic pain. Rev. Dor. Sao Paulo. 2016; 17: 35-42.