Efficiency and Safety of Topical Use of the Gentamicin-Impregnated Collagen Matrices in Surgical Treatment of Patients with Diabetic Foot Syndrome
Author'(s): Vladimir N. Obolensky*
1Associate Professor, Department of General Surgery and Radiation Diagnostics, Faculty of General Medicine, N. I. Pirogov’s Russian National Research Medical University, Moscow, Russia.
*Correspondence:
Vladimir N. Obolensky, Associate Professor, Department of General Surgery and Radiation Diagnostics, Faculty of General Medicine, N. I. Pirogov’s Russian National Research Medical University, Moscow, Russia, E-mail: gkb13@mail.ru.
Received: 21 October 2017 Accepted: 14 November 2017
Citation: Vladimir N. Obolensky. Interferon-Based Antiviral Treatment of Chronic Hepatitis C in Combination with Metformin in Patients with HCV-1 Genotype and Insulin Resistance. Diabetes Complications. 2017; 1(4): 1-6.
Abstract
The article presents literature review (web-site PubMed is used) on the use of gentamicin-impregnated collagen matrices in prevention and treatment of surgical site infections – background information, clinical and pharmacological reasoning of the use of this composite product, clinical efficiency. Meta-analysis of published research on the application of this product in treatment of patients with diabetic foot syndrome has been carried out. The authors analyzed the results of their own research of topical application of antibiotic-impregnated collagen matrices in surgical treatment of 161 patients with diabetic foot syndrome and Charcot foot in purulent stage and with osteomyelitis.
Keywords
Surgical site infections (SSI) are one of the most frequent complications among the inpatients. According to published data, on average almost 3 % of all postoperative patients suffer from wound infection after surgery. SSI increases hospital stay and rehabilitation period, significantly increases treatment cost and can provoke the development of other pathological processes and increase mortality rate [1]. After adverse and undesirable reactions to injected drugs, SSI firmly holds the second place in the complications rating in hospitalized patients [2]. Affected patients are 60 % more likely to spend time in the intensive care unit [3]. In the research published by Liau et al. [4], average surplus direct cost of long stay in the hospital, nursing care, drug products and supplies per each patient with SSI was estimated at 1,530 USD.
It is widely recognized that several factors (e. g., smoking, obesity, diabetes, surgical intervention, duration of surgery, repeated surgery, blood transfusion, ALV duration, length of stay in the intensive care unit) predispose to a higher risk of both superficial and deep wound infections [5].
Indisputably, the basis for the prevention of infectious wound complications in surgery in general is compliance with aseptic regulations, rational technique of wound closure and the use of preoperative systemic preventive antibiotics, and systemic antibiotic therapy is recommended in the risk groups [6,7].
However, amid the general consensus that SSI prevention is clearly more beneficial than the treatment of this catastrophic complication, wound infections continue to occur even despite the compliance with all the rules of aseptic and antiseptic treatment and the use of antibiotic prophylaxis and antibiotic therapy [8].
The emergence of multi-resistant bacterial strains created a difficult situation: beta-lactam antibiotics have become ineffective against most clusters of coagulase-negative staphylococcus and staphylococcus aureus (CoNS and S. aureus), and routine use of vancomycin as a prophylactic is impractical to avoid further increase in vancomycin resistance of staphylococci [9,10].
Furthermore, CoNS have an internal ability to adhere to foreign bodies (any implants) and produce biofilms thus increasing their antibiotic-resistance and the likelihood of chronic infection [11].
Topical application of antibiotics, such as gentamicin, tobramycin, tetracycline, minocycline, teicoplanin and sulbactam-cefoperazone, has been performed in several surgical areas. Gentamicin has gradually become the most widely used molecule for this purpose due to a combination of such properties wide spectrum, low cost, favorable pharmacokinetics and pharmacodynamics in topical application [12].
Gentamicin is an aminoglycoside antibiotic widely used in treatment of various infections. Although its spectrum is mainly directed to gram-negative species, gentamicin is also effective against several gram-positive strains. In addition, gentamicin also demonstrates synergy with beta-lactam antibiotics, especially against gram- positive species, such as S. aureus and CoNS [10]. However, the main factor limiting its systemic use is toxicity: with intravenous or intramuscular administration gentamicin accumulates in the renal cortex and in the endolymph and perilymph of the inner ear causing damage to the kidneys and hearing loss [13]. These disadvantages can be partially eliminated by topical administration of gentamicin which reduces its systemic toxicity.
According to Pagkalis S. et al., when gentamicin-impregnated collagen matricesare used locally for sternotomy the drug concentration in serum does not exceed 1 mg/l, while the concentration in the mediastinum exudate remains above 300 mg/l for 36 hours. Besides, gentamicin has a dose-dependent effect, especially against gram-negative rods: this means that high drug concentration in the surgical site can lead to a bactericidal effect not only with respect to sensitive bacteria, but also to the weak-sensitive or even resistant; acute peak local concentration in combination with low drug level in blood plasma has a therapeutic effect with minimal systemic side effects [13,14].
Surgical implants impregnated with gentamicin started to be used in the 1970s, primarily in orthopedic surgery, with the goal of treating or preventing prosthetic infections. The first devices had a disadvantage: they were not made from reabsorbable materials – therefore they had to be surgically removed after they “worked” in the wound [15].
As a result, biodegradable polymers have been designed [16,17], and since the 1980-s collagen implants started to be used in several surgical specialties for the delivery of local antibiotics, mainly due to the biocompatibility of collagen and pharmacokinetic versatility.
As for pharmacokinetics, collagen is a unique polymer due to its complex, well-known three-dimensional structure with different hierarchical levels: primary, secondary, tertiary and quaternary [18]. Physicochemical properties of the final polymer can be altered by interfering into it’s the molecular structure (e. g., intra- and intermolecular cross-links), as well as by binding collagen with other polymers to obtain different drug release time curves
Therefore, when collagen is used as a drug carrier any structural modification may result in different pharmacokinetic profiles [12,19,20].
Collagen biocompatibility and absorption are essential characteristics for infection prevention or treatment since they allow avoiding additional surgery for the drug removal (which may also be complicated by infection).
Gentamicin molecule is highly soluble in water, and in vitro studies have shown that the effect of soaking a gentamicin-impregnated collagen matrix in normal saline leads to a 6.7 %, 40.5 % and 100 % loss of gentamicin after 2 seconds, 1 minute and 6 hours, respectively. That is why manufacturers do not recommend soaking gentamicin-impregnated collagen implant before use [21,22].
The location of the gentamicin-impregnated collagen matrix in the wound is also important: thus, the subcutaneous localization is indicated for the prevention of superficial wound infections, while the deep location is mainly for the prevention of deep wound infections [23,24].
Diabetes is considered an important risk factor for the development of SSI after general surgical procedures [25]. However, in the research by Chia C.L.K. et al. [26] none of the patients with diabetes (n=19) has developed wound infection after using an antibiotic-containing collagen implant in various general surgical interventions.
The evidence behind the use of prolonged local antibacterial therapy (we propose PLAT abbreviation) for the prevention of infectious wound complications is proved by many randomized studies and Meta-analyzes [27–31] (Table 1).
The aim of the authors’ own research: evaluate clinical efficiency of topical application of antibiotic-impregnated collagen matrices in surgical treatment of patients with diabetic foot syndrome in purulent stage and with osteomyelitis.
Materials and Methods
Retrospective analysis of treatment results of 161 patients with diabetic foot syndrome and osteomyelitis, operated with the use of gentamicin-collagen implant (Collatamp EG, EUSA) in the period 2012–2016 has been carried out with postoperative follow-up period of more than 1 year.
Taking into account published data on diabetes as a risk factor for the development of infectious wound complications and the effectiveness of PLAT in the prevention and treatment of wound infections and osteomyelitis, we did not attempt to perform reconstructive bone-plastic interventions and especially osteosynthesis without prolonged local antibiotic therapy in patients with diabetic foot syndrome in the purulent stage, therefore there were no control experimental groups.
All the patients were diagnosed with stage 3 according to the classification by Wagner FW., 1979 [32]. Pathological process character according to the classification by Eichenholtz S. N., 1966 [33], determined the choice of surgical technique for the affected bones removal: in the fragmentation stage the bones were “shelled out” with subsequent resection of the articular surfaces, and in the fusion stage – a wedge resection with an oscillatory saw was performed.
By localization and the presence of foot deformation (classification Sanders L. & Frykberg R., 1991, and Rogers L. C., 2012 [34,35]) and, accordingly, by the treatment tactics the patients were divided into 4 groups (Table 2).
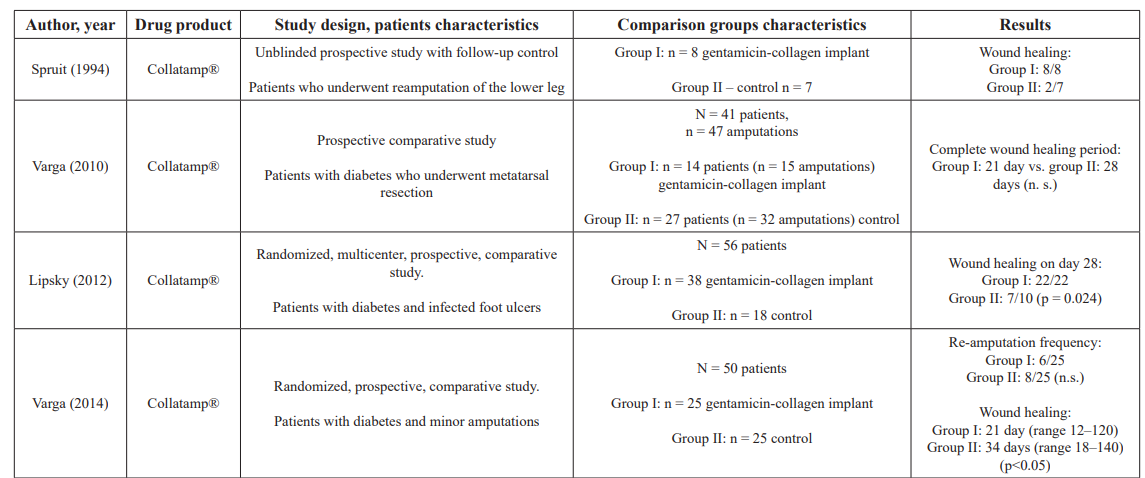
Table 1: Review of scientific publications: application experience of gentamicin-containing collagen implant in diabetic foot surgery.
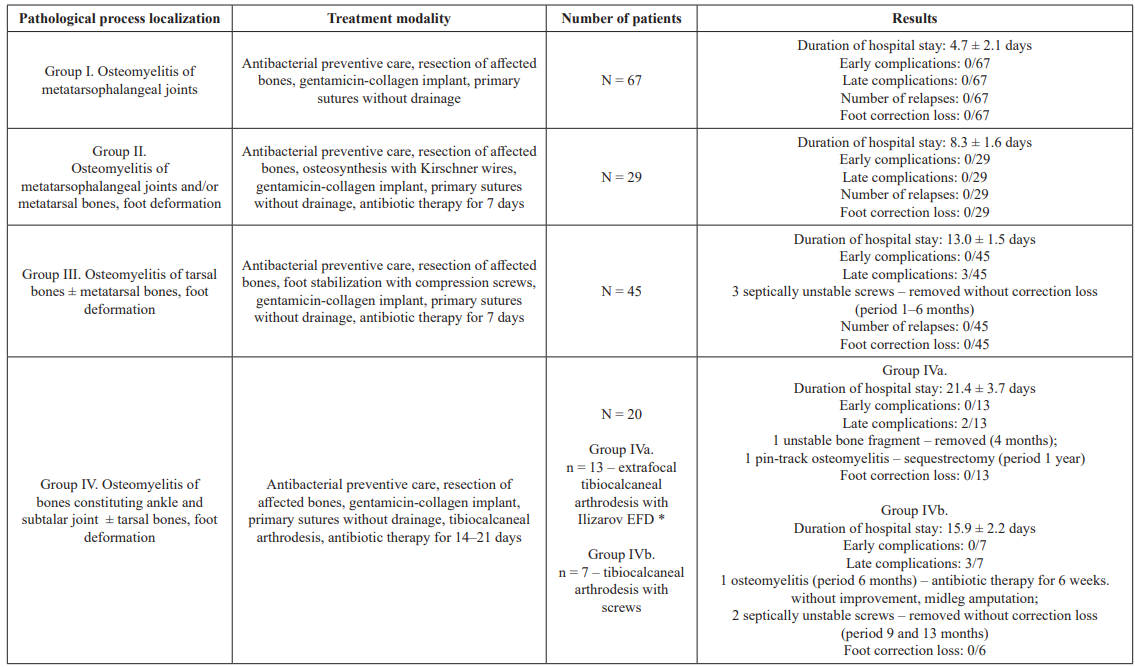
Table 2: Analysis of treatment results of 161 patients with diabetic foot syndrome and osteomyelitis. *: EFD – external fixation device.
It should be noted that none of the patients has developed side effects (nephro- and ototoxic) including those with with diabetic nephropathy, terminal chronic renal failure and chronic hemodialysis. In total, of all the treated patients in period from 14 to 20 months from the moment of surgical treatment, three patients with type 1 diabetes, chronic renal failure and chronic dialysis died from multiorgan insufficiency, but without complications from the operated limb.
Clinical examples of intraoperative use of gentamicin-impregnated implants and long-term results in patients of different study groups are shown in Figures 1, 2 and 3.
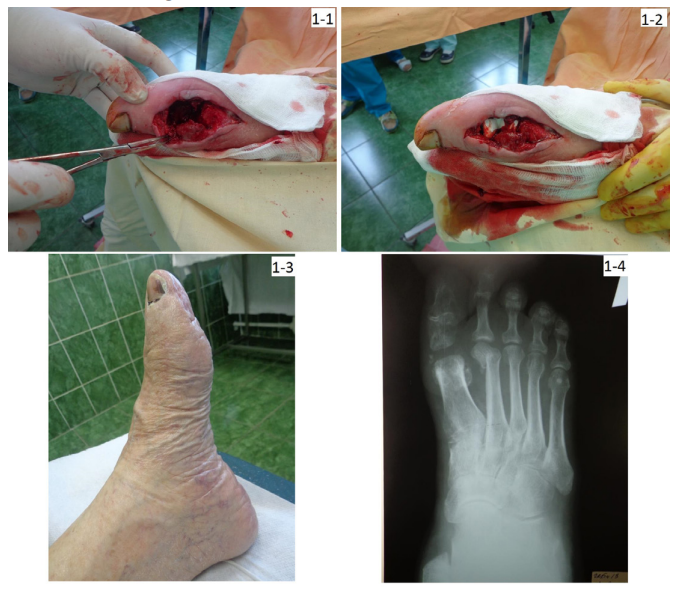
Figure 1: Patient L., age 86, diabetes mellitus of the 2 type, osteomyelitis of bones constituting 1 metatarsophalangeal joint; one-time AB prevention, 3 days in the hospital, foot offloading for 1.5 months; observation period 3.5 years:
1-1) view of the wound after joint resection; 1-2) wound filling with gentamicin-impregnated collagen implant, primary sutures without drainage; 1-3) view of the foot after 4 months; 1-4) foot X-ray after 8 months.

Figure 2: Patient K., age 58, diabetes mellitus of the 2 type, osteomyelitis of 2, 3 and 4 metatarsophalangeal joints, foot ulcer of the 3 degree acc. to Wagner’s classification, varus deformity of the 1 toe; AB prevention, antibiotic therapy for 7 days, 7 days in the hospital, foot offloading for 1.5 months; observation period 2 years 3 months:
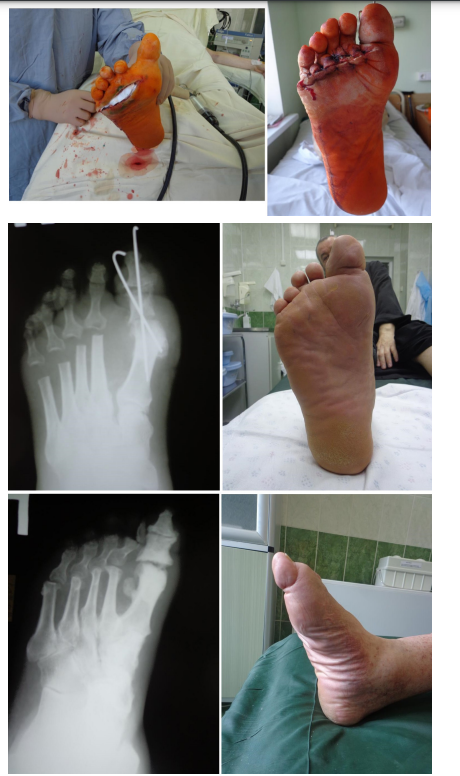
2-1) view of the foot in the initial consultation; 2-2) view of the foot before the surgery; 2-3) surgery type – panresection of metatarsophalangeal joints with ulcerectomy, osteosynthesis of the 1 metatarsophalangeal complex with Kirschner wires, wound filling with gentamicin-impregnated collagen implant, primary sutures without drainage; 2-4) view of the foot the next day; 2-5) X-ray control in 2 weeks; 2-6) view of the foot in 8 months; 2-7) X-ray control; 2-8) view of the foot in 1 year.
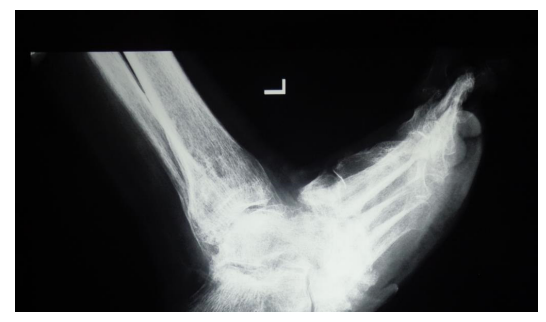
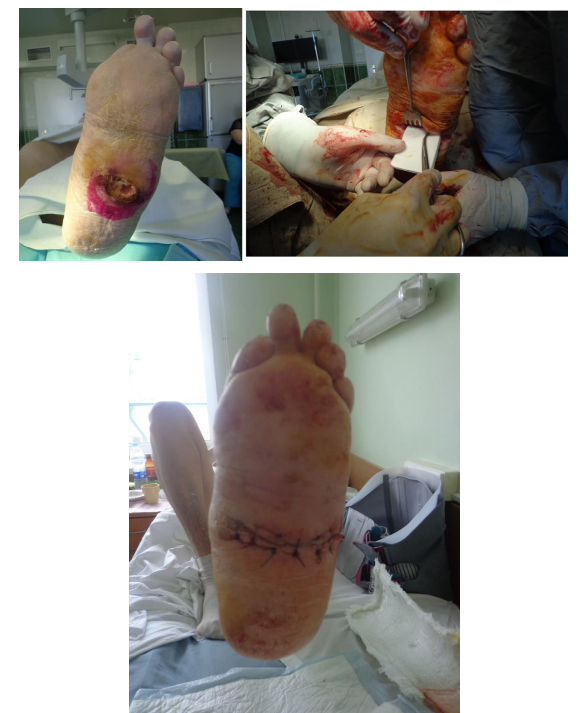
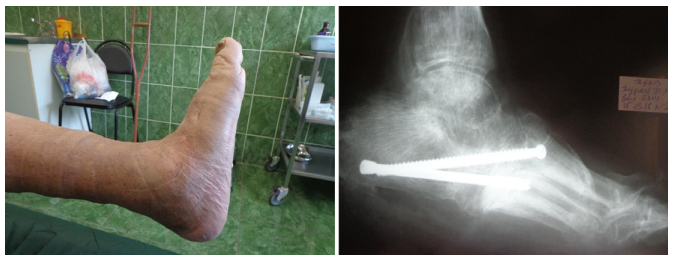
Figure 3: Patient K., age 60, diabetes mellitus of the 2 type, Charcot foot; previous resection of the 1 metatarsophalangeal complex; osteomyelitis of tarsal bones and subtalar joint bones, foot ulcer of the 3 degree acc. to Wagner’s classification, foot deformation; AB prevention, antibiotic therapy for 1 days, 17 days in the hospital, foot offloading for 3 months; observation period 1 year 8 months:
3-1) foot X-ray 3 months before the surgery; 3-2) view of the foot before the surgery; 3-3) tarsal bones resection, osteosynthesis with screws, wound filling with gentamicin-impregnated collagen implant, primary sutures without drainage; 3-4) view of the foot on the 3-rd day; 3-5) view of the foot and 3-6) X-ray control after 6 months.
Discussion
The obtained results can be considered positive, since patients with pathology classified in groups I and II normally undergo minor amputations (fingers or metatarsal amputations), while patients with pathology classified in groups III and IV, i. e. with Charcot's foot – major amputations.
Summarizing the results of our study, we would like to note the following:
- reduction of the total volume of systemic antibacterial therapy (accepted period of systemic antibacterial therapy in patients with osteomyelitis 4–12 weeks) –
- a.no systemic antibiotic therapy in patients of group I ;
- b.short course (1 week) of systemic antibiotic therapy in patients of groups II and III;
- c.reduced course (2–3 week) of systemic antibiotic therapy in patients of group IV;
- no early and late complications and relapses in patients of groups I and II;
- no early and small percentage (6.7 %) of late complications in patients of group III;
- no early complications and 25 % of late complications in patients of group IV;
- only one amputation in all of the treated patients
- and no foot correction loss in all of the rest 160
Long-term systemic antibiotic therapy can cause side effects and the risk of developing antibiotic resistance. At the same time local use of antibiotic-impregnated matrices allows to release high local concentrations of antibacterial drug for a long time with minimal blood concentration. This technology reduces the use of systemic antibiotics [9]. The use of collagen as a carrier matrix has a positive effect on tissue repair [36].
Conclusions
Local use of antibiotic-impregnated collagen matrices – PLAT – in surgical treatment of patients with diabetic foot syndrome in the purulent stage reduces the duration of systemic antibiotic therapy, minimizes the number of complications, improves the results of treatment and enhances the patient’s quality of life.
References
- Griffin FA. Best-practice protocols: preventing surgical site infection. Nurs Manage. 2005; 36: 22-26.
- Brennan TA, Leape LL, Laird NM, et Incidence of adverse outcomes and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. N Engl J Med. 1991; 324: 370-376.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical site infections in the 1990s attributable mortality, excess length of hospitalization, and extra Infect Control Hosp Epidemiol. 1999; 20: 725-730.
- Liau KH, Aung KT, Chua N, et al. Outcome of a strategy to reduce surgical site infection in a tertiary-care hospital. Surg Infect Larchmt. 2010; 11: 151-159.
- Rapetto F, Bruno VD, Guida G, et Gentamicin-Impregnated Collagen Sponge Effectiveness in Preventing Sternal Wound Infection in High-Risk Cardiac Surgery. Drug Target Insights. 2016; 10: 9-13.
- Schimmer C, Özkur M, Sinha B, et al. Gentamicin collagen sponge reduces sternal wound complications after heart surgery a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg. 2012; 143: 194-200.
- Schersten Modified prophylaxis for preventing deep sternal wound infection after cardiac surgery. APMIS. 2007; 115: 1025-1028.
- Beiner JM, Grauer J, Kwon BK, et Postoperative wound infections of the spine. Neurosurg Focus. 2003; 15: E14.
- Friberg Ö, Dahlin LG, Källman J, et al. Collagen-gentamicin implant for prevention of sternal wound infection; long-term follow-up of effectiveness. Interact Cardiovasc Thorac Surg. 2009; 9: 454-458.
- Friberg Ö, Svedjeholm R, Söderquist B, et al. Local gentamicin reduces sternal wound infections after cardiac surgery: a randomized controlled Ann Thorac Surg. 2005; 79: 153- 161.
- Darouiche Treatment of infections associated with surgical implants. N Engl J Med. 2004; 350: 1422-1429.
- Ruszczak Z, Friess Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliv Rev. 2003; 55: 1679- 1698.
- Pagkalis S, Mantadakis E, Mavros MN, et Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs. 2011; 71: 2277-2294.
- Leyh RG, Bartels C, Sievers Adjuvant treatment of deep sternal wound infection with collagenous gentamycin. Ann Thorac Surg. 1999; 68: 1648-1651.
- Wahlig Gentamicin-PMMA beads: a drug delivery system in the treatment of chronic bone and soft tissue infections. J Antimicrob Chemother. 1982; 10: 463-465.
- Sendil D, Gürsel I, Wise DL, et al. Antibiotic release from biodegradable PHBV J Control Release. 1999; 59: 207-217.
- Türesin F, Gürsel I, Hasirci V. Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic release. J Biomater Sci Polym Ed. 2001; 12: 195-207.
- Brown JC, Timpl The collagen superfamily. Int Arch Allergy Immunol. 1995; 107: 484-490.
- Ramshaw JA, Werkmeister JA, Glattauer Collagen-based biomaterials. Biotechnol Genet Eng Rev. 1996; 13: 335-382.
- Rao Recent developments of collagen-based materials for medical applications and drug delivery systems. J Biomater Sci Polym Ed. 1995; 7: 623-645.
- Bennett-Guerrero E, Ferguson TB, Lin M, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA. 2010; 304: 755-762.
- Lovering AM, Sunderland J. Impact of soaking gentamicin- containing collagen implants on potential antimicrobial efficacy. Int J Surg. 2012; 10: S2-S4.
- Schersten Modified prophylaxis for preventing deep sternal wound infection after cardiac surgery. APMIS. 2007; 115:1025-1028.
- Koziol M, Targoñska S, Stazka J, et Gentamicin-impregnated collagen sponge for preventing sternal wound infection after cardiac surgery. Kardiochir Torakochirurgia Pol. 2014; 11: 21-
- Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular Ann Surg. 2008; 248: 585-591.
- Chia CLK, Shelat VG, Low W, et al. The Use of Collatamp G Local Gentamicin-Collagen Sponge, in Reducing Wound Infection. Int Surg. 2014; 99: 565-570.
- Spruit, M, Bosman CH. Revision of failed below knee Local debridement with gentamicin collagen. Eur J Surg. 1994; 160: 267-270.
- Varga M, Sixta B, Bem R, et al. Influence of gentamicin- collagen sponge on wound healing after metatarsophalangeal joint resection in diabetic In: 20th conference of the European Wound Management Association, Geneva, Switzerland. 2010.
- Lipsky BA, Kuss M, Edmonds M, et al. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity a randomized, controlled, multicenter clinical trial. J Am Podiatr Med Assoc. 2012; 102: 223-232.
- Varga M, Sixta B, Bem R, et al. Application of gentamicin- collagen sponge shortened wound healing time after minor amputations in diabetic patients a prospective, randomised trial. Arch Med Sci. 2014; 10: 283-287.
- Knaepler H. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic Int J Surg. 2012; 10: S15- 20.
- Wagner A classification and treatment program for diabetic, neuropatic and dysvascular foot problems. In The American Academy of Ortopaedic Surgeons instructional course lectures. St. Louis: Mosby Year Book. 1979; 143-165.
- Eichenholtz SN. Charcot joints. With a foreword by P.D. Wilson. Springfield Ill Charles C. Thomas; 1966. Shibata T, Tada K, Hashizume The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am. 1990; 72: 749-756.
- Sanders L, Frykberg R. Diabetic neuropathic osteoarthropathy: The Charcot foot. In: Frykberg RG, editor. The high risk foot in diabetes mellitus. 1991; 325-333.
- Rogers LC, Frykberg RG, Armstrong DG, et al. The Charcot foot in diabetes. Diabetes Care. 2011; 34: 2123-2129.
- Stemberger A, Grimm H, Bader F, et al. Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur J Surg. 1997; S578: 17-26.