Epidemiological and Prognostic Aspects of Secondary Brain Insults of Systemic Origin in Patients Operated for Intracranial Tumor in Low-Resource Settings
Author'(s): Paul Owono Etoundi1,2*, Roddy Stephan Bengono Bengono1, Albert Ludovic Amengle1, CristellaIroume1, Bonaventure Jemea1,2 and Ze Minkande Jacqueline1
1Department of Surgery and Specialties, Anaesthesiology, University of Yaoundé 1, Cameroon.
2Department of Anaesthesiology, Yaoundé Central Hospital,Cameroon.
*Correspondence:
Owono Etoundi Paul, Department of Surgery and Specialties/ Anaesthesiology, University of Yaoundé 1, P.O. Box 1364 Yaoundé, Cameroon, Tel: +237 677 981 951.
Received: 15 Nov 2022; Accepted: 18 Dec 2022; Published: 22 Dec 2022
Citation: Etoundi PO, Bengono RSB, Amengle AL, et al. Epidemiological and Prognostic Aspects of Secondary Brain Insults of Systemic Origin in Patients Operated for Intracranial Tumor in Low-Resource Settings. Anesth Pain Res. 2022; 6(2): 1-4.
Abstract
Background: Secondary Brain Insults of Systemic Origin (SBISO) are factors that can aggravate a primary brain injury regardless of its origin (traumatic, vascular, infectious, tumoral, or surgical). The aim was to describe the type and frequency of SBISO occurring after intracranial surgery indicated for intracranial tumors, and to establish the prognosis of patients presenting with these SBISO.
Methods: This was a prospective and descriptive study carried out at the ICU of the Yaoundé Central Hospital between January 1st, 2012 to December 31st, 2019. We enrolled patients operated for intracranial tumors and admitted in the ICU. The variables studied were data from the preoperative, intraoperative and postoperative periods. Data analysis was performed using Epi Info 3.5.4 software. The chi-square test was used, the p-value being considered significant if < 0.05.
Results: 93 patients were included during the period of study. The mean age was 45 ± 17.1 years and a sex- ratio of 1.27. We found 604 SBISO in 70 patients (75.3%). The most common SBISO were hypoxia (44.7%) and hyperglycemia (30%). The risk factors for the occurrence of SBISO were age ≥ 60 years (p=0.02), history of hypertension (p=0.005), patients classified ASA II (p=0.007) and intraoperative complications (p ? 0.001). The occurrence of SBISO was associated with a high mortality risk.
Conclusion: SBISO are common in patients operated for intracranial tumors. Mortality was increased by the presence of SBISO.
Keywords
Introduction
Brain tumors are common. Data available report an incidence of primary central nervous system tumors around 6 to 20 per 100,000 people per year. The apparent increase in the incidence of brain tumors over the last decades observed by some authors seems mainly to be related to the improvement in early diagnosis since the advent of CT scan and MRI [1,2]. The surgical managemen of intracranial tumors is well codified. Common considerations in anesthetic management of brain tumors are: to maintain cerebral autoregulation and reactivity to CO2, optimization of brain compliance, to protect against Secondary Cerebral Brain Insults of Systemic Origin (SBISO) [3]. SBISO are factors that can aggravate a primary brain injury regardless of its origin (traumatic, vascular, infectious, tumoral, or surgical) [4].
In France, Proust et al. found 35% of metabolic and hemodynamic complications in patients operated of brain tumor in 2010 [5]
The management of operated patients in the intensive care unit during the postoperative period permits early recognition of any neurological deterioration and helps keep a good homeostasis and neurologic stability. The aim was to describe the type and frequency of SBISO occurring after intracranial surgery indicated for intracranial tumors, and to establish the prognosis of patients presenting with these SBISO.
Patients and Methods
Study Design, Setting and Population
This was a prospective descriptive cohort study conducted at the ICU of Yaoundé Central Hospital from January 1, 2012 to December 31, 2019. After approval by the National Ethics Committee and obtaining written informed consent from patients or their legal guardians, patients operated for brain tumor were admitted to the ICU after surgical intervention, done under general anesthesia. During their hospitalization in ICU, the control and detection of SBISO were done clinically and paraclinically. The appearance of possible ACSOS was noted and their management was ensured. Then the daily evaluation of these patients was done until the exit of the ICU.
Study Variables and Data Analysis
The variables studied were: the preoperative data (age, sex, comorbidities, ASA classification, tumor location), intraoperative data (intraoperative complications, length of surgery) and postoperative data (identified SBISO and treatment outcomes). Data analysis was performed using Epi info 3.5.4 statistical software. Means and standard deviations of numerical variables were reported. Categorical variables were analyzed using the Fisher's exact test or the Pearson's Chi-square test where appropriate. Qualitative variables were analyzed using the Student t-test or the Kruskal-Wallis test where appropriate. The threshold for statistical significance was set a p-value less than 0.05.
Results
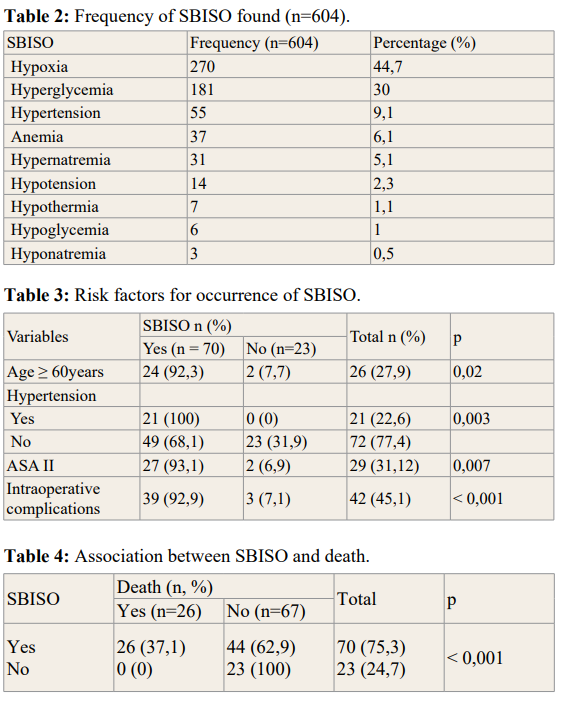
During the study period, 93 patients were included. The mean age of the population was 45.8 ± 17.1 years (extreme 18 and 76 years) and the sex ratio was 1.27 (Table 1). The main comorbidity encountered was arterial hypertension (22.6%) and diabetes (17.2%). The majority of patients were classified ASA I. Tumor location, intraoperative complications and duration of surgery are illustrated in table 1. We highlighted 604 ACSOS in 70 (75.3%) patients. The most frequent were hypoxia (44.7%) and hyperglycemia (30%) (Table 2). The risk factors for the occurrence of SBISO were age ≥ 60 years (p=0.02), history of hypertension (p=0.005), patients classified ASA II (p=0.007), and the presence of intraoperative complications (p Ë? 0.001) (Table 3). Mortality rate was 28% (n=26). The occurrence of SBISO was associated with a high risk of death (Table 3).
Discussion
Intracranial surgery has been performed at the Yaoundé Central Hospital for several years. The present study objective was to present our experience in the management of patients, which
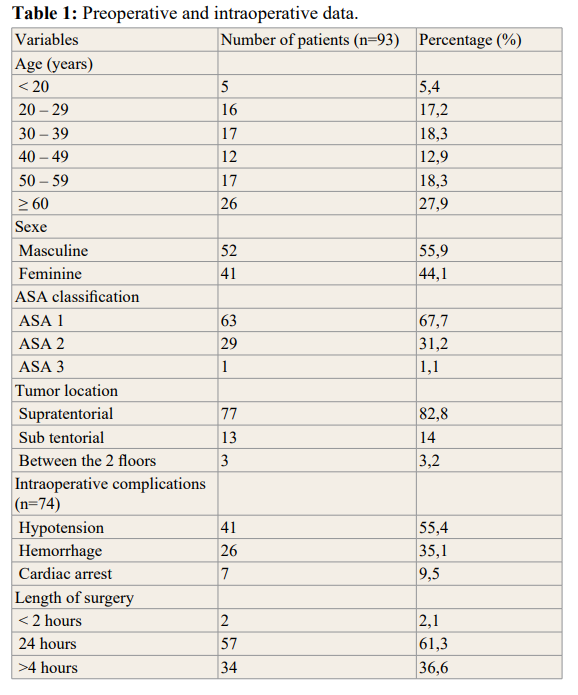
presented SBISO after intracranial surgery indicated for intracranial tumors. Among the 93 operated patients, 70 presented SBISO. The mean age in our study was 45.8 years. Patients aging 60 years and above was the most represented age group (27.9%) with a sex ratio of 1.27. This is similar to the results of the studies of Egbohou et al., finding a mean age of 49 ± 20 years with a masculine predominance (52.4%). The incidence of brain tumors in adults increases steadily with age. Brain tumors are more common in men with a sex ratio of 1.1 to 1.6, the only exception being meningiomas [1,6]. Supratentorial tumors were predominant (82.8%). This agreed with data from the literature. Tumors in adults are mainly located at the supratentorial level according to the literature [1,6,7].
The main intraoperative complications were bleeding and hypotension. Management of this hypovolemia induced by bleeding was handled with fluid resuscitation with normal saline and colloids, but also with homologous transfusions. This was similar to that observed by Egbohou et al, whose intraoperative complications consisted essentially of bleeding, with significant blood loss causing hypotension, hemorrhagic shock and cardiocirculatory arrest [3].
The incidence of SBISO was 75.3% in our series. This was comparable to studies by Ilunga et al. in Congo in 2014 who found 79% of SBISO [8]. Proust et al. in 2010 found a 35% incidence of SBISO [5]. On the other hand, Brell et al. in a population of 200 patients operated for brain tumors reported an incidence of SBISO of 27.5% [9]. This was justified by the fact that the definition of SBISO varied according to the authors, both in terms of threshold and duration [10]. Major complications after intracranial surgery occur in 13–27% of patients [2,11]. These complications can be neurologic, hemodynamic, metabolic or respiratory [12,13]. The main SBISO were hypoxia (44.7%), hyperglycemia (30%), hypertension (9%), anemia and hypotension (8.4%). According to Lonjaret, hypertension was the most common SBISO found in the postoperative period (9%). Hyperglycemia was found in 6% of patients. No respiratory complications were found [11]. Anemia and hypotension were related to intraoperative hemodynamic impairments (intraoperative bleeding, use of halogen). Hypotension is a serious SBISO that should be managed early, as it leads to the reduction of cerebral perfusion with a risk of cerebral ischemia. A mean blood pressure of 80 mmHg is recommended for a sufficient cerebral perfusion pressure (CPP) of 60 mmHg to avoid cerebral ischemia [3,14,15]. Postoperative cerebral ischemia is an important complication that alters the outcome of brain surgery. Moreover, these secondary ischemic lesions, of surgical origin, can be favored and aggravated by certain systemic conditions such as hypotension, hypoxemia, and hypercapnia [16].
The risk factors for the occurrence of SBISO were age ≥ 60 years, history of hypertension, patients classified ASA II, and the presence of intraoperative complications. The length of surgery had no impact on the occurrence of SBISO. According to the study made by Sawaya et al. age is a factor that can influence th
incidence of postoperative complications of brain tumor surgery. Indeed, patients who were above 61 years old had a high frequency of complications [17]. Asano et al. reported a risk of developing postoperative complications, namely SBISO in the 84 patients aged 70 years and above operated for brain tumors [18]. Age appears to be a determining factor in the onset and worsening of SBISO factors. In a retrospective study carried out in France by Marescal [19], it was found that the incidence of SBISO increases significantly with the age of patients with head trauma, hence it is the injured patient aged above 40 years who are secondarily aggravated due to systemic disorders. The presence of a serious pathology or an underlying comorbidity increases the risk of postoperative complications according to the study by Brell et al. [9]. All patients with SBISO had at least a past medical history, including hypertension (n=48, 37.5%). This also explained the fact that the ASA II class represented a risk factor. The higher the ASA class, the more the patient runs anesthetic risks and therefore deserves keen attention [20]. Western studies revealed that the risk factors for intraoperative complications after craniotomy were elderly patients, diabetes, length of surgery > 4 hours and major intraoperative bleeding [11,21,22].
Mortality risk is significantly high for patients with SBISO with a p-value < 0.001. The occurrence of SBISO postoperatively is strongly associated to mortality. All deceased patients had SBISO. Likewise, in the Proust [5] and Ilunga [8] studies, SBISO were mainly associated to mortality.
Limitation
The limitations of our study were the monocentric nature and the lack of equipment necessary for the detection of SBSO such as capnogram, arterial blood gas analysis. This equipment defect would contribute to the non-discovery of certain SBISO which, uncorrected, participated in the increase in the death rate.
Conclusion
SBISO are common in patients operated for brain tumor. Age ≥ 60 years, past medical history of hypertension, ASA class II and intraoperative complications influence the occurrence of SBISO. The presence of SBISO increases mortality risk.
Acknowledgement
They go to all the authors who have participated in an active, honest and regular way in the effective realization of this work. Their rigor in the analysis of the data highlighted the results presented. Thanks also go to all patients hospitalized in intensive care during the study period.
References
- Gray F, Mokhtari K, Poirier Epidémiologie. Tumeurs cérébrales du diagnostic au traitement. Edition Masson Paris. 2004; 3-6.
- Lantos PL, Vandenberg SR, Kleihues Tumours of the nervous system. in: Digraham plantos, greenfidds. Neuropathology Arnold. 1997; 2: 583-879.
- Egbohou P, Mouzou T, Beketi K, et al. Les méningiomes intracrâniens opérés au CHU Sylvanus Olympio de Lomé: aspects anesthésiologiques et complications: à propos de 21 Pan African Medical Journal. 2017; 28: 42-48.
- Moeschler O, Boulard G, Ravussin P. Concept d’agression cérébrale secondaire d’origine systémique (ACSOS). Ann Fr Anesth Réan. 1995; 14: 114-121.
- Proust S, Houdemont E, De Carli M, et al. Complications postopératoires des tumeurs cérébrales pédiatriques : expérience du CHU d’Angers. Archives de Pédiatrie. 2010; 17: 26.
- Vallat AV, Pairier J, Gray F, et Tumeurs du système nerveux central, classifications histologiques et topographiques, épidémiologique. EMC Neurologie. 2015; 17: 197-199.
- Gray F, Poirier Classifications et grading des tumeurs cérébrales. Tumeurs cérébrales du diagnostic au traitement. Paris. 2004; 7-15.
- Ilunga Agressions cérébrales secondaires dorigine systémique chez les cérébrolésés en réanimation des cliniques universitaires de Kinshasa. Rev Afr Anesthésiol Med Urgence. 2017; 22: 89-93.
- Brell M, Ibanes J, Caral L, et al. Factors influencing surgical complications of intraaxial brain tumors. Acta neuchirurgia. 2000; 142: 739-750.
- Schoettker Chioléro Agression cérébrale secondaire d’origine systémique. La reanimation neurochirurgicale. Springer Science Business Media. 2003; 72-78.
- Lonjaret L, Guyonnet M, Berard E, et Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017; 36: 213-218.
- Badiane SB, Sokho Y, Bam C, et Méningiomes intracrâniens: expérience dakaroise à propos de 79 cas. Neurochirurgie. 1999; 45: 134-139.
- Thiam AB, Kessely YC, Thioub M, et Notre expérience de méningiomes intracrâniens à Dakar: à propos de 50 cas. Pan Afr Med J. 2015; 16: 379-383.
- Bruder Anesthésie et hypertension intracrânienne sur œdème cérébral. Ann Fr Anesth Reanim. 2003; 22: 226-234.
- Devys JM, Bonnet F, Lembert Anesthésie en neurochirurgie. Le livre de l’interne Anesthésiologie. Paris Flammarion. 2006; 478-485.
- Giljenbeek JMM, Hop WCJ, Braakman R, et al. Surgery for intracranial meningiomas in the elderly Clinical Neurology and Neurosurgery. 1993; 95: 291-295.
- Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Clinical article Journal of 1998; 42: 1044-1055.
- Asano K, Nakano T, Takeda T, et Risk factors for postoperative systemic complications in elderly patients with brain tumors. Clinical article. Journal of Neurosurgery. 2009; 111: 258-264.
- Marescal C, Adnet P, Bello N, et al. Agressions cérébrales secondaires d’origine systémique chez les enfants traumatisés crâniens et cérébraux graves. Ann francaise d Anest Réan. 1998; 17: 234-239.
- Jawad M, Baigi A, Oldner A, et Swedish surgical outcomes study: An observational study on 30-day and 1-year mortality after surgery. EJA. 2016; 33: 317-325.
- Rhondali O, Genty C, Halle C, et al. Do patients still require admission to an intensive care unit after elective craniotomy for brain surgery? J Neurosurg Anesthesiol. 2011; 23: 118-
- Hanak BW, Walcott BP, Nahed BV, et Postoperative intensive care unit requirements after elective craniotomy. World Neurosurg. 2014; 81: 165-172.