Evaluation of Ferritin, Vitamin D, Total Oxidant and Antioxidant Status in Patients With Behçet's Disease
Author'(s): Gülten Toprak1 , Ilhan Sabancilar2, Nida özcan3 and Beran YokuÅ?2
1Dicle University, Medical Faculty, Dept. of Medical Biochemistry
2Dicle University, Veterinary Faculty, Dept. of Biochemistry.
3Dicle University, Medical Faculty, Dept. of Medical Microbiology.
*Correspondence:
Nida özcan, Dicle University, Medical Faculty, Dept. of Medical Microbiology, Sur, Diyarbakir, Turkey, Tel: 905052710103.
Received: 30 April 2020; Accepted: 21 May 2020
Citation: : Gülten Toprak, Ilhan Sabancilar, Nida özcan, et al. Evaluation of Ferritin, Vitamin D, Total Oxidant and Antioxidant Status in Patients With Behçet's Disease. Clin Immunol Res. 2020; 4(1): 1-5.
Abstract
Background & Aim: Behçet's Disease (BD) is a chronic vascular-inflammatory multisystemic disease. Infectious, psychological, genetic and immunological factors are thought to play role in BD. Recent studies have shown that oxidative stress is associated with various diseases. In this study, some oxidative stress parameters were investigated in patients with BD to investigate the relationship of the disease with oxidative stress.
Materials & Methods: The study included 35 patients with BD who admitted to the dermatology clinic of Dicle University Hospital and 37 healthy individuals as the control group. Total Oxidant Status (TOS), Total Antioxidant Status (TAS), Paraoxonase (PON-1), Ferritin, Folic Acid (folate) and D-vit levels were examined in the serum samples of both groups included in the study.
Results: Compared to the control group, serum TOS, ferritin and folate levels were significantly higher in patients with BD, while TAS and D-vit levels were significantly lower(p<0.05). PON-1 level was found lower in BD patients than the control group, but the difference was not statistically significant.
Conclusion: High oxidative stress and degraded antioxidant system parameters in patients with BD, may indicate the role of oxidative stress in the pathogenesis of the disease.
Keywords
Introduction
Behçet's Disease (BD) is a multisystemic inflammatory disease characterized by genital ulcer, skin lesions and recurrent ocular symptoms [1]. Although the etiology of BD is not fully known, genetic, environmental and immunological factors are thought to cause this disease [2]. Reactive oxygen metabolites produced by activated neutrophils during the inflammatory response increases the oxidative stress in BD. It has been reported that decreased antioxidant system activity and increased level of free radicals in BD may play a role in tissue damage [3]. Increased chemotoxic and phagocytic functions of neutrophils and excessive production of reactive oxygen metabolites such as superoxide anions may be responsible for oxidative tissue damage in BD. On the other hand, nonenzymatic antioxidants such as vitamins and ferritin can prevent tissue damage caused by reactive oxygen species [4]. Previous studies suggested that inflammation may cause oxidant / antioxidant imbalance of molecular and cellular mediators in Behçet's disease. Thus upregulation of the oxidant system triggered by the inflammation process may lead to decreased antioxidant capacity [5].
Paraoxanase (PON-1) is a calcium-dependent esterase associated with high-density lipoprotein. PON-1 metabolizes lipid peroxides and protects low-density lipoprotein from oxidation. PON-1 contains cysteine thiol groups that mediate in protecting low- density lipoproteins against atherogenic oxidative damage [6].
Iron is essential for the proliferation and growth of the living but abundant iron may be harmful by leading to produce toxic reactive oxygen species (ROS). The chief regulatory mechanism to control intracellular iron levels is the storage of iron in ferritin. Ferritin plays a major role as an antioxidant in iron metabolism since it can store iron in non-toxic and harmless forms [7,8].
Vitamins are useful for the catalysis of many enzymatic reactions in human body [9]. D-vit deficiency is a common healthcare issue and is thought to play a role in many autoimmune, vascular and metabolic diseases [10]. It was reported that D-vit deficiency can cause vascular dysfunction by means of endothelial inflammation [1].
This study aims to investigate the levels of total oxidant status (TOS), total antioxidant status (TAS), ferritin, PON-1, folate and D-vit in BD and to determine the relationship of these parameters with the pathogenesis of the disease.
Materials and Methods
Study Groups
Two groups were compared in the study; patients with BD constituted the case group, while there were healthy volunteers of similar age in the control group.The patients in the case group were 19 female and 16 male patients aging 18-67 years, who applied to Dicle University Hospitals Dermatology outpatient clinic. The control group consisted of 37 healthy people aging between 22 and 65 years, 24 of which were women and 13 were men.
The diagnosis of Behçet's Disease was made according to the Behçet's Disease criteria of The International Study Group for Behçet’s Disease [11]. The study was initiated after approval by Dicle University Medical Faculty Non-Interventional Ethics Committee. All patients and control subjects gave informed consent for the study.
Biochemical Analysis
Venous blood samples were collected from the case and control groups under aseptic conditions and transferred to tubes without anticoagulants. The blood samples were centrifuged at 4000 rpm for 5 minutes, and the sera were stored in ependorf tubes at -80°C till the testing time.
At the time of the test, all samples dissolved at once, TAS, TOS, ferritin, folate, PON-1 and D-vit levels were analysed in both groups.
Folate, D-vit and ferritin were analyzed with Cobas 601 (Roche Diagnostics, USA) autoanalyser by electro-chemiluminescence method. TAS, TOS and PON-1 levels were studied by Erel method with Rel Assay Diagnostics (Mega Medicine, Gaziantep, Turkey) kits. The analysis was performed on the ARCHITECT c16000 Abbott Diagnostics, USA) autoanalyser system. TAS and TOS results were expressed as mmol Trolox equivalent/l and μmol h3O2 equivalent/l, respectively [12,13].
Statistical Analysis
SPSS (version 21.0 for Windows; IBM Corporation, Armonk, NY, USA) package program was used for statistical evaluation of the data. The results were expressed as mean ± standard deviation using descriptive statistics. Student's t test was used to compare the case and control groups. Correlations between the data of each group were calculated with Pearson's r coefficient. P <0.05 value was accepted for statistical significance level.
Results
The mean age of the case and control groups were 32.66 ± 6.93 and 30.20 ± 8.80, respectively. There was no significant difference between the average age of the groups. Compared to the control group, the case group had a significantly higher TOS level (p <0.05) and significantly lower TAS level (p <0.05).
The low PON-1 level in the case group was not statistically significant (p>0.05). Compared to the control group, ferritin and D-vit levels were found to be significantly lower (p = 0.012; p = 0.022, respectively), while folate level was significantly higher (p = 0.002) (Table 1).
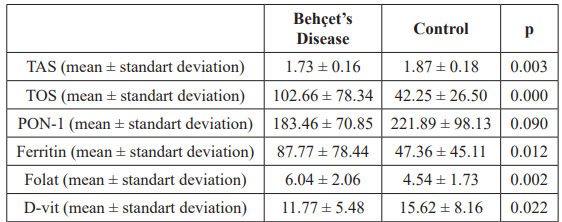
Table 1: Comparison of Behçet's Disease and control group in terms of oxidative stress parameters.
The correlations of the parameters in both groups were investigated with the Pearson r Test. TAS and D-vit parameters were positively correlated among healthy subjects (r=0.601). In the patients group, a positive correlation was found between D-vit and folate as well as ferritin and TAS with r values of 0.421 and 0.375, respectively. The correlation tests of control group and BD patients are summarised in Table 2 and 3.
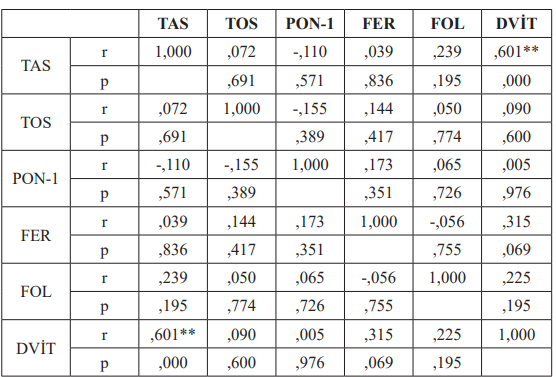
Table 2: Correlation of the parameters in the control group (Pearson r Test).
were significantly lower in both active and inactive BD patients compared to healthy subjects [21]. Decreased serum PON-1 activity in BD results from increased reactive oxygen production and inactivation of PON-1 activity. It has been suggested to be explained by the increase observed in lipid peroxidation and oxidative stress [3].
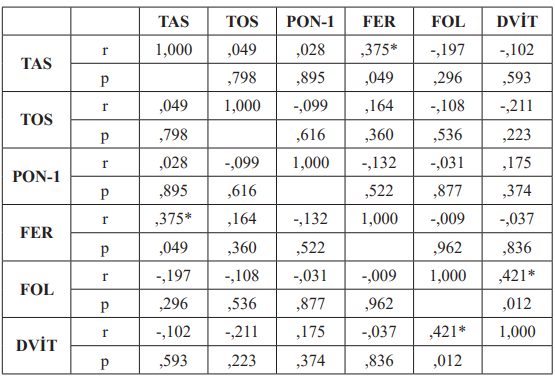
Table3: Correlation of the parameters in Behçet’s Disease group (Pearson r Test).
Discussion
Environmental, genetic and immunological factors contribute to BD etiopathogenesis. Reactive oxygen species lead to peroxidation of double-chain fatty acids in the cell membrane, thereby causing cellular damage and oxidative stress. In contrast, the antioxidant defense system protects cells from possible damage of oxidants [14]. TAS shows the general function of enzymatic and nonenzymatic antioxidants in plasma. It is also a sign of superoxide radical capture activity in plasma [15]. As a marker of antioxidant activity, TAS was significantly lower in BD patients compared to healthy groups.
However TOS, indicating oxidative status, was higher in BD patients than control group. Our results were compatible with previous studies. Turkmen et al., evaluated 49 patients with BD and 26 healthy subjects. TAS levels were lower in BD patients than control subjects [16]. Onur et al., revealed mean TAS levels in BD patients and control group as 1.19 ± 0.34 and 3.29 ± 0.89 mmol/L, respectively. The difference was statistically significant (p<0.05) [17].
In BD, reactive oxygen species are produced in high amounts since the functions of neutrophils in peripheral blood increase [18,19]. Reactive oxygen species can adhere to almost all cellular components and give damage to the surrounding tissues. This mechanism has a very important and possibly causative role in the pathogenesis of many diseases.
PON-1 is an antioxidant that hydrolyzes lipid peroxides [20]. In current study, it was found lower in patients with BD compared to healthy subjects but the difference was not statistically significant. Karakucuk et al., evaluated paraoxonase1 (PON-1) and malondialdehyde (MDA) levels in 16 patients with BD and 15 healthy subjects. Compared to the control group; PON 1 was found significantly lower, while MDA was significantly higher in patients with BD [3].
In another study conducted by Mungan et al., PON-1 levels Ferritin expression is regulated by intracellular iron, inflammatory cytokines and oxidative stress. Ferritin is reported as an important regulator of the immune system and serum ferritin level is increased in some autoimmune diseases, especially with disease activity [7,8]. In our study, serum ferritin level in BD group was found to be significantly higher than the control. Elevated serum ferritin level may be associated with inflammatory status and oxidative stress. Challacombe et al. reported that approximately 15% of BD had a low ferritin prevalence and serum ferritin was stated as a biomarker for BD [22].
OdabaÅ? et al. found that serum ferritin was significantly higher in active BD and suggested that ferritin could be an activity criteria for BD [23]. Gönül et al., reported that serum ferritin levels were not different between BD and control groups but were significantly higher in BD patients with papulopustular lesions and thrombophlebitis [3,24].
Serum folate level was significantly lower in Behçet patients compared to the control group in current study. Mungan et al. reported serum folate levels within the reference ranges in BD patients [21]. In a study conducted by YeÅ?ilova et al., serum folate levels were found lower in BD patients compared to the control group. Also folate levels were found lower in BD patients with eye involvement and thrombosis compared to those without. The authors attributed low serum folate levels to increased catabolism due to inflammation [25]. In contrast, Türsen et al., reported high serum folate levels in BD patients. They attributed the low incidence of vascular and ocular involvement of patients included in the study to the beneficial effect of high folate levels [26]. Folate level differences between studies may be due to the duration of the disease, medications or vitamin supplements.
Vitamin D has an important regulatory role in the immune system [27]. Vitamin D level was found significantly lower in patients with BD compared to healthy subjects. Our findings were compatible with previous studies. Hamzaoui et al. evaluated some inflammatory mediators and vitamin D levels in active and passive BD patients. Vitamin D levels were low in active BD patients compared to inactive patients and healthy controls. Vitamin D levels were low in active BD patients compared to inactive patients and healthy controls [2].
In a study conducted in 2012, serum vitamin D levels were determined in Behçet's patients, and then the effect of vitamin D supplementation on disease activity and endothelial functions was investigated. Vitamin D levels were low in 61.1% of Behçet's patients and in 35.3% of the control group. Endothelial function was evaluated by brachial artery flow-mediated dilation (FMD) and ultrasonic examination. It was observed that vitamin D supplementation lasting three months had positive effect on endothelial functions in Behçet patients with vitamin D deficiency.
It has been stated that serum vitamin D level was low in active Behçet patients and vitamin D deficiency may have a role in disease activation and inflammatory process [28]. Kandi et al., evaluated some vitamin levels among patients with active and inactive BD. Serum Vitamin D levels were lower in patients with BD than control group subjects but the difference wasn’t statistically significant [9]. In a cross-sectional study conducted by Khabbazi et al., serum Vitamin D levels were measured in 48 BD patients and 47 control subjects. The mean Vitamin D level in patients with BD was found lower than the control subjects. In the study, vitamin D deficiency was reported to be higher in the patient group compared to the control group [29].
Conclusion
Our findings suggests that patients with BD are exposed to oxidative stress reflected by increased TOS and ferritin levels. In addition, we have noted a degraded antioxidant capacity reflected by low TAS, folate and Vitamin D levels. Our findings may assist the literature on BD pathogenesis. Oxidative stress parameters may be useful in clinical and treatment monitoring of the disease.
Acknowledgement
Approval for this study was obtained from the Non-Interventional Ethics Committee of Dicle University in 2020 (No: 90).
References
- Güngör Å?, Gökdemir G, Çiçek YG, et The effect of 25(OH) D on endothelial and immunological markers in Behçet’s disease. J Dermatolog Treat. 2016; 27: 254–259.
- Hamzaoui K, Dhifallah IB, Karray E, et al. Vitamin D modulates peripheral immunity in patients with Behçet’s disease. Clin Exp Rheumatol. 2010; 28: 50–57.
- Karakucuk S, Baskol G, Oner AO, et al. Serum paraoxonase activity is decreased in the active stage of Behçet’s Br J Ophthalmol. 2004; 88: 1256–1258.
- Dormohammadi Toosi T, Shahram F, Ghodsi SZ, et al. Iron deficiency state in resistant oral aphthosis of Behcet’s Int J Rheum Dis. 2014; 17: 425–429.
- Sepici-Dinçel A, Özkan Y, Yardim-Akaydin S, et al. The association between total antioxidant status and oxidative stress in Behçet’s Rheumatol Int. 2006; 26: 1005– 1009.
- Aviram M, Billecke S, Sorenson R, et al. Paraoxonase Active Site Required for Protection Against LDL Oxidation Involves Its Free Sulfhydryl Group and Is Different From That Required for Its Arylesterase/Paraoxonase Activities. Arterioscler Thromb Vasc Biol. 1998; 18: 1617–1624.
- Orino K, Watanabe K. Molecular, physiological and clinical aspects of the iron storage protein ferritin. Vet J. 2008; 178: 191–201.
- Orino K, Lehman L, Tsuji Y, et Ferritin and the response to oxidative stress. Biochem J. 2001; 357: 241–247.
- Kandi B, Çicek D, Ilhan Vitamin levels in Behçet’s disease.J Dermatolog Treat. 2007; 18: 69–75.
- Ravid A, Koren R. The Role of Reactive Oxygen Species in the Anticancer Activity of Vitamin In: Vitamin D Analogs in Cancer Prevention and Therapy. Recent Results Cancer Res. 2003; 357–367.
- Duffy O, Kökmen E, editors. International Study Group for Behçet’s Disease Contributors. Evaluation of Diagnostic (“Classification”) Criteria in Behçet’s Disease: Toward Internationally Agreed Criteria. In: Behçet’s Disease Basic and Clinical Aspects. New York: Marcel Dekker Inc; 1991. 11–39.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 38: 1103–1111.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; 37: 277–285.
- IÅ?ik A, Koca S, Üstündag B, et Decreased total antioxidant response and increased oxidative stress in Behcet’s disease. Tohoku J Exp Med. 2007; 212: 133–141.
- Bozkurt M, Yüksel H, Em S, et al. Serum prolidase enzyme activity and oxidative status in patients with Behçet’s Redox Rep. 2014; 19: 59–64.
- Turkmen S, Ayabakan HB, Buldanlioglu S, et Nitric oxide, lipid peroxidation and antioxidant defence system in patients with active or inactive Behçet’s disease. Br J Dermatol. 2005; 153: 526–530.
- Onur E, Kabaroglu C, Inanir I, et al. Oxidative stress impairs endothelial nitric oxide levels in Behçets’ Cutan Ocul Toxicol. 2011; 30: 217–220.
- Niwa Y, Miyake S, Sakane T, et al. Auto-oxidative damage in Behçet’s disease--endothelial cell damage following the elevated oxygen radicals generated by stimulated Clin Exp Immunol. 1982; 49: 247–255.
- Takeno M, Kariyone A, Yamashita N, et Excessive function of peripheral blood neutrophils from patients with behcet’s disease and from hla-b51 transgenic mice. Arthritis Rheum. 1995; 38: 426–433.
- Köse K, Yazici C, Çambay N, et al. Lipid Peroxidation and Erythrocyte Antioxidant Enzymes in Patients with Behçet’s Disease. Tohoku J Exp Med. 2002; 197: 9–16.
- Mungan A, Can M, Acikgöz S, et al. Lipid peroxidation and homocysteine levels in Behcet’s disease¸A disease¸ disease¸A. Clin Chem Lab Med. 2006; 44: 1115–1123.
- Challacombe SJ, Scully C, Keevil B, et al. Serum ferritin in recurrent oral ulceration. J Oral Pathol Med. 1983; 12: 290–
- Odabas AR, Karakuzu A, Cetinkaya R, et Increased serum ferritin levels in active Behçet’s disease. Int J Clin Pract. 2002; 56: 310–311.
- Gonul M, Gul U, Cakmak S, et al. Serum iron and ferritin levels in Behηet′s Indian J Dermatol Venereol Leprol. 2010; 76: 85.
- Yesilova Z, Pay S, Oktenli C, et Hyperhomocysteinemia in patients with Behçet’s disease: is it due to inflammation or therapy? Rheumatol Int. 2005; 25: 423–428.
- Türsen Ü, Çimen B, Api H, et al. Serum Erythropoietin, Interleukin-4, Ferritine, Iron, Total Iron Binding Capacity, Vitamin B12, Folate Levels in Patients with Behçet’s DiseaseArticles. J Turkish Acad Dermatology. 2011; 5: 1–5.
- Szodoray P, Nakken B, Gaal J, et al. The Complex Role of Vitamin D in Autoimmune Scand J Immunol. 2008; 68: 261–269.
- Can M, GüneÅ? M, Haliloglu O, et al. Effect of vitamin D deficiency and replacement on endothelial functions in Behçet’s disease. Clin Exp Rheumatol. 2012; 30: 57–61.
- Khabbazi A, Rashtchizadeh N, Ghorbanihaghjo A, et al. The status of serum vitamin D in patients with active Behcet’s disease compared with controls. Int J Rheum Dis. 2014; 17: 430–444.