Evaluation of Performance of Robotic-Assisted Coronary Angioplasty System Over Conventional/Manual PCI
Author'(s): N John Camm1* and Sherrie Singh2
1Klinikum Nurnberg Hospital, Germany
2Leicester Medical School, UK.
*Correspondence:
Dr. N John Camm, Klinikum Nurnberg Hospital, Germany.
Received: 01 Oct 2022; Accepted: 15 Nov 2022; Published: 20 Nov 2022
Citation: Camm NJ, Singh S. Evaluation of Performance of Robotic-Assisted Coronary Angioplasty System Over Conventional/Manual PCI. Cardiol Vasc Res. 2022; 6(5); 1-5.
Abstract
Most percutaneous coronary intervention (PCI) might present hazards for patients, procedure operators, and the laboratory staff [1-3]. Due to complex nature of lesions and procedures, both patients and laboratory team may be subjected to exposure of longer procedural duration and radiation [3]. The long hours of standing for operators while wearing a lead apron commonly leads to exhaustion and injuries that frequently results in reduced performance and sub optimal clinical results [1]. A remote-control, robotic-assisted angioplasty system was developed to address some of the procedural challenges and occupational hazards associated with traditional PCI in addition to enhancing the degree of precision and control for the interventional procedure. A remote- control, robotic-assisted angioplasty system is used to address some of the procedural challenges and occupational hazards associated with traditional PCI. The objective of this study was to assess the safety and feasibility of the operating robotic system in patients undergoing elective PCI. This review summarises the safety and feasibility of a robotic angioplasty system in delivery and manipulation of coronary guidewires, balloons, and stents in patients undergoing PCI. Patients with coronary artery disease and clinical indication for elective PCI were enrolled. The coronary angioplasty procedure was performed with the CorPath 200 robotic system (Corindus, Inc.) The operating system consists of a remote interventional cockpit and a multicomponent bedside unit that contains advance, retract, and rotate guidewires within rapid exchange catheters. The primary endpoint was device clinical success (30% residual stenosis) with- out in-hospital major adverse cardiac events. The procedural success was defined as the ability of the system to complete all the planned angioplasty steps based on procedural segments.30 days follow up after angioplasty procedure was done. A total of 47 patients were enrolled in the study. Primary endpoint had been achieved in all patients. The success of the robotic system was 98.1% in completing 48 of 49 planned steps. No device or procedure-related complications were reported and no in-hospital or 30-day major adverse cardiac events were observed. We rated the robotic system performances as equal to as or better than manual procedures in 97.7% of the cases. Cardiologist’s radiation exposure was 97% lower than found at the standard table position. Our procedure results demonstrated better safety, feasibility and procedural effectiveness than manual operation. Besides, total operator exposure to radiation was quite low. However, a larger study is warranted to confirm the safety and effectiveness of robotic-assisted percutaneous coronary intervention.
Keywords
Methods
Study Population
47 Patients with documented CAD obstruction and myocardial ischemia were enrolled in the study. All registered patients had a coronary target lesion, with a maximum of 25 mm in length, in vessels with diameter of 2.5 to 4.0 mm. Major clinical exclusion criteria included planned coronary artery bypass graft surgery or PCI within 30 days of target procedure, congestive heart failure or left ventricular ejection fraction of 30%, recent MI, and recent stroke or coagulation disorder. Exclusion criteria included prior stenting, ostial or bifurcation lesion, CTO, and severe tortuos or calcified lesion. A proper follow up was executed for all patients for any clinical event within 30 days after procedure.
Robotic PCI system
The CorPath 200 System (Corindus, Inc.) is a novel robotic system that was developed for coronary and endovascular procedures (Figure 1). The system consists of 2 major components: the interventional cockpit and a bedside unit. Unit has interventional cockpit that is a radiation- shielded mobile workstation which can be positioned anywhere in the catheterization laboratory. It facilitates operators to perform the PCI remotely from the console while sitting at the cockpit unit. The robotic system is an open- architecture system that is compatible with 0.014-inch guidewires, rapid exchange catheter systems, and other Granadaetal. 461 Novel Robotic-Assisted Coronary Angioplasty System standard catheterization laboratory hardware and imaging systems (Figure 1B). Unit allows manipulation of the guidewire, balloon and stent catheter with one hand and operating the automatic contrast media injector with the other hand. Hardwares manipulation is controlled through the designated knobs at the console (Figure 2A). The fluoroscopy, electrocardiography, and hemodynamic monitors are “slaved” to duplicate monitors inside the cockpit, enabling visualization from a closer distance (Figure 2B). The bedside unit is consists of the bedrail-mounted articulated arm supporting the robotic drive with the attached single-use cassette. The robotic drive is connected to the control console with a communication cable.
Robotic-Assisted Angioplasty Procedure
We evaluated angiograms and target lesion for robotically assisted PCI before intervention. The procedure was initiated by obtaining vascular access through percutaneous catheterization techniques. A standard guiding catheter was manually introduced, and the Cardiologist with standard interventional techniques cannulated the target coronary artery. Guide catheter was manually connected to the Y-connector, which is placed manually into the Y-connector holder of the cassette. Guide catheter between the Y-connector and incision site was supported by robotic extension arm. Guidewire was manually introduced through the Y-connector into the guiding catheter and loaded the distal end of the guidewire into the cassette. Cardiologist, via the control console knobs is capable of controlling the cassette, which provide both linear as well rotational motions, so the devices can be advanced, rotated and retracted. Then operator loaded a coronary angiography balloon into the system and advanced the device with the robotic system to perform pre-dilation of the target lesion. Thereafter, the angioplasty balloon was retracted with the robotic system and exchanged for a rapid stent delivery system. Subsequently, stent insertion, deployment, and retrieval was performed with the robotic system. Final angiography was performed from the cockpit to assess stent implantation and rule out any complication.
Study Endpoints
Primary endpoint was clinical success that was defined as <30% post procedure final diameter stenosis to deliver a balloon and deploying stent and successfully retracting the delivery system without in hospital major adverse cardiac events (MACE), defined as death, Q-wave or non–Q- wave myocardial infarction, or clinically driven target vessel revascularization. Technical success was defined completing all the technical steps based on the number of procedural segments required to complete the introduction and retrieval of all devices. Radiation exposure to the operator at the cockpit and at the procedure table, a site of cardiologist was monitored with the electronic direct dosimeters. The robotic- system procedural attributes were recorded immediately after the procedure and were rated by all cardiologist as better, equal, or worse than manual separately for the guidewire, balloon catheter, and stent catheter in following performance parameters: introduction, tractability, pushability, crossing and retrieval.
Statistical Analysis
All the data were collected and evaluated with average (mean), SD, and median. The radiation exposure for the operator at the interventional cockpit and at the procedure table as well as creatine kinase-myocardial band values from before and 24 h after procedure were analyzed.
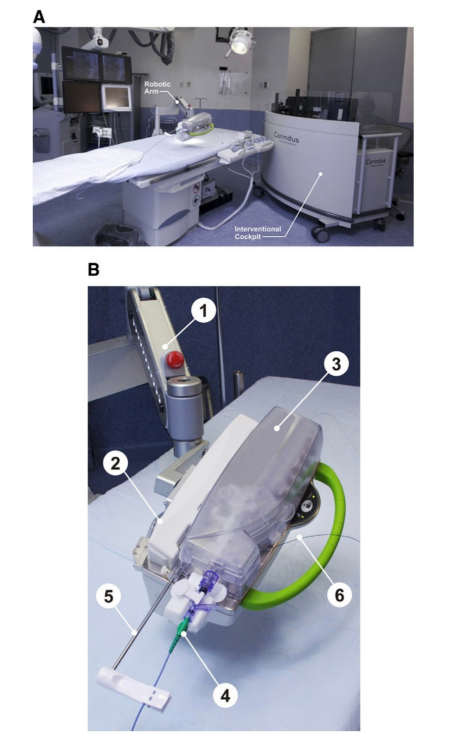
Figure 1: CorPath 200 System. Description of the CorPath 200 System.
(A) Typical set up of the equipment in the catheterization laboratory: bedside unit mounted on a bedrail, and the Inter- ventional Cockpit is positioned at the foot of the procedure table. (B) The bedside unit is composed of: 1–an articulated arm containing 2–the robotic drive; and 3–a single-use cassette. The 4–single-use cassette, shown with 5–attached guide catheter supported with guide catheter arm and 6–loaded balloon catheter.
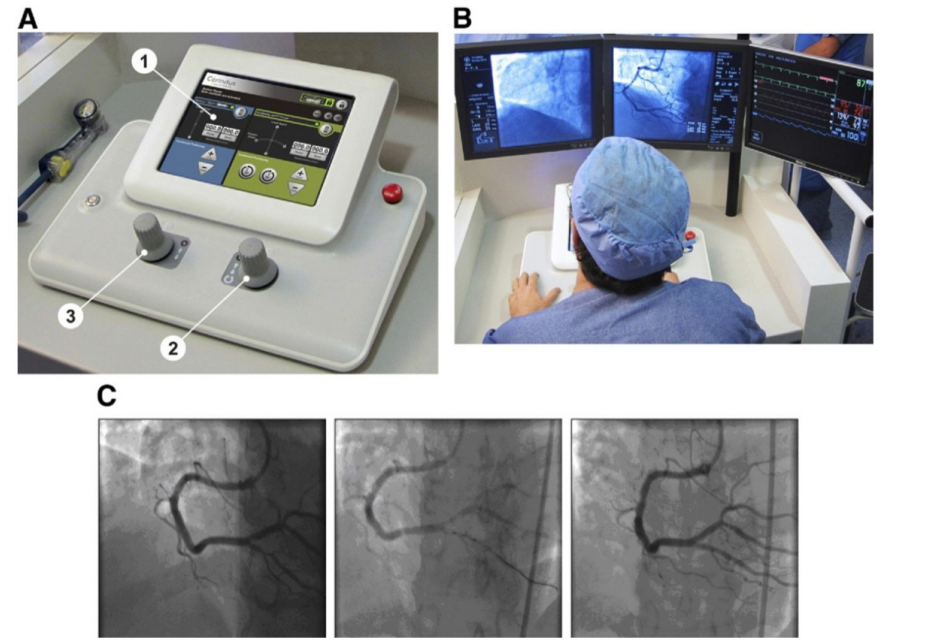
Figure 2: CorPath 200 System Console (A) Representative picture of the control console: 1–touch screen controls; 2–guidewire joystick; and 3– balloon/stent catheter joystick. (B) The operator seated at the interventional cockpit with control console for manipulating percutaneous transluminal coronary angioplasty devices and the angiography and hemodynamic signs monitors positioned at his eye level. (C) A guidewire and then a 2.5- 9-mm balloon were advanced through a proximal posterior descending artery lesion with the robotic angioplasty arm to per- form pre-dilation. A 2.5- 16-mm stent was delivered to the target lesion with the robotic system and deployed in a standard fashion.
Results
Total of 47 patients who met inclusion and exclusion criteria and had signed the informed consent form underwent PCI with the robotic system.
Table 1 demonstrates the demographic and baseline characteristics of all included patients. The left anterior descending artery was treated in 6 patient (12.5%), the right coronary artery was treated in 20 patients (50%), and the left circumflex artery was treated in 15 (37.5%). 40 selected lesions were classified as type A, and 7 were classified as Type B1 lesions, in accordance with American College of Cardiology/American Heart Association grading criteria. The mean lesion length was 11.4 ± 6.1 mm, mean reference vessel diameter was 3.0 ± 0.74 mm, and the mean diameter stenosis was 63.1 ± 15%. A 6-F guide catheter and a single type 0.014-inch balanced middleweight wire were used in all procedures were performed. Pre-dilation was done with the monorail balloon.
The stents used were either bare- metal or the everolimus-eluting stent. At the end of the procedures, all patients had final residual stenosis of <10% with TIMI flow grade 3.
Table 1: Demographic Data and Procedural Characteristics of All Patients Intervened.
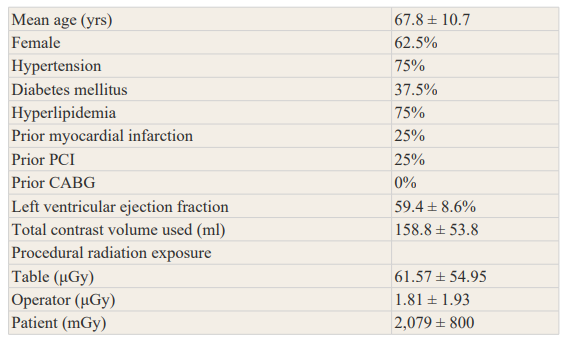
Values are mean ± SD or %.
CABG = coronary artery bypass graft surgery; PCI = percutaneous coronary intervention.
PCI procedures were performed with the robotic system without periprocedural complications. Lesions treated is demonstrated in Figure 2B. The mean procedure time was 43.0 ± 18.6 min with mean robotic-system procedure time of 26.5 ± 8.0 min and with a mean fluoroscopy time of 11.5 ± 3.7 min. In all patients, procedure included successful navigation and crossing the target lesion. Guidewire proceeded smoothly, without any dissection or perforation. Pre-dilation balloon was successfully delivered to the lesion, inflated as clinically indicated, and successfully retrieved by the robotic system back into the guiding catheter.
The stent was successfully delivered to the lesion by the system. Subsequently, the stent delivery system was successfully retrieved into the guiding catheter. In all but 3 patient, the guidewire was successfully retrieved into the guide catheter. In 3 patient, the guidewire was retrieved by the robotic system to the distal part of the guide catheter, but because of a partial system malfunction, the rest of the retrieval was performed manually. This conversion to manual operation was immediate and not associated with any unwarranted event like myocardial ischemia, hemodynamic compromise, or any other complications.
In summary, 100% of the interventional components were successfully delivered, and 95.8% (23 of 24) were successfully retrieved, for an overall technical success rate of 97.9% (44 of 47). No clinical adverse effects related to the use of the robotic system were noted. Thus, the primary endpoint defined as device clinical success (≤30% residual stenosis) without in- hospital MACE was achieved in all 8 patients (100%). At 24 h after procedure, no patient had increase in the creatine kinase-myocardial band levels (mean levels changed from 14.6 ± 3.2 at baseline to 16.1 ± 3.6 at 24 h, p < 0.35). No in-hospital MACE was reported, and at 30-day follow- up, all patients remained asymptomatic with no MACE. Total radiation exposure to the operator at the cockpit was 97% lower than at the procedure table (1.81 ± 1.93 μGy vs. 61.57 ± 54.95 μGy; p < 0.012). The robotic system performance during PCI procedure was rated as equal to that of the manual procedure in 92.5% of the cases for all devices and procedural steps. In 8 cases, the guidewire advancement/retrieval was rated as better than manual, and in the single case of guidewire retrieval failure, it was rated as worse than manual.
Discussion
In this study, we report our experience with the CorPath 200 System, a novel robotic-assisted angioplasty system. The system achieved a technical success rate of 97.9%, completing 47 of 48 procedural segments, and there was no MACE or any other adverse events associated with the system. Patients were discharged from hospital within 24 hours after procedure. In two cases, at the time of the final wire retrieval, there was a recoverable cassette failure, and the operator decided not to complete the procedure with the robotic-assisted system and remainder of the wire (approximately 2 cm) was removed manually. This was minor system malfunction was considered and did not affect the overall performance and safety of the procedure at any given point.
Apart from these two cases, the cardiologists scored the robotic- assisted system equal to manual operation. All the patients completed the 30-day follow-up without any MACE. Robotic systems have been suggested to enhance the precision of cardiovascular procedures with increased accuracy [4-10].
Technical features of the robotic system include capability to control and accurately position (1-mm steps) the stent delivery system and robotically assisted system facilitates the positioning of the stent delivery system with a high degree of accuracy. Robotic systems have also been suggested to reduce radiation exposure [11-13]. The retrospective evaluation study of lens injuries and dose (RELID) study revealed that operators have cataract-type eye opacities 3 times more than age-matched controlled group [2]. Besides, mean fluoroscopy time from our study compares favorably with the results of the other study of 9,650 patients with single-vessel PCI (11.5 min vs. 18.3 min) [14] and with another sub- group of 7,242 patients where fluoroscopy time was ≤ 23 min (11.5 min vs. 12.7 min) [14]. Main advantage therefore, has been shorter fluoroscopy time leading to reduced radiation exposure for the patient and the operator and reduced contrast fluid usage. In our study, 1 of the most important findings was the significant (97.1%) reduction in radiation exposure to the operator performing the robotic PCI procedure.).
Although the study was not designed to show differences in contrast volume usage, the total contrast agents used in this study seemed to be less, compared with what has been reported in other clinical series [14]. The low contrast usage is attributed to the complete procedure control enabled by the interventional cockpit environment (Figure 2).
Another important finding was the technical “comfort” expressed by the operators. Although no specific measurement of this variable was used in this study, both operators perceived this system to be more comfortable compared with the typical technique used for coronary intervention. Most of the cases were performed under a controlled environment, in which the operator had the opportunity to focus on the performance of the procedure, having a close proximity of the monitors and not distracted by the physical strain of the lead apron and standing position. Despite the significant evolution in interventional device technologies, the actual procedural methodology and workflow in the catheterization laboratory has remained unchanged in the last 25 years. As the current practice of interventional cardiology evolves into more complex PCI procedures, interventional cardiologists, the professional societies [15], Cardiologists around the world have called for enhanced catheterization laboratory safety by reducing radiation exposure to both patients and operators [16] and making the overall catheterization laboratory environment more ergonomically friendly through technological innovations [3]. Incorporating a remote control, robotic-assisted PCI system into the catheterization laboratory addresses some of the procedural deficiencies and hazards associated with conventional PCI in addition to contributing to a higher degree of precision and control for the interventional procedure. In this clinical experience, the use of the robotic system seems not to reduce the overall periprocedural times compared with manual PCI of single lesions [14].
However, as this technology underwent initial clinical evaluation, the rigor of the study procedure in addition to the time required to register the data added additional procedural time. From the operator point of view, there is a learning and technology adaptation curve that improves over time, leading to shorter procedural times. This study is an early feasibility study in a small cohort of patients, and the use of this technology in complex anatomies like severe tortuosity, severe calcification, or interventions requiring multiple wires and balloons needs to be further studied. Therefore, a larger, prospective, multicenter pivotal clinical trial designed to test the robotic angioplasty system in a larger number of patients is required.
References
- Goldstein JA, Balter S, Crowley M, et al. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004; 63: 407-411.
- Duran AD, Duran GD, Ramirez RR, et al. Cataracts in interventional cardiology personnel: Retrospective evaluation study of lens injuries and dose. Eur Heart J. 2009; 30: 872.
- Kline LW, Miller DL, Balter S, et al. Occupational hazards in the interventional laboratory: time for a safer environment. Catheter Cardiovasc Interv. 2009; 73: 432-438.
- Di Biase L, Wang Y, Horton R, et al. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: single-center experience. J Cardiovasc Electrophysiol. 2009; 20: 1328-1335.
- Beyar R, Wenderow T, Lindner D, et al. Concept design and pre-clinical studies for remote control percutaneous coronary interventions. EuroIntervention. 2005; 1: 340-345.
- Beyar R, Gruberg L, Delleanu D, et al. Remote-control percutaneous coronary interventions: Concept, validation, and first-in-humans clinical trial. J Am Coll Cardiol. 2006; 47: 296-300.
- Solomon SB, Patriciu A, Bohlman ME, et al. Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology. 2002; 225: 277-282.
- Marescaux J, Leroy J, Gagner M, et al. Transatlantic robot- assisted telesurgery. Nature. 2001; 413: 379-380.
- Argenziano M, Katz M, Bonatti J, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg. 2006; 81: 1666-1674.
- Subramanian VA, Patel NU, Patel NC, et al. Robotic assisted multivessel minimally invasive direct coronary artery bypass with port-access stabilization and cardiac positioning: paving the way for outpatient coronary surgery. Ann Thorac Surg. 2005; 79: 1590-1596.
- Costa MA, Angiolillo DJ, Tannenbaum M, et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of multicenter prospective STLLR trial). Am J Cardiol. 2008; 101: 1704- 1711.
- Garcia-Garcia HM, Tsuchida K, Meulenbrug H, et al. Magnetic navigation in a coronary phantom: experimental results. Eurointervention. 2005; 1: 321-328.
- Steve D, Servatius H, Rostock T, et al. Reduced fluoroscopy during atrial fibrillation ablation: benefits of robotic guided navigation. J Cardiovasc Electrophysiol. 2010; 21: 6-12.
- Nikolsky E, Pucelikova T, Mehran R, et al. An evaluation of fluoroscopy time and correlation with outcomes after percutaneous coronary intervention. J Invasive Cardiol. 2007; 19: 208-213.
- Hirshfeld JW, Balter S, Brinker JA, et al. ACCF/AHA/HRS/ SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2004; 44: 2259-2282.
- Rehani MM. Training of interventional cardiologists in radiation protection-the IAEA's initiatives. Int J Cardiol. 2007; 114: 256-260.