Fundaments of Thermal Analgesia in Humans: Exploring New Methods of Pain Relief
Author'(s): Charles Chabal*
Chief Science Officer Soovu Labs Inc., Seattle WA, USA.
*Correspondence:
Charles Chabal, MD, Soovu Labs Inc. 2439 8TH Ave N, Seattle WA. USA, 98109-2212, 206-579-4910.
Received: 16 December 2020; Accepted: 19 January 2021
Citation: Chabal C. Fundaments of Thermal Analgesia in Humans: Exploring New Methods of Pain Relief. Anesth Pain Res. 2021; 5(1):1-8.
Abstract
Heat is often used for pain relief but basic information related to the potential mechanisms are not well understood in humans. This manuscripts reviews clinical studies of heat induced pain relief in humans, analyses basic laboratory studies, and offers possible mechanisms of action based on these animal findings particularly as related to TRPV1 channels. Many of the laboratory studies done on TRPV1 channels likely have strong correlations in humans. Recent human studies are discussed that provide insights on onset of action, duration of pain relief, and relationship of thermal energy delivered to analgesic effect with correlation to these laboratory findings.
The term analgesic nociceptive boundary is offered to describe the amount of thermal energy needed to cause maximal pain relief while not causing nociception or tissue damage. This boundary provides a framework for future clinical development.
Keywords
Introduction
Humans have long used heat to provide pain relief and comfort with many noting that a flare up of back or muscle pain is significantly improved by a hot shower or soak. For many, a hot shower after a workout is a comforting indulgence. Many remain in the shower well past the time needed to wash off the sweat of a workout. The water cleans but the heat reduces pain, spasms, and stress providing a level of comfort and well-being, an oasis in everyday life. As Sylvia Platt remarked, “There might be things that a hot soak won't cure but I don't know many of them.”
The pain relieving qualities of heat are well recognized. A recent article in the Annals of Internal Medicine presented evidence based treatment guidelines for the management of low back pain [1]. One of the initial recommended treatments is the use of superficial heat. The guidelines note that evidence supports that opioids generally produce a small reduction in reported pain that is often not maintained in those with chronic back pain and are not generally recommended as a first line option [2]. Acetaminophen recently was shown to be ineffective in relieving low back pain [3] and overdose is the leading cause of liver failure in many countries [4]. Nonsteroidal anti-inflammatory medications (NSAIDs) such as ibuprofen provide some reduction in pain [5] but have significant potential side effects. NSAIDs can raise blood pressure, effect kidney function [6] and cause gastrointestinal bleeding [7]. In 2015 the FDA [8] issued a strong warning about the use of NSAIDs and the increased risk of heart attack and stroke. The FDA warning noted:
- The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID.
- NSAIDs can increase the risk of heart attack or stroke in patients with or without heart disease leading to progressive and sometimes sudden and fatal heart disease.
- Patients treated with NSAIDs following a first heart attack were more likely to die in the first year after the heart attack than non-users of NSAIDs.
- There is an increased risk of heart failure with NSAID use.
The limited efficacy and the risk of complications associated with common pain medications, has led to a search for effective non drug treatments. Unfortunately this background has opened opportunities for marginal or poorly supported pain therapies and in some cases outright quackery. Reviewing well designed studies and potential mechanisms of action provides the reader with a scientific approach to better understanding a drug-free approach: thermal induced pain relief.
The evidence supporting heat as a pain relieving strategy
Heat is one of the most common treatments used by those in pain. Surprisingly there are very few human studies that have examined fundamental concepts of thermal analgesia (the reduction of pain intensity associated with heat) such as the optimal temperature for pain relief, how long does the pain relief last, and what is a safe amount of heat that does not cause tissue damage. More importantly, little was known about the mechanism of action in humans. As such, the National Institutes of Health awarded our group two significant grants to study the mechanism of action of heat and its clinical application.
Some speculate that heat reduces pain by increasing local blood flow, improving muscle relaxation, and reducing muscle spasm [9]. While these mechanisms are likely, the onset of pain relief from heat occurs very rapidly, generally within 5 minutes. To put this in perspective, this is faster than the onset of intravenous morphine and significantly faster than any orally ingested pain medications which generally have an onset of action ranging from 15 to 20 minutes [10]. Heat’s action is so rapid that it is likely involves a neurologic pathway. This manuscript examines the pain relieving effects of heat through published clinical studies and then discusses potential mechanisms of action (MOA). By studying the MOAs, one should be able to identify physiological pathway(s) and therefore interventions or strategies that can be enhanced to improve pain relief.
Clinical studies in humans
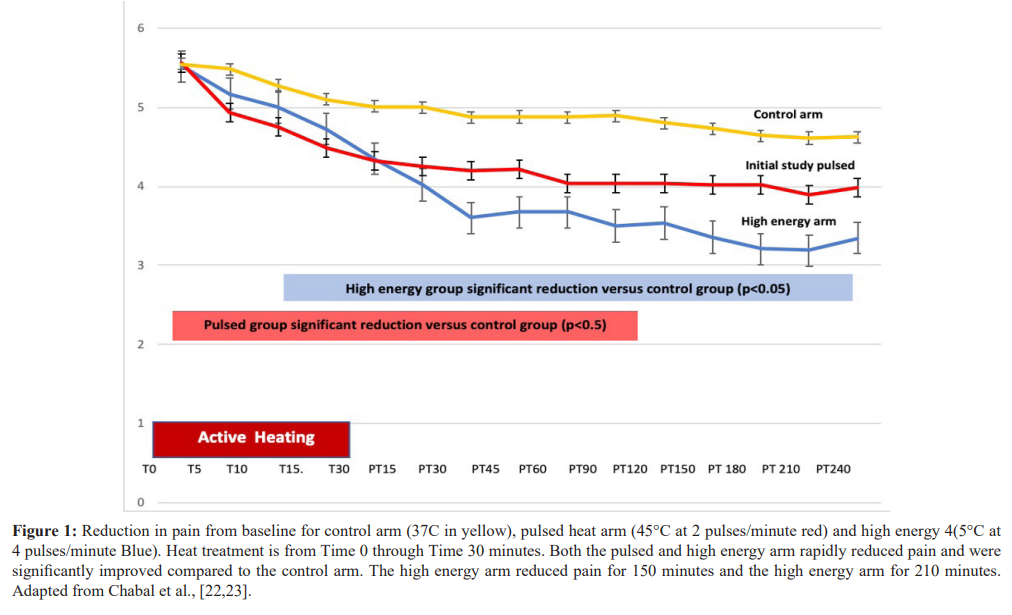
In a Cochrane review of superficial heat or cold for acute low back pain, French et al. 2006 [11] identified four published trials judged as higher quality using heat in low back pain. In one study, Nadler et al., 2002 [12] compared a chemical heat wrap to oral placebo, ibuprofen, or acetaminophen in subjects with acute to subacute (3 months or less) low back pain. The heat wrap provided up to 8 hours of 40° C heat produced by a chemical oxidation reaction and produced superior pain relief when compared to both ibuprofen and acetaminophen. Similar studies in acute low back pain found superior pain relief compared to oral placebo or ibuprofen when the wraps were used overnight as well over a five day period [13,14]. Nuhr et al., 2004 [15] evaluated heat provided by electrical blankets in subjects with acute low back pain to treatment with an unheated blanket. The heated electrical blanket produced significantly greater pain relief than an unheated blanket. Finally, Mayer et al., 2005 [16] found that chemical hot packs in acute back pain combined with exercise were better than either intervention alone. More recent studies also support the use of heat to relieve pain in neck and back strain [17,18], knee pain [19], dysmenorrhea [20], and delayed muscle soreness related to exercise [21]. All of the above studies were in acute or semi acute pain. However, two recent publications in subjects with chronic low back pain, demonstrated that high temperature pulses to 45° C produced significantly better and longer lasting pain relief as compared to lower level steady heat at 37° C [22,23].
The literature cited above, and human experience supports that heat produces pain relief and comfort, yet our understanding of thermal analgesia and its MOA, remains relatively thin. Until recently, little was known about the onset of action or how quickly heat reduces pain, which for analgesics, is a critical measure. The recent studies support that the onset of thermally induced reduction of pain at 45° C occurs very rapidly, within 5 minutes of treatment [22,23]. In contrast low level steady heat at 40° C produces pain relief but takes over 95 minutes for the onset of pain relief and over 200 minutes to reach significant pain relief [24]. In the studies using heat pulsing up to 45° C not only was the onset very rapid but the duration of pain relief last for over 2 hours after 30-minute treatment period [22,23]. This prolonged duration of analgesia to high level heat has not been carefully measured for low level heat. The rapid onset of action is very suggestive of a mechanism of action, involving the peripheral nervous system with animal studies showing that hotter temperatures to a point are correlated with increased firing of thermal receptors as measured by C fiber stimulation [25,26]. While studies support the effectiveness of heat for the management of both acute and chronic pain, superficial heat also has significant effects on muscle temperature, blood flow and muscle and tendon flexibility.
In addition to thermally induced pain relief, superficial heat has a number of other effects on blood flow, oxygen delivery, muscle stretch and tension that may aid in recovery, repair and contribute to pain relief. Applied heat has been studied in athletic workouts and injured athletes. Petrofsky et al., [27] compared superficial heat via a commercially available chemical heat wrap (40°C) and commercially available menthol patch and cream applied over the quadriceps muscle for up to two hours in a three arm crossover study. Muscle temperature was measured by a thermal probe inserted 2 ½ cm into the quadriceps muscle and blood flow using an infrared laser Doppler flow meter with probes inserted into the belly of the quadriceps. Quadriceps temperature and blood flow were decreased by the menthol containing patches and cream. In contrast deep muscle temperature was increased 2.7°C and muscle blood flow was increase 1.5 fold [27]. In addition, studies by the same group showed that superficial heat had the effect of increasing muscle relaxation and improving the flexibility of underlying tendons, specifically the anterior and posterior cruciate ligament of the knee [28]. The same effects were not seen with the application of cold.
Heat has also been used to reduce pain and speed recovery associated with intense exercise and joint injury. After intense exercise or training, delayed onset muscle soreness (DMOS) usually begins with in 8 hours and may last 5-7 days. DMOS is often accompanied with muscle weakness that can last up to 2 weeks. While there are many theorized etiologies for DMOS and muscle weakness one theory hypothesizes muscle injury and is linked to elevated biomarkers or injury metabolites such as elevated heat shock proteins, lactate, creatinine phosphokinase, and myoglobin. With increased muscle blood flow and reduction of toxic metabolites, topical heat demonstrated a significant reduction in DMOS [29- 31]. In fact, heat applied after exercise was shown to reduce in fascial edema, white cell counts, heat shock protein [30] serum skeletal muscle troponin I, creatine kinase, and myoglobin [32]. Those that used no heat or used cold baths showed no reduction in these markers [32]. In a study examining DMOS and muscle strength after exercise [21], showed that superficial heat applied after rigorous exercise of the lower extremity rapidly increased the recovery and quadriceps strength as compared to those subjects who received no heat or 24 hour delayed heat therapy. As expected, the pain associated with DMOS was also reduced by heat treatment.
Finally, the use of heat with or without ibuprofen produced better pain relief, increased range of motion and improved patient adherence to therapy in those who were undergoing either physical therapy for neck pain [18] or knee injuries [19]. As such, heat may have future applications in recovery from strenuous exercise, promote healing of injured muscles, reduce injuries by muscle and tendon relaxation, and potentially facilitate out of clinic physical therapy.
Heats effect on peripheral nerves and receptors
In humans the sensation of heat is largely transmitted from the periphery to the central nervous system by C fibers with contributions from Aδ fibers. C fibers are thin unmyelinated fibers that are slow conductors of electrical impulses. C fibers and Aδ fibers are classically activated by thermal energy or noxious stimuli with Aδ requiring a higher temperature for activation (46.9± 1.7°C) and C fibers activated by lower temperatures (39.8 ±1.7°C) [33]. In addition, it is estimated that the ration of C to Aδ fibers is about 4:1 providing a rich sensory conduit to the central nervous system [34].
C fibers a pathway to the amygdala
A somewhat underappreciated role in thermal analgesia is that of the limbic system of the amygdala. The limbic system plays a key role in emotional responses such as fear, anxiety, and pain [35]. It is also one of the areas of the brain that is responsible for the perception of comfort and relaxation. One example of the limbic response is the comfort and feeling of well-being associated with coming in from the outdoors wet and cold, and getting wrapped in a warm blanket. It's been demonstrated that the unmyelinated C- fibers provide a gateway into the limbic system. In fact, stimulation of subpopulations of the C fibers induces pleasant sensations such as relaxation, comfort, reduce anxiety and stress [36]. Given that warmth and heat are associated with a feeling of comfort and well-being, stimulation of C fibers can help induce these pleasant sensations and reduce anxiety [36]. Some have called this pleasant sensation associated with heat the hedonic response, and it constitutes a key yet underappreciated, component of thermal stimulation [37]. In contrast to other forms of peripheral stimulation such as transcutaneous electrical nerve stimulation (TENS), heat has the ability to both relieve pain and to provide a pleasant form of comfort, security and to reduce anxiety. Given these facts, the methods and parameters of activating C and Aδ fibers remain an area of significant interest.
Transient receptor potential channels, vanilloid subtype (TRPV) as sensory mediators
The transient receptor potential cation channels (TRPV) were first identified in Drosophila in 1969 [38]. There are six broad classifications of TRPV channels ranging from TRPV1 through TRPV6 (see Table 1). TRPV are ion channels and are found in animals and humans. In humans they are widely distributed throughout the body in cells and tissues like the brain [39], lungs heart, spleen, kidney, placenta and peripheral nervous system. The channel most relevant to pain relief in humans is the TRPV1 channel. TRPV1 are located on afferent sensory fibers, C and Aδ, the dorsal root ganglia as well as multiple other locations in the body. With depolarization, there is an influx Na+ and Ca+ across the cell membrane leading to cellular depolarization and the initiation of an action potential. This action potential can initiate the nociceptive process and drive painful sensations. TRPV1 channels are involved in multiple pain conditions like visceral, inflammatory, neuropathic, migraine, and some forms of cancer related pain [38]. There has been an extensive search for TRPV1 agonists but success has been limited as TRPV1 channels are widespread throughout the body and involved with temperature homeostasis which could cause dangerous side effects.
Currently the most commonly used TRPV1 activator is topical capsaicin. While initially the mechanism of action was thought to be depletion of substance P, more recent research indicates that capsaicin works by desensitization of nociceptive fibers in a process known as “defunctionalization” [39]. This defunctionalization of

the TRPV1 channel leads to prolonged block of action potentials and may actually reduce the longer term transport of neurotrophic substances [39]. While initially approved for post herpetic pain, capsaicin is now approved for diabetic neuropathy. TRPV1 channels may be involved with the transmission and maintenance of other chronic pain states.
In summary, activation of TRPV1 channels lead to an action potential that can initiate pain impulses. Once activated or depolarized, the channel may become defunctionalized thereby blocking or reducing ongoing depolarization and generation of new action potentials thereby reducing pain sensations.
Heat a selective TRPV1 activator and defunctionalization
Clinical studies cited previously demonstrate that heat is an effective treatment for both acute and subacute pain. In addition, heat increases muscle blood flow and reduces muscle and tendon spasm. Recently a pair of studies published demonstrated that heat is an effective treatment in subjects with longstanding chronic low back pain. In these studies, heat that pulsed to 45°C at the rate of 2 pulses per minute produced a rapid onset of pain relief, within 5 minutes. The 30 minutes of treatment reduced pain for over 120 minutes post treatment, compared to subjects who received steady state heat at 37°C. In addition, a subset of subjects who received twice as much thermal energy (four pulses per minute 45°C versus 2 pulses per minute) showed an increase in pain relief as well as extended pain relief out to 180 minutes after the cessation of treatment. These studies are one of the very few published studies examining the effect of heat in chronic low back pain [22,23]. In contrast to previously published studies in acute and sub-acute pain that used steady low level heat at 40 °C from chemical hot packs, this study used pulses of heat up to 45°C delivered by a novel medical device (Soovu TM Pain Relief System, Soovu Labs Inc.). The results demonstrated that high levels of pulsed heat provided the onset of thermally induced pain relief within 5 minutes: further, there was a dose response relationship between the amount of thermal energy delivered and effectiveness including the duration of pain relief from a single 30 minute treatment session. The results of these studies are summarized in figure 1.
Pulsed high level heat: a new method to treat pain?
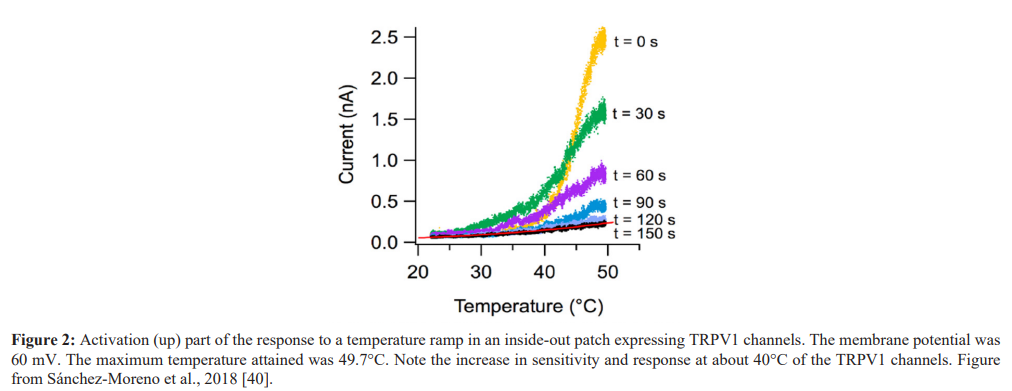
The rapid onset and long duration of pain relief by heat offers some insight into potential mechanisms of action. The MOA while not studied in humans has been elucidated in a variety of animal models. The range of thermal activation of TRPV1 channels lies in a range from 37°C to approximately 48°C. Some of this variation is likely due to the method by which TRPV1 channel activation was measured. For example, a recent study [40], using a cell patch technique, demonstrated that activation of TRPV1 channels were temperature dependent (see figure 2). At about 40°C there was increased sensitivity and a rapid depolarization of these cells. If one extrapolates these findings from in-vitro cell patch techniques to human skin that contains layers of dermis, fat, and blood vessels, one would expect that in vivo temperatures in the range of 43°C would be a realistic skin temperature to stimulate TRPV1 channels. This may explain why the heat pulses at 45°C in the cited articles caused such a rapid and profound analgesic response [22,23]. In the second study, that delivered twice the amount of thermal energy, the degree of analgesia delivered, and the duration of the response was greater than that delivered with the less frequently pulsed heat arm. If one visualizes intact human skin and underlying tissue, one can think of a thermal pulse radiating out in a three dimensional cone to penetrate to wider deeper layers of the skin with activation of more TRPV1 channels. A higher energy pulse at four pulses per minute in contrast to two pulses per minute delivers twice the amount of thermal energy and the volume of tissue stimulated would be significantly greater in the higher energy group thereby recruiting more TRPV1 channels. This is a possible explanation why the high energy group had significantly greater analgesic results than the lower energy group. This may also help explain why low level steady heat from chemical hot packs at 40°C require up to three hours to provide analgesia. In addition, it is not known if such low level heat provides lasting pain relief as demonstrated in the high level pulsed heat experiment.
The high-level pulsed heat results suggest that there is the possibility of even better analgesia than seen in the previous studies [22,23]. The upper range or ceiling of thermally induced analgesia where increasing levels of thermal stimulation provide increasing and longer durations of pain relief is limited by an energy point where the heat causes nociception or tissue damage. We have termed this the analgesic nociceptive boundary (ANB) [23]. In the pulsed heat studies the increased energy produced better and longer lasting analgesia without any suggestion of discomfort or tissue damage indicating that the analgesic nociceptive boundary was not reached and providing the opportunity to enhance this analgesia response in the future. Another question related to the ANB is whether additional short pulses of energy during the extended period of pain relief could further prolong the duration of relief.
TRPV1 activation: From animal data to human clinical testing
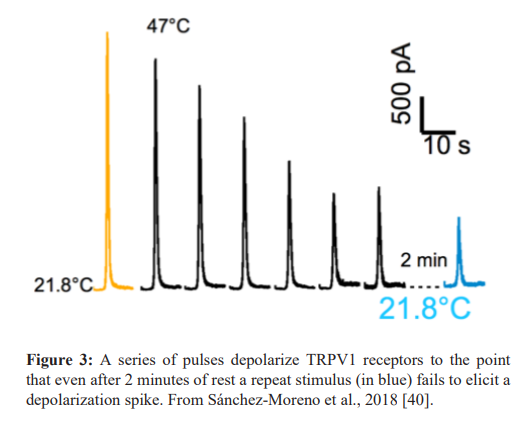
In the clinical trials described earlier the use of pulsed high level heat was chosen based on a number of factors. It is generally accepted that the selective agonist of TRPV1, capsaicin, binds to the TRPV1 channel and can cause prolonged deactivation. In the case of high concentrations of capsaicin that deactivation combined with the loss of some terminal branches of C fibers can produce analgesia lasting up to three months. Heat may also be thought of a selective TRPV1 agonist with some studies showing that a brief dose of high level heat can produce prolonged pain relief that greatly exceeds the actual duration of heat. This concept is reinforced by a recent study by Sánchez-Moreno et al 2018 [40]. In this study the effect of heat on TRPV1 channels was observed using a cell patch technique. Relatively short durations of heat in the mid 40°C range produced irreversible depolarization of the TRPV1 receptor (see figure 3). The clinical studies using high level pulsed heat are likely related to the prolonged deactivation of the TRPV1 channel thereby producing a period of prolonged analgesia and may be one of the reasons for the prolonged pain relief after 30 minutes of treatment described by Chabal et al., [22,23] in humans.
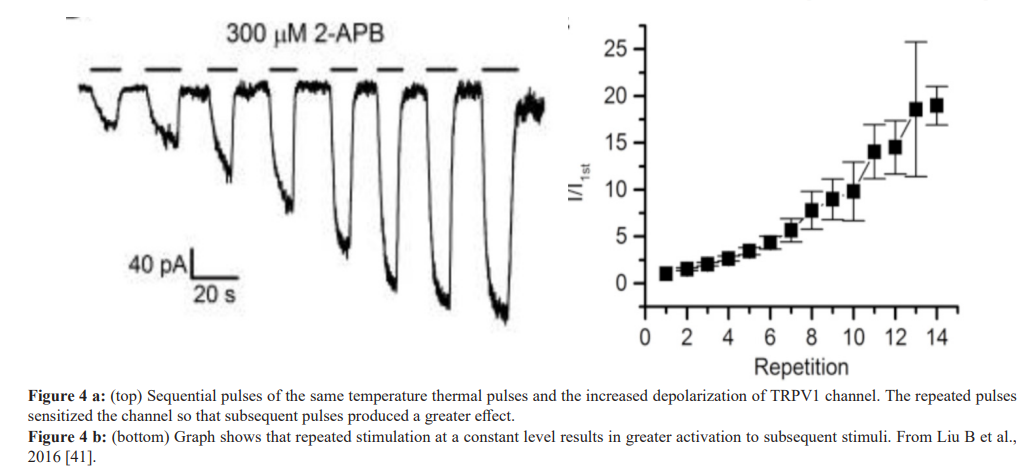
Another reason that repeated pulses were used in the clinical studies, relates to a study by Liu B et al, 2016 [41]. In this study sequential pulses of high level heat were applied to TRPV1 and TRPV2 receptors. The result showed that a series of pulses produced sensitization of the TRPV channel whereas subsequent thermal pulses produced greater depolarization of the channel (See figure 4a, 4b). As noted, the series of thermal pulses sensitize the channel to the point that a thermal pulse at the same temperature produces a significantly greater action potential. Finally pulsed heat stimulation provided significant thermal stimulation yet minimized the amount of energy transferred to the skin offering increased safety.
the analgesic nociceptive boundary (ANB). The ANB is defined as the amount of thermal energy needed to cause maximal pain relief while not causing nociception or tissue damage. This boundary provides a framework for future clinical development. Exploration and a better understanding of the thermal analgesic boundary offers a model that may result in significant clinical improvement and understanding of thermal analgesia.
• Stimulation of C-fibers are associated with changes in the limbic system of the amygdala are under appreciated. Reinforcing stress management techniques with the physical activation of C-fiber afferents offers a potentially powerful synergy by coupling activation of neurological pathways with psychological exercises to reduce pain or anxiety.
It is hoped that this manuscript will lead to a closer examination of thermal analgesia and to methods of better drug free pain relief.
References
1.Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2017; 166: 514-530.
2.Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013; 27: 49-59.
3.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomized controlled trial. Lancet. 2014; 384: 1586-1596.
4.Yan M, Huo Y, Yin S, et al. Mechanisms of acetaminophen- induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018; 17: 274-283.
5.Roelofs PD, Deyo RA, Koes BW, et al. Non-steroidal anti- inflammatory drugs for low back pain. Cochrane Database Syst Rev. 2008; 33: 1766-1774.
6.Radi ZA, Khan KN. Cardio-renal safety of non-steroidal anti- inflammatory drugs. J Toxicol Sci. 2019; 44: 373-391.
7.Moore N, Scheiman JM. Gastrointestinal safety and tolerability of oral non-aspirin over-the-counter analgesics. Postgrad Med. 2018; 130: 188-199.
8.FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes July 9, 2015.
9.McCarberg B, Connor A. A New Look at Heat Treatment for Pain Disorders, Part 1 and 2. APS Bulletin. 2004; 14: 6.
10.Smith H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs. 2012; 26: 509-535.
11.French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006; 25: 47-50.
12.Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low- level heatwrap therapy for treating acute nonspecific low back pain. Arch Phys Med Rehabil. 2003; 84: 329-334.
13.Nadler SF, Steiner DJ, Petty SR, et al. Overnight use of continuous low-level heat wrap therapy for relief of low back pain. Arch Phys Med Rehabil. 2003; 84: 335-342.
14.Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low- level heat wrap therapy provides more efficacy than Ibuprofen and acetaminophen for acute low back pain. Spine. 2002; 27; 1012-1017.
15.Nuhr M, Hoerauf K, Bertalanffy A, et al. Active warming during emergency transport relieves acute low back pain. Spine. 2004; 29: 1499-1503.
16.Mayer JM, Mooney V, Matheson LN, et al. Continuous low- level heat wrap therapy for the prevention and early phase treatment of delayed-onset muscle soreness of the low back: a randomized controlled trial. Arch Phys Med Rehabil. 2006; 87: 1310-1317.
17.Garra G, Singer AJ, Leno R, et al. Heat or Cold Packs for Neck and Back Strain: A Randomized Controlled Trial of Efficacy. Academic Emergency Medicine. 2010; 17: 484-489.
18.Petrofsky JS, Laymon M, Alshammari F, et al. Use of low level of continuous heat and Ibuprofen as an adjunct to physical therapy improves pain relief, range of motion and the compliance for home exercise in patients with nonspecific neck pain: A randomized controlled trial. J Back Musculoskelet Rehabil. 2017; 30: 889-896.
19.Petrofsky JS, Alshammari FS, Lee H. Use of Low Level of Continuous Heat as an Adjunct to Physical Therapy Improves Knee Pain Recovery and the Compliance for Home Exercise in Patients With Chronic Knee Pain: A Randomized Controlled Trial, Journal of Strength and Conditioning Research. 2016; 30: 3107-3115.
20.Jo J, Lee SH. Heat therapy for primary dysmenorrhea: A systematic review and meta-analysis of its effects on pain relief and quality of life. Sci Rep. 2018; 8:16252.
21.Petrofsky J, Berk L, Bains G, et al. The Efficacy of Sustained Heat Treatment on Delayed-Onset Muscle Soreness, Clinical Journal of Sport Medicine. 2017; 27: 329-337.
22.Chabal C, Dunbar PJ, Painter I, et al. Journal of Pain Research. 2020; 13: 2083-2092.
23.Chabal C, Dunbar P, Painter I. Is Thermal Analgesia, Exploring the Boundary Between Pain Relief and Nociception Using A Novel Pulsed Heating Device? Anesth Pain Res. 2020; 4:1-7.
24.Stark J, Petrofsky J, Berk L, et al. ThermaCare Heat Wraps for lower back pain and muscle stiffness. The Journal of Pain. 2012; 13.
25.Hensel H, Kenshalo DR. Warm receptors in the nasal region of cats. J Physiol. 1969; 204: 99-112.
26.Hoffmann T, Sauer SK, Horch RE, et al. Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci. 2008; 28: 6281-6284.
27.Petrofsky JS, Laymon M, Berk L, et al. Effect of ThermaCare Heat Wraps and Icy Hot Cream/Patches on Skin and Quadriceps Muscle Temperature and Blood Flow. Journal of Chiropractic Medicine. 2016; 15: 9-18.
28.Petrofsky JS, Laymon M, Lee H. Effect of heat and cold on tendon flexibility and force to flex the human knee. Med Sci Monit. 2013; 19: 661-667.
29.Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wraps therapy and/or exercise: a randomized controlled trial. Spine J. 2005; 5: 395- 403.
30.Petrofsky JS, Laymon M, Berk L, et al. Pilot study: physiological evidence that heat reduces pain and muscle damage in delayed onset muscle soreness. Clin Pract. 2012; 9:639-650.
31.Petrofsky J, Berk L, Bains G, et al. Moist heat or dry heat for delayed onset muscle soreness. J Clin Med Res. 2013; 5: 416-425.
32.Hassan ES. Thermal therapy and delayed onset muscle soreness. J Sports Med Phys Fitness. 2011; 51: 249-254.
33.Churyukanov M, Plaghki L, Legrain V, et al. Thermal detection thresholds of Aδ- and C-fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PLoS One. 2012; 7: 35817.
34.Ochoa J, Mair WG. The normal sural nerve in man. I. Ultrastructure and numbers of fibers and cells. Acta Neuropathol. 1969; 13: 197-216.
35.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol. 2015; 227: 261-284.
36.Liljencrantz J, Olausson H. Tactile C fibers and their contributions to pleasant sensations and to tactile allodynia. Front Behav Neurosci. 2014; 8: 37.
37.Marshall AG, Sharma ML, Marley K, et al. Spinal signaling of C-fiber mediated pleasant touch in humans. eLife. 2019; 8: 51642.
38.Mickle AD, Shepherd AJ, Mohapatra DP. Nociceptive TRP channels: sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals. 2016; 9: 72.
39.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch BJA: British Journal of Anaesthesia. 2011; 107: 490-502.
40.Sánchez-Moreno A, Guevara-Hernández E, Contreras- Cervera R, et al. Irreversible temperature gating in trpv1 sheds light on channel activation. Elife. 2018; 7: 36372.
41.Liu B, Qin F. Use Dependence of Heat Sensitivity of Vanilloid Receptor TRPV2. Biophys J. 2016; 110: 1523-1537.
42.Tominaga M. The Role of TRP Channels in Thermosensation. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis. 2007.