Genuine Non Compaction of the Left Ventricle: A Case Report
Author(s): Dohou Serge Hugues Mahougnon1 , Sonou Arnaud2, Adoco Joseph2, Lawani Bolarin Pierre3, Hounkponou Murielle2, Agbo-Ola clarisse1 and Codjo Houétondji Léopold2
1Cardiology Teaching and Research Unit, Faculty of Medicine, University of Parakou, Parakou, Benin.
2Cardiology Teaching and Research Unit, Health Sciences Faculty, University of Abomey-Calavi, Cotonou, Benin.
3Hospital center of Dax (France).
*Correspondence:
Dohou Serge Hugues Mahougnon, Cardiology Teaching and Research Unit, Faculty of Medicine, University of Parakou, Parakou, Benin.
Received: 30 Aug 2023; Accepted: 01 Oct 2023; Published: 07 Oct 2023
Citation: Hugues Mahougnon DS, Arnaud S, Joseph A, et al. Genuine Non Compaction of the Left Ventricle: A Case Report. Cardiol Vasc Res. 2023; 7(5): 1-4.
Abstract
Left ventricular noncompaction (LVNC) is a rare unclassified heart disease characterized by the presence of multiple left intraventricular trabeculations. We report the case of a 52-year-old man admitted to hospital with congestive heart failure, which revealed non-compaction of the left ventricle on the basis of the echocardiographic criteria of Jenni. The patient was treated and progressed favourably. Outpatient follow-up was organised.
Keywords
Introduction
Non-compaction of the left ventricle (NCVG) is a rare cause of congenital cardiomyopathy resulting from the arrest of normal myocardial embryogenesis and characterized by the persistence of prominent ventricular trabeculations separated by deep recesses. Cardiac imaging, in particular trans-thoracic echography (TTE) and/or magnetic resonance imaging (MRI) of the heart, is used for diagnosis. We report in this work a case of LVNC revealed by a picture of global cardiac insufficiency.
Medical Observation
The patient was 51 years old and had a history of five (05) episodes of decompensation in the space of one year. He presented with progressively worsening exertional dyspnoea, progressing from New York Heart Association (NYHA) stage II to stage IV, which had been present for a fortnight prior to admission. The dyspnoea was associated with a decubitus cough, orthopnoea and progressive onset and cessation of rest palpitations. For one (01) month prior to admission, he had also presented with progressive enlargement of the abdomen and oedema of the lower limbs.
His physical examination on admission showed a deterioration in general condition, signs of left heart failure with tachycardia at 110 bpm, a left gallop, and crepitating rales in the lower 1/3 of both lung fields. Signs of florid right heart failure included oedema of the lower limbs rising to the thigh, moderate ascites, painful hepatomegaly with a smooth surface and foamy lower border, and hepatojugular reflux.
His electrocardiogram (Figure 1) showed complete tachyarrhythmia due to atrial fibrillation with a mean frequency of 104 bpm, a QS appearance in the anteroseptoapical region and peripheral microvoltage. The chest X-ray (Figure 2) showed cardiomegaly, bilateral alveolar-interstitial syndrome and blunting of the right cost diaphragmatic cul-de-sac. On cardiac ultrasound (Figures 3 and 4), the left ventricle was dilated (LVEDD: 60 mm LVOT: 150 ml), There was global hypokinesia with a severely reduced left ventricular ejection fraction (LVEF) of 15% on Simpson biplane (SBP). There was spontaneous left intraventricular contrast with no visible thrombus. In 2D mode, there was extensive trabeculation at the apex of the left ventricle and on the anterolateral wall, with deep recesses giving a spongy appearance to the myocardium. On color Doppler, the intertrabecular filling by blood flow was visualized. The ratio of non-compacted to compacted myocardium was greater than 2. The mitral profile was restrictive. There was major
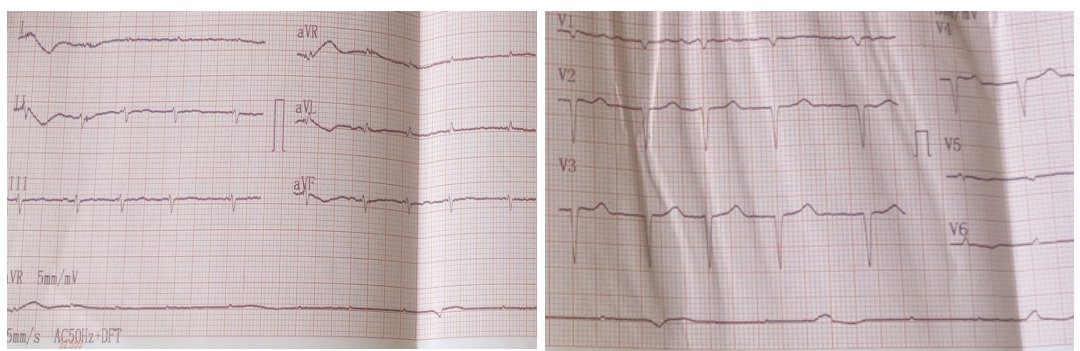
Figure 1: Electrocardiogramme.
Bi-atrial dilatation (VOG: 62ml/m² SOD: 28cm²), right ventricular dysfunction (TAPSE: 11mm VD shortening fraction: 20% S'vd:6cm/s). There was pulmonary hypertension with pulmonary artery pressure estimated at 50 mmHg on tricuspid insufficiency flow, an initial non-dilated aorta, a 30 mm dilated and non-complicating inferior vena cava and a dry pericardium.
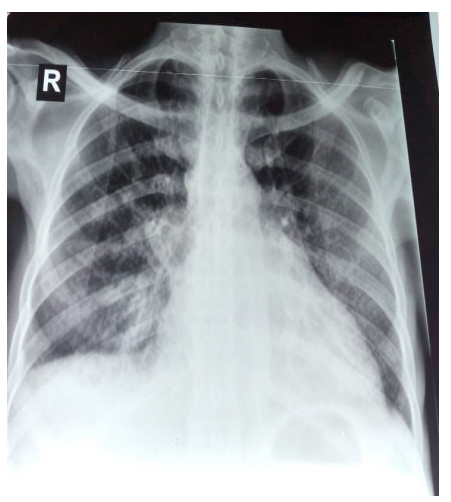
Figure 2: Chest X-ray (front).
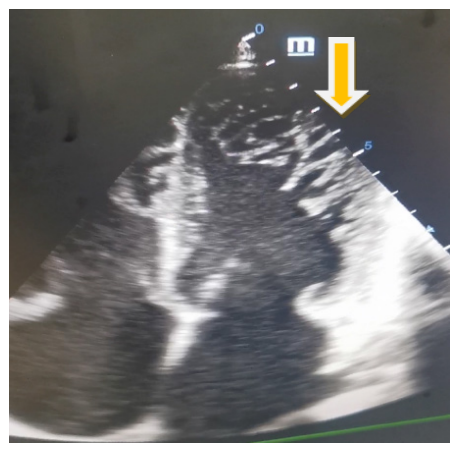
Figure 3: 2D apical 4-cavity section Showing multiple trabeculations at apex and anterolateral wall.
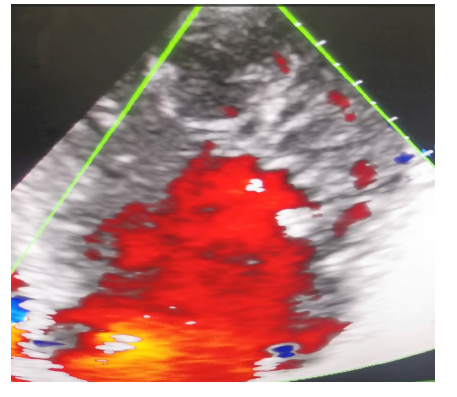
Figure 4: Apical 4-cavity color Doppler section centered on the left ventricle.
The Holter EKG showed paroxysmal atrial fibrillation and ventricular hyperexcitability with triplets of ventricular extrasystoles. The diagnosis was that of a 6th cardiac decompensation of a dilated cardiomyopathy of the non- compaction type of the left ventricle with severe bi-ventricular dysfunction and spontaneous intra-VG contrast, complicated by supraventricular and ventricular hyperexcitability.
The factors leading to decompensation were essentially a change in diet, therapeutic insufficiency and accelerated AF. Her treatment consisted of diuretics, potassium supplementation and curative anticoagulation. The course was marked by a good response to diuretics, regression of congestive symptoms and weight loss of 8 kg compared with admission.
Therapeutic education was then provided to ensure compliance with the low-salt diet. Treatment with beta-blockers, converting enzyme inhibitors and anti-aldosterone agents was started at a low dose on discharge and then titrated to optimum doses for the next 6 weeks. The patient was advised not to take part in strenuous or competitive sporting activities. Only activities with a low static and dynamic component were allowed as part of his exercise rehabilitation.
Discussion
Non-compaction of the left ventricle is a rare cause of cardiomyopathy first described in 1926 by Grant et al. It is a rare congenital cardiomyopathy resulting from the arrest of normal myocardial embryogenesis, classified by the WHO in the group of unclassified cardiomyopathies [1-3]. It is the consequence of an arrest in the embryological phenomenon of compaction of the ventricular myocardium, which normally occurs between the 5th and 8th weeks.
Epidemiology
This is a rare condition with an incidence of 0.05% [4]. The age range most represented at the time of diagnosis according to the French registry of NCVG published in 2017 is 40-50 years, which corroborates well the age of our patient [5]. A genetic origin is found in 30 to 40% of cases [6]. The prevalence of left ventricular involvement is approximately 0.014%, while the prevalence of right ventricular involvement is unknown [7-9].
Clinical presentation
The clinical presentation at the time of discovery of LVNC in our case was heart failure complicated by rhythm disturbance and spontaneous intra-VG contrast. There is no pathognomonic clinical presentation for LVNC, but three clinical pictures have been shown to be the most frequent in cases of LVNC: heart failure (HF), arrhythmias and embolic events [10,11], which is consistent with the clinical case reported here.
Diagnosis
The diagnosis is confirmed by transthoracic ultrasound or magnetic resonance imaging. The criteria used for TTE (Jenni 2001) are:
- Multiple trabeculations
- Intertrabecular recesses
- Double layer structure
- Classically, the diagnosis is accepted if the myocardial ratio NC/C>2 in the parasternal short axis in systole [12].
The clinical case we report presents these jenni criteria, which allowed us to retain the diagnosis. However, the inaccessibility of MRI remains a shortcoming of our working conditions. Cardiac MRI still has its place as a complement to TTE in cases where doubt persists on TTE.
Prognosis
Certain characteristics appear to be associated with patients at higher risk of mortality: New York Heart Association class III/ IV dyspnoea, chronic atrial fibrillation, bundle branch block [13], sustained ventricular arrhythmias, larger left atrium and larger LV end-diastolic diameter at initial presentation [14]. Patients with these features may require closer follow-up and more intensive clinical management.
Treatment
There are currently no recommendations for the management of patients with LVNC. The current treatment of LVNC is therefore that of any cardiomyopathy, based on conventional treatments for heart failure [14-16].
This is a cardiomyopathy with rhythmic potential and therefore at risk of sudden death. In the case of complicated LVNC with reduced ejection fraction, drug treatment for heart failure with reduced LVEF is indicated with the same level of recommendation as for CMD with reduced LVEF. Our patient was therefore treated with a betablocker, ACE inhibitor and anti-aldosterone. This treatment remains incomplete according to the ESC 2023 recommendations, which can be explained by the inaccessibility of certain molecules such as SGLT2 inhibitors and the combination of valsartan and sacubitril in our working context. The indications for ICDs in the primary prevention of sudden death are not codified.
In secondary prevention, the indication is the same as for CMDs. With regard to sporting activities, in the case of symptomatic LVD at risk of sudden death, the ESC 2020 recommendations advise against competitive sports. Only group (IA) sports are authorised for leisure activities. Curative anti-coagulation, although debatable, is the best treatment for paroxysmal fibrillation. Clinical and para- clinical monitoring is based on an ECG, a TTE, a holter EKG, EE with VO2 at least once a year and more frequently in the event of new symptoms or if a therapeutic adjustment is required.
Conclusion
NCVG is one of the rare causes of heart failure in CMD as reported in this work. Despite the absence of MRI, the existence of the Jenni criteria has facilitated confirmation of its diagnosis in our country.
References
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006; 113: 1807-1816.
- Arbustini E, Narula N, Dec GW, et al. The MOGE (S) classification for a phenotype- genotype nomenclature of cardiomyopathy. Global Heart. 2013; 8: 355-382.
- Westphal JG, Rigopoulos AG, Bakogiannis C, et al. The MOGE(S) classification for cardiomyopathies: current status and future outlook. Heart Fail Rev. 2017; 22: 743-752.
- Nugent AW, Daubeney PEF, Chondros P, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national populationbased study. Circulation. 2005; 112: 1332-1338.
- www.filiere-cardiogen.fr.
- Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity?. Eur Heart J. 2011; 32: 1446-1456.
- Lahmiti S, Aboussad A. non-compaction du ventricule droit : à propos de deux cas. annales de Cardiologie et d'angéiologie. 2012; 61: 299-302.
- Weiford bC, subbarao Vd, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004; 109: 2965-2971.
- Rao g, Tauras J. Biventricular noncompaction Cardiomyopathy in an adult with unique Facial dysmorphisms: Case report and brief review. Case Rep Cardiol. 2015: 831341.
- Jenni R, Oechslinen, van der loo b. Isolated ventricular noncompaction of the myocardium in adults. Heart (british Cardiac society). 2007; 93: 11-15.
- Aras D, Tufekcioglu O, Ergun K, et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. Journal of cardiac failure. 2006; 12: 726-733.
- Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart (british Cardiac society). 2001; 86: 666-671.
- Ichida F, Tsubata S, bowles KR, et al. et al. novel gene mutations in patients with left ventricular noncompaction or barth syndrome. Circulation. 2001; 103: 1256-1263.
- Lofiego C, biagini E, Pasquale F, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart (british Cardiac society). 2007; 93: 65-71.
- Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. physiological genomics. 2003; 15: 165-176.
- 16. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the aCC/aHa 2005 guidelines for the diagnosis and Management of Heart Failure in adults: a report of the american College of Cardiology Foundation/ american Heart association Task Force on practice guidelines: developed in collaboration with the international society for Heart and lung Transplantation. Circulation. 2009; 119: e391- 479.