Hepatocellular Carcinoma Induced by Viruses: Epidemiological, Clinical and Therapeutic Aspects In Pointe Noire (Congo Brazzaville)
Author'(s): Ngatali Christian F.S1, Boumba A.L.M2, Ebatetou A2, Moukassa D3, Nkoua Mbon J.B4 and Ibara J-R5
1Department of oncology and Internal Medicine, LoandjiliGeneral Hospital, Congo.
2Pathology anatomy laboratory, Loandjili General Hospital, Pointe Noire, Congo.
3General Hospital of Edith Lucie Bongo, Congo.
4Department of oncology, CHU Brazzaville, Congo.
5Department of Gastroenterology CHU Brazzaville, Congo.
*Correspondence:
Dr. Ngatali Christian, Faculty of Health Sciences Brazzaville and Loandjili General Hospital.
Received: 27 December 2019; Accepted: 18 January 2020
Citation: Ngatali Christian F.S, Boumba A.L.M, Ebatetou A, et al. Hepatocellular Carcinoma Induced by Viruses: Epidemiological, Clinical and Therapeutic Aspects In Pointe Noire (Congo Brazzaville). Cancer Sci Res. 2020; 3(1); 1-5.
Abstract
Objective: To determinate the epidemiological, clinical and therapeutic aspects of hepatocellular induced by virus at Pointe Noire.
Patients and Methods: This was a cross-sectional descriptive study that took place in the cancer department of the general hospital of loandjili in Pointe Noire during the period from January 1, 2013 to December 31, 2018, for a duration of 6 years. Have been included in our study: All patients diagnosed with hepatocellular Carcinoma (HCC) with viral etiology B and/or C or both viruses. The variables studied were: Sociodemographic parameters: age, sex, level of study. Clinical parameters: telltale sign, tumor size, number of tumors, the stage of extension according to Barcelona liver Cancer Clinic (BLCC) classification. The level of alpha fetoprotein (AFP), viral etiology. The type of treatment. Bivariate analysis was done between size of tumor and the level of AFP.
Results: At the end of our study, 37 files of Hepatocellular Carcinoma patients fulfilling the criteria of inclusions were collected. The average age was 40.15 ± 14.40 years old. The extremes were 21 years and 70 years old. The most represented age group was the age group from 31 years to 40 years in 32% of cases. Men were most represented than women with respectively the rate of 59% and 41%. The most represented level of study was the primary level in 43% of cases. The telltale sign most represented was hepatomegaly in 73% of. The size of tumor was more than 5 cm in 81% of cases. Most of patients had single tumor in 81%. Most of the patient had a level of alpha fetoprotein more than 400 ng/ml in 86% of cases. The most represented viral etiology of hepatocellular Carcinoma was hepatitis B in 46% of cases. The most represented stage of extension was advanced stage of the Barcelona liver Cancer Clinic (BLCC) in 62.16% of cases. The most represented treatment was supportive therapy in 86% of cases, only 14% of patients received a specific treatment. The bivariate analysis between the tumor size and the level of alpha fetoprotein showed that there was relationship p<5%.
Conclusion: HCC is one of the increasing major health problems in both developing and developed countries. The most important risk factor is cirrhosis which is mainly due to hepatitis B virus and hepatitis C virus. Thus, much effort should be put into the field of prevention and treatment of viral hepatitis infections and chronic liver disease. Screening programs should be done to get rid of the problem, and most importantly, there must be an acceptable
and effective therapy for HCC.
Keywords
Introduction
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018, with about 841,000 new cases and 782,000 deaths annually [1]. Rates of both incidence and mortality are 2 to 3 times higher among men in most world regions ; thus liver cancer ranks fifth in terms of global cases and second in terms of deaths for males. Incidence rates are 2-fold greater among men in transitioned countries, but the highest rates are observed mainly in lower HDI (high development indice) settings, with liver cancer the most common cancer in 13 geographically diverse countries that include several in Northern and Western Africa (Egypt, the Gambia, Guinea) and Eastern and South-Eastern Asia (Mongolia, Cambodia, and Vietnam [2].
Primary liver cancer includes hepatocellular carcinoma (HCC) (comprising 75%-85% of cases) and intrahepatic cholangiocarcinoma (comprising 10%-15% of cases) as well as other rare types. The main risk factors for HCC are chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), aflatoxin-contaminated foodstuffs, heavy alcohol intake, obesity, smoking, and type 2 diabetes [3,4]. The major risk factors vary from region to region. In most high risk HCC areas (China, Eastern Africa), the key determinants are chronic HBV infection and aflatoxin exposure, whereas in other countries (Japan, Egypt), HCV infection is likely the predominant cause. In Mongolia, HBV and HCV virus and coinfections of HBV carriers with HCV or hepatitis δ virus, as well as alcohol abuse, all contribute to the high burden[4]. The rising obesity prevalence is considered a contributory factor to the observed increasing incidence of HCC in low-risk HCC areas [5].
Globally, both infections (HBV and HCV) are reported to contribute to greater than ca. 80% of HCC cases [6-8]. In developing countries they account for >90% of all HCC cases, whereas in developed countries - for 40% [9]. Comparing HCC incidence rates due to viral infections versus other etiologies revealed that an increase of HBV or HCV prevalence by 1% elevates by 14% and 10%, respectively, the incidence of liver cancer [10]. To our knowledge , few studies has been done in Congo about this subject. Therefore, our purpose was to determine the epidemiological, cilinical and therapeutic aspect of hepatocellular Carcinoma induced by virus in Pointe Noire.
Patients and Methods
This was a cross-sectional descriptive study that took place in the cancer department of the general hospital of loandjili in Pointe Noire during the period from January 1, 2013 to December 31, 2018, for a duration of 6 years. Have been included in our study: All patients diagnosed with hepatocellular Carcinoma (HCC) with viral etiology B and/or C or both viruses. The diagnosis of HCC has been established based on cytology or histology. Diagnosis of HCC was based on clinical, imaging and cytological histopathological findings. Diagnosis was achieved by ultrasonographic guided biopsy from the lesions, HCC radiological criteria in triphasic CT or presence of lesion by sonar and rising alpha fetoprotein more than 400 ng/ml. Following advances in understanding HCC-specific radiological features during phasic vascular perfusion of contrast during cross-sectional imaging with CT and MRI, the diagnosis of HCC in patients with cirrhosis who are under surveillance can now be made reliably without biopsy.
The variables studied were: Sociodemographic parameters: age, sex, level of study. Clinical parameters: telltale sign, tumor size, number of tumors, the stage of extension according the Barcelona liver cancer Clinic (BLCC) classification. The level of alpha fetoprotein (AFP), viral etiology. The type of treatment. survival. Bivariate analysis was done between size of tumor and the level of AFP.
Patients were also subdivided into five stages according to the BLCC as (0) very early stage—a mass of less than 20 mm in diameter, Child Pugh (CP) class A; (A) early stage—a mass of less than 50 mm in diameter or three masses with diameters of less than 30 mm, CP class A or B with absence of vascular invasion;(B) intermediary stage—a mass greater than 50 mm in diameter or more than three masses, CP class A or B with absence of vascular invasion; (C) advanced stage—any mass, CP class A or B and the presence of vascular invasion and/or extrahepatic metastasis and(D) terminal stage—any mass and CP class C.
The data entry was made from the Excel version 2016 software. Qualitative variables were represented in numbers and percentages. Quantitative variables were represented in numbers and on average. Statistical analysis and data processing were performed by the Excel 2016 software and graphpad Prism version 7 software. The statistical test used was Fisher's exact test for finding relationship between variables. The results were statistically significant for p<5%.
Results
At the end of our study, 37 files of Hepatocellular Carcinoma patients fulfilling the criteria of inclusions were collected. The average age was 40.15 ± 14.40 years old. The extremes were 21 years and 70 years old. The most represented age group was the age group from 31 years to 40 years in 32% of cases followed by the age group from 61 to 70 years in 24%, the group age from 21 to 30 years in 24% of cases and the group age from 41to 50 years (Table 1). Men were most represented than women with respectively the rate of 59% and 41% (Table 2).
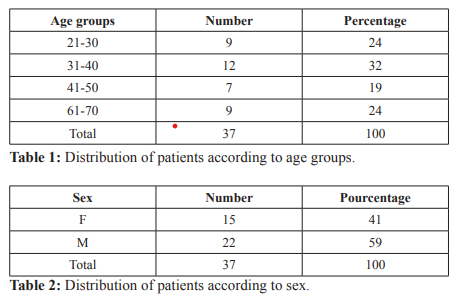
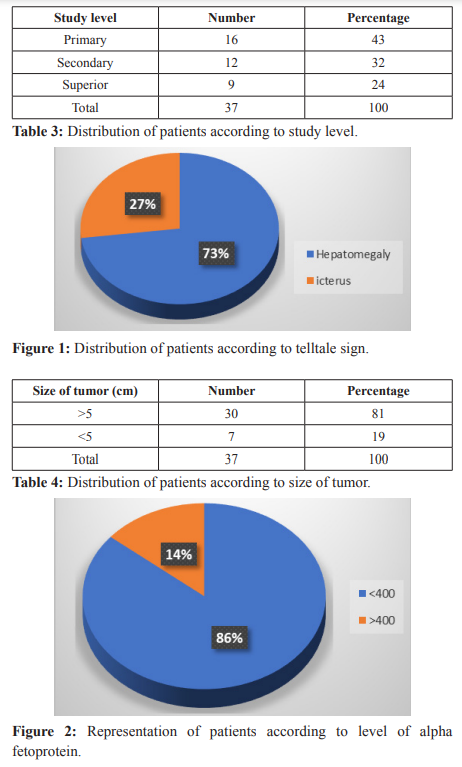
The level of education was the primary level in 43% of cases followed by the Secondary level of study in 32% and the Superior level in 24% of cases (Table 3). The telltale sign most represented was hepatomegaly in 73% of cases followed by icterus in 27% of cases (Figure 1). The size of tumor was more than 5 cm in 81% of cases and less than 5 cm in 19% of cases (Table 4). Most of patients had single tumor in 81%, multiple tumors represented 19% (Table 5). Most of the patient had a level of alpha fetoprotein more than 400 ng/ml in 86% of cases and alpha fetoprotein level less than 400 ng /ml was represented in 14% of cases (Figure 2).
The most represented viral etiology of hepatocellular Carcinoma was hepatitis B in 46% of cases followed by hepatitis C in 30%. Of cases. The association or coinfection of hepatitis B and hepatitis C was represented in 24% (Table 6). The most represented stage of extension was advanced stage of the Barcelona liver Cancer Clinic (BLCC) in 62.16% of cases followed by terminal stage in 32.43% of cases and intermediary stage in 5.41% of cases (Table 7). The most represented treatment was supportive therapy in 86% of cases, only 14% of patients received a specific treatment Table .The bivariate analysis between the tumor size and the level of alpha fetoprotein showed that there was relationship between two variables p<5%. Table 9.
Discussion
Although small in size with a sample of 37 patients and being an hospital study, our study reflects the situation of HCC in our context of resource-limited countries. Thus, At the end of our study, which took place in the oncology department of loandjili General Hospital in Pointe Noire, we collected 37 cases of patients with hepatocellular Carcinoma who met the inclusion criteria of our study. The average age in our study was 40.15 ± 14.40 years with extremes of 21 years and 70 years. This average age and these extremes are close to those found in the literature [11,12].
The younger onset of hepatocellular carcinoma (HCC) diagnoses in Africa could simply be a reflection of the younger population structure in sub-Saharan Africa and the effect of country of birth on age at the time of HCC diagnosis in the USA using the Surveillance, Epidemiology, and End Results program (SEER) 18 registry was evaluated [13]. The age of onset of HCC varies in different parts of the world. HCC tends to occur later in life in Japan, North America and European countries, where the median age of onset is above 60 years. In contrast, in parts of Asia and most African countries, HCC is commonly diagnosed in the age range 30–60 years [14]. The HCC BRIDGE study of 18,031 patients with HCC from 42 sites in 14 countries showed that the mean age at HCC diagnosis was 69,65 and 62 years in Japan, Europe and North America, respectively, whereas it was 59 and 52 in South Korea and China, respectively [14].
In Africa, high-quality data from population-based studies are lacking, but a tertiary-referral-centre-based cohort study published in 2015 showed that the age of onset of HCC is low in sub-Saharan Africa. A study of 1,552 patients with HCC from 14 centres in seven African countries showed a median age at HCC diagnosis of 45 years [15]. For HBV-induced HCC, the age range having the most frequent HCC diagnoses was 32.5–37.5 years [15]. The early onset of HCC in individuals born in sub-Saharan Africa was also seen in an analysis of 59,907 patients with HCC diagnosed in the USA between 2000 and 2012 in the Surveillance, Epidemiology, and End Results (SEER) programme. Very early-onset HCC at <40 years of age was most strongly associated with being born in West Africa (adjusted odds ratio (AOR) 16.3, 95% CI 9.2–27.9; P<0.01), Central/South/other Africa (AOR 11.0, 95% CI 4.5–23.7; P<0.01), Oceania (AOR 4.9, 95% CI 2.9–8.0; P<0.01) and East Africa (AOR 3.5, 95% CI 1.5–6.8; P<0.01) [16].
The number of African-born Americans in the SEER studies is small, leading to wide confidence intervals in the AOR estimates. Importantly, the countries of West Africa, where the earliest onset of HCC is seen, include 384 million people, approximately 30% of the population of Africa. High-quality, population-based studies are needed to improve our understanding of HCC epidemiology in Africa. The age group most represented in our study was the age group from 31 to 40 years ; this results is corroborated with the literature [17]. In Africa there are variation about age group [17- 20]. In our study men were most represented than women. This result was corroborated with literature [17-20].
The most represented study level was primary study level that lead to the lack of education ,information about hepatitis which was the leading etiology of HCC in our study. In other studies which showed an increase in HCV infection with an increase in older age, education below secondary level and low parity [21-23]. Patients generally present with symptoms of advancing cirrhosis, as know 80% - 90% of HCC is a background of cirrhosis [24] and these symptoms are mimics symptoms of cirrhosis and it includes pruritus, jaundice, splenomegaly, cachexia, hepatomegaly, variceal bleeding (Hematemesis/Melena) right upper quadrant pain, ascites, hepatic encephalopathy, periumbilical collateral veins, asterixis. While the ultrasound was incidentally finding with unrelated complains. In our country, patients came to the hospitals at the end stage of liver disease with more advanced symptoms due to uninterested to go to physicians when they become sick due to their poor socio-economic condition, life style as well as lack of awareness of the disease [25].
The Number of tumor was single in most of cases with an tumor size more than 5 cm in our study. In most of studies the number of tumor was single and the size tumor most represented was greater more than 5 cm [26,27]. On evaluating the characteristics of tumors in a Spanish multicenter study, of 705 HCC cases, it was found that in this study, 60.5% (133/220) of patients had masses less than or equal to 50 mm in diameter or three masses smaller than 30 mm in diameter [28].
In the Latin American study [29], of 240 cases, 63.3% had single masses and 12.5%, 6.7% and 13.3% had two, three and more masses, respectively. In the Raphael Raphe study [27], 64.2% had single masses, 11.5% had two masses and 4% had three masses. Multifocal disease was diagnosed in 20% of the sample. In our study most of patient had the level of alpha fetoprotein more than 400 ng/ml and there was relationship between tumor size and the level of alpha fetoprotein.
According to the studies of Trevisani et al. [30], Marrero et al. [31] and Lok et al. [32] who compared the accuracy of alpha-fetoprotein as a biomarker in the early detection of HCC with cut-off values between 10 and 20 ng/dL, the diagnostic sensitivity is about 60%. In the Raphael Raphe study, 29.2% of the cases presented normal alphafetoprotein levels, that is, less than 20 ng/dL. Values above 200 ng/dL were observed in 46% of the cases. In respect to the size of the tumor, it was observed that tumors smaller than 30 mm have normal level of alpha-fetoprotein and in tumors larger than 50 mm, the level was greater than 200 ng/dL in 67.4% of the cases [27].
The most represented viral etiology in our study was hepatitis B. This result corroborates with the literature. The hepatitis B virus is the most represented cause of liver cancer due to a virus in Africa (southern sub-Saharan Africa, western sub-Saharan Africa); on the other hand, the hepatitis C virus is the leading cause of liver cancer due to a virus in North Africa and the Middle East [33]. The alleged causes of the severity of this situation in Africa are multiple and can be summarized as follows: late access to care and drugs; poverty and weakness of infrastructure technical patient care facilities, especially regarding the use of specific high cost diagnosis and treatment; lack of skills of health workers; and lack of political drive [34,35].
Despite the commitment by African countries to allocate 15% of their Gross Domestic Product (GDP) to health improvement in 2001 (Declaration of Abuja, Nigeria), only four out of 53 countries have reached the target set in 2008 (Rwanda 18.9%; Liberia, 16.8%; Tanzania and Zambia 16.2% and 15.2%, respectively)[36]. As a result, the economic consequences of cancer in Africa remains at a high estimate of 849 million USD in 2009 [37].
The Majority of patients of our study arrived in advanced stage of extension of Barcelona Liver Cancer Clinic (BLCC).
Most of patients in our study received supportive care, only14% were treated by specific treatment (Sorafenib) in our study. In our outlook, most of patients came to the hospital to advanced stage or end of the liver disease and they didn’t receive a definitive treatment of HCC due to the unavailable of these treatment in our setup and there is no specialist center for liver disease for these treatment of HCC, but the patients received a supportive treatment both early stage and advanced stage due to the limited recourses of the country, so missing of specialist center in the country. This result corroborates with literature [25].
Conclusion
Hepatocellular Carcinoma is common in Pointe Noire. The mean risk factors in our context of limited resources is the infection of hepatitis B and C. Men are most affected than women by disease in young age. Patients arrive in advanced stage that leads to treat by the important using of supportive care instead of specific care. Thus, much effort should be put into the field of prevention and treatment of viral hepatitis infections and chronic liver disease. Screening programs should be done to get rid of the problem, and most importantly, there must be an acceptable and effective therapy for HCC.
References
- Freddie Bray, Jacques Ferlay, Isabelle Soerjomataram, et al. Global Cancer Statistics 2018 GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 CA CANCER J CLIN. 2018; 68: 394-424.
- Garcia M, Jemal A, Ward EM, et Global Cancer Facts & Figures 2007. Atlanta GA: American Cancer Society. 2007.
- London WT, Petrick JL, McGlynn KA, et al. Liver cancer. Cancer Epidemiology and Prevention. 4th eds. New York Oxford University 2018; 635-660.
- Chimed T, Sandagdorj T, Znaor A, et Cancer incidence and cancer control in Mongolia results from the National Cancer Registry 2008-12. Int J Cancer. 2017; 140: 302-309.
- Marengo A, Rosso C, Bugianesi E. Liver cancer connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 2016; 67: 103-117.
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016; 122: 1312-1337.
- Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012 a synthetic analysis. Lancet Glob 2016; 4: 609-616.
- De Martel C, Maucort-Boulch D, Plummer M, et al. World- wide relative contribution of hepatitis B and C viruses in hepatocellular Hepatology. 2015; 62: 1190-1200.
- Sartorius K, Sartorius B, Aldous C, et Global and country underestimation of hepatocellular carcinoma HCC in 2012 and its implications. Cancer Epidemiol. 2015; 39: 284-290.
- Omland LH, Jepsen P, Krarup H, et al. Liver cancer and non- Hodgkin lymphoma in hepatitis C virus-infected patients results from the DANVIR cohort study. Int J Cancer. 2012; 130: 2310-2317.
- Nnadi I, Olu-Eddo A, Obaseki D. Hepatocellular Carcinoma in Benin City Nigeria A Twenty-Five 1987-2011 Year Retrospective Histopathological Health. 2019; 11: 1177-1185.
- Mutuma GZ, Mbuchi MW, Zeyhle E, et al. Prevalence of Hepatitis B Virus HBV Surface Antigen and HBV-Associated Hepatocellular Carcinoma in Kenyans of Various African Journal of Health Sciences. 2011; 18 : 53-61.
- Yang JD, Altekruse SF, Nguyen MH, et Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017; 123: 81-89.
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death the bridge Liver Int. 2015; 35: 2155-2166.
- Yang JD, Gyedu A, Afihene MY, et Hepatocellular carcinoma occurs at an earlier age in Africans particularly in association with chronic hepatitis B. Am. J. Gastroenterol. 2015; 110: 1629-1631.
- Yang JD, Altekruse SF, Nguyen MH, et Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017; 123: 81-89.
- Seleye-Fubara D, Jebbin NJ. Hepatocellular Carcinoma in Port Harcourt Nigeria Clinicopathologic Study of 75 Cases. Annals of African 2007; 6: 54-57.
- Ndububa DA, Ojo OS, Adeodu OO, et Primary Hepatocellular Carcinoma in Ile-Ife, Nigeria A Prospective Study of 154 Cases. Nigerian Journal of Medicine. 2001; 10: 59-63.
- Chinombe N, Chavhunduka E, Matarira HT. Seroprevalence of HBV and HCV in Primary Hepatocellularcarcinoma Patients in Zimbabwe. Infect Agents 2009; 4: 15-20.
- Nwokediuko S, Ijoma U, Obienu O. Liver Cancer in Enugu, Southeast Insight Bioinformatics. 2011; 1: 1-5.
- Khamis HH, Farghaly AG, Shatat HZ, et al. Prevalence of Hepatitis C Virus Infection among Pregnant Women in a Rural District in Tropical Doctor. 2016; 46; 21-27.
- Hutchinson SJ, Goldberg DJ, King M, et Hepatitis C Virus among Childbearing Women in Scotland Prevalence, Deprivation, and Diagnosis. Gut. 2004; 53: 593-598.
- Murad EA, Babiker SM, Gasim GI, et al. Epidemiology of Hepatitis B and Hepatitis C Virus Infections in Pregnant Women in Sana’a, Yemen. BMC Pregnancy and Childbirth. 2013; 13: 127.
- Perz, JF, Armstrong GL, Farrington LA, et The Contributions of Hepatitis B Virus and Hepatitis C Virus Infections to Cirrhosis and Primary Liver Cancer Worldwide. Journal of Hepatology. 2006; 45: 529-538.
- Hassan-Kadle The Diagnostic Challenges, Possible Etiologies and Lack of Researches of Hepatocellular Carcinoma in Somalia. Open Journal of Gastroenterology.2017; 7: 115-123.
- Wu E, Hernandez B, Wong L. Hepatocellular Carcinoma in Micronesians a Growing Pacific Islander Population in the Open Journal of Gastroenterology. 2018; 8: 223-233.
- Raphael Raphe, Willian J. Duca, Paulo C. Arroyo Jr, et al. Hepatocellular Carcinoma Risk Factors, Diagnosis, Staging and Treatment in a Referral Journal of Cancer Therapy. 2013; 4: 10.
- Mazzaferro V, Regalia E, Dogi R, et Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. New England Journal of Medicine. 1996; 334; 693-369.
- Fassio E, Díaz S, Santa S, et al. Etiology of Hepatocellular Carcinoma in Latin America: A Prospective, Multicenter, International Annals of Hepatology. 2010; 9: 63-69.
- Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum Alpha-Fetoprotein for Diagnosis of Hepatocellular Carcinoma in Patients with Chronic Liver Disease Influence of HBsAg and Anti-HCV Status. Journal of Hepatology. 2001; 34: 570-
- Marrero JA, Feng Z, Wang Y, et Alpha-Fetoprotein DesGamma Carboxyprothrombin, and Lectin-Bound AlphaFetoprotein in Early Hepatocellular Carcinoma. Gastroenterology. 2007; 137; 110-118.
- Lok AS, Sterling RK, Everhart ME, et Des-Gamma- Carboxyprothrombin and Alpha-Fetoprotein as Biomarkers for the Early Detection of Hepatocellular Carcinoma. Gastroenterology. 2010; 138: 493-502.
- Akinyemiju T, Abera S, Ahmed M, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global Regional and National Level Results From the Global Burden of Disease Study JAMA Oncol. 2017; 3: 1683-1691.
- Ly Enjeux et perspectives de la prévention des cancers dans les pays en développement. J. Afr. Cancer. 2011; 3: 268-272.
- Ly M, Ly A, Rodrigues M, et al. Le cancer en Afrique, un nouveau défi sanitaire. Exemples du Mali et de l’association Bull Cancer. 2010; 97: 965-968.
- Guerin S, Hill C. L’épidémiologie des cancers en France en 2010 comparaison avec les États-Unis. Bull. Cancer. 2010; 97: 47-54.
- Dangou JM, Sambo BH, Moeti M, et al. Prévention et lutte contre le cancer dans la région Afrique de L’OMS un appel à l’action. Afr. Cancer. 2009; 1: 56-60.