Human Placental Mesenchymal Stem Cells Potency and Differentiation
Author'(s): Doaa Aboalola1,2,3,*
1King Abdullah International Medical Research Center, Jeddah,Western Region, Saudi Arabia.
2King Saud bin Abdulaziz University for Health Sciences, Jeddah,Western Region, Saudi Arabia.
3Ministry of National Guard Health Affairs, Jeddah, Western Region, Saudi Arabia.
*Correspondence:
Doaa Aboalola, King Abdullah International Medical Research Center, Saudi Arabia.
Received: 27 March 2020; Accepted: 18 April 2020
Citation: Doaa Aboalola. Human Placental Mesenchymal Stem Cells Potency and Differentiation. Cancer Sci Res. 2020; 3(2); 1-4.
Keywords
Introduction
The first report of the presence of adult stem cells was in 1961 by Canadian scientists McCulloch and Till [1]. Bone marrow MSCs were the first to be isolated in 1968 by Friedenstein [2]. To define the MSC population in vitro, the International Society for Cellular Therapy established three criteria, namely that a MSC must be a) plastic- adherent when maintained in standard culture conditions, b) express CD105 (a stromal and vascular marker), CD73, and CD90 (fibroblast markers) and lack hematopoietic markers such as CD45 and CD34, and c) be capable of differentiation into osteoblasts, adipocytes, and chondroblasts [3]. Although traditionally isolated from bone marrow [4], more recent reports have described the isolation of cells with MSC characteristics from other mature organs and tissues such as skeletal muscle [5], adipose tissue [6], deciduous teeth [7], umbilical cord [8] and peripheral blood [9], fetal liver and lung, amniotic fluid, synovium and the circulatory system [10].
In culture, MSCs are defined as plastic-adherent, fibroblast-like cells which are able to self-renew and differentiate into different mesodermal cell lineages including bone, adipose and cartilage tissue [3]. Moreover, MSCs are very important candidates for regenerative medicine because most adult tissues possess multipotent MSCs, which are crucial for tissue maintenance and repair [11]. Also in contrast to ESC, human MSCs have been studied in phase 1 and 2 clinical trials with the possibility of being used in the clinic in the very near future [10].
The placenta is a vital organ for the embryo, acting as the interface between the fetal and maternal environments [12] unlike the early embryo from which embryonic stem cells are derived, the
placenta is a non-controversial source of adult mesenchymal stem cells (PMSCs) and is a readily available source of MSCs for possible tissue regeneration therapy for human patients [12-15]. The embryonic mesenchymal cells infiltrate the cytotrophoblast layer at 6-7 days post fertilization, where these cells in conjunction with trophoblast stem cells, invade the maternal endometrium for implantation and initiation of placental development [16,17].
The main functional units of the placenta are the chorionic villi, which carry the embryonic blood and allow the fetal-maternal exchange [18]. Moreover, stem cells derived from the chorionic villi have greater cell expansion compared to the adult bone marrow MSCs [19].
In this study, cells previously obtained from preterm placental tissue (15 and 20 weeks) and representative of late first and second trimester pregnancy, were characterized for mesenchymal stem cell markers and differentiation ability.
Materials and Methods
PMSCs isolation
PMSC isolation and experiments were conducted with the approval from the Health Sciences Research Ethics Board of Western University (REB# 12154). Informed consent was obtained from healthy women undergoing therapeutic termination of pregnancy, and the PMSCs used in this study were isolated from 15 and 20 week preterm placental tissue. After surgery, chorionic villi were dissected, washed, and minced with surgical scissors and forceps, and then subjected to enzymatic digestion with collagenase IV (369 IU/mg), hyaluronidase (999 IU/mg), and DNase I (2,000 IU/mg) followed by 0.05% trypsin. The sample was then washed for 10 minutes with 10% FBS in DMEM/F12 medium and the resulting single cell suspension was separated by density centrifugation over a Percoll gradient using a modified protocol by Worton et al [20,21].
Cell culture
Cells from Percoll gradient fractions #3, #4, and #5 were plated on to T75 flasks, cultured, and maintained using DMEM/F12 media supplemented with 15% FBS and FGF-2 (50 ng/mL) containing 100 U/mL Penicillin, 100 μg/mL Streptomycin, and 0.25 μg/mL Amphotericin-B. The non-adherent cells were discarded at the time of media change, which was performed every 72 hours. Cells were then passaged 1:2 approximately once per week using 0.05% Trypsin for 10 min at 37°C for 3 passages.
Osteogenic differentiation
Cells were grown in 6-well plates in osteogenic differentiation media (MesenCult basal medium, osteogenic differentiation promoting supplements, ascorbic acid, β-glycerophosphate and dexamethasone) purchased from Stem Cell Technologies. Media was changed every 3 days. After three weeks, cells were fixed using 100% ice cold ethanol for 30 minutes, then dried at room temperature.
The wells were stained with 1 mL of 0.01% of alizarin red for 40 minutes at room temperature. The staining was observed under an inverted Zeiss light microscope. Cells grown in 10% FBS media were used as negative controls. The staining of the cells was solubilized using 1mL of 10% cetylpyridium phosphate in 10 nM sodium phosphate pH 7.0 and the absorbance of a 200 μL reaction volume was read at λ=570 nm using a plate reader- spectrophotometer.
Adipogenic Differentiation
Cells were grown in 6-well plates in adipogenic differentiation media (StemPro basal medium, adipogenesis supplements, Gentamicin reagent) from Gibco-Invitrogen for three weeks. Media was changed every 3 days and cells were fixed with 100% ice cold ethanol for an hour, then 60% Isopropanol was added for 5 minutes before staining with oil red O for an hour. Finally, hematoxylin stain was added for one minute to stain nuclei. Cells grown in 10% FBS media were used as negative controls.
Flow Cytometry Analysis
Cells were trypsinized for 10 minutes using TripleXExpress diluted in PBS, at 37ºC. After the cells were detached from the flask, trypsin was neutralized with 10% FBS in DMEMF/12 medium, cells were washed and incubated for one hour with fluorochrome labeled primary antibody against MSCs markers. CD73 (#550256) (BD Pharmingen), PE conjugated CD105 (#12- 1057-73) (eBioscience), and CD-117/c-Kit (sc-13508) (Santa Cruz Biotechnology) were used.
Statistical Analysis
All experiments were performed in triplicate. GraphPad Prism Software 5.0 was used to generate all graphs and analyses. A two- way ANOVA followed by a Bonferroni’s multiple comparison test was used to calculate significant differences when p<0.05. Graphic representation values are presented as mean ± SEM (shown as variance bars).
Results
PMSCs Isolated from two different gestational ages Placental Tissue Showed Stem Cell Characteristics
Mesenchymal stem cells from preterm placentae were isolated from the late first trimester of pregnancy (15 weeks) (Figure 1) and from the second trimester (20 weeks) (Figure 2). Cells were collected from layers 3, 4, and 5 based on the Percoll gradient density (Figures 1A and 2A). In vitro, the cells isolated were plastic adherent (Figure 1B and 2B). Stem cell markers CD73, CD105, and CD117 were measured using flow cytometry (Figures 1C and 2C).
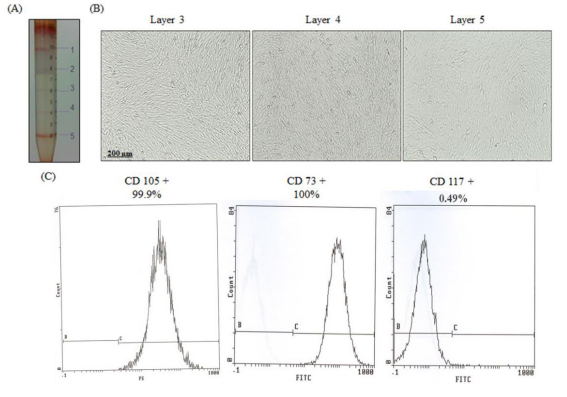
Figure 1: PMSC Isolation from 15 weeks preterm placenta. (A) The dissected villous tissue was digested enzymatically and cells were separated using a discontinuous Percoll gradient. Five cell fractions were typically obtained corresponding to five different densities and cells were isolated from layers 3, 4, and 5. (B) Phase contrast images of the isolated PMSCs, from all three layers, grown in culture after 4 weeks.
(C) PMSCs from passage 4 of all three layers were positive for CD73 and CD105 (>98%), and were negative for CD117 (<1%) (measured by flow cytometry). Flow cytometry histograms are representative of all 3 layers from one placental tissue as they showed the same results.
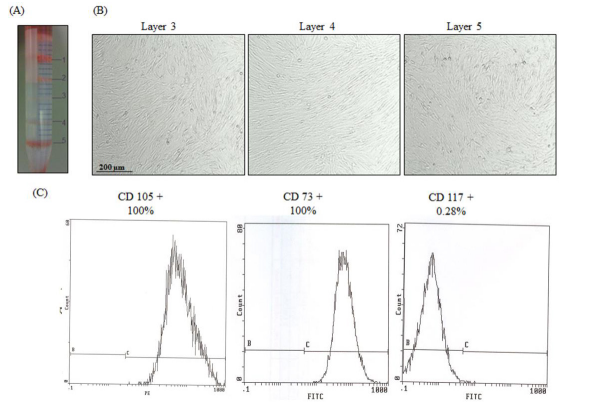
Figure 2: PMSC Isolation from 20 weeks preterm placenta. (A) Successful cell separation using Percoll gradient. Five cell fractions were obtained and cells were isolated from layers 3, 4, and 5. (B) Phase contrast images of the isolated PMSCs, from all three layers, grown in culture after 4 weeks. (C) PMSCs from passage 4 of all three layers were positive for CD73 and CD105 (>98%), and were negative for CD117 (<1%) (measured by flow cytometry). Flow cytometry histograms are representative of all 3 layers from one placental tissue as they showed the same results.
PMSCs Demonstrate Osteogenic and Adipogenic Differentia- tion Competence
After 20 days in culture, cells were fixed and stained for differentiation markers. Alizarin red was used for osteogenic differentiation and oil red O for adipogenic differentiation.
All three layers of isolated PMSCs formed multilayered structures in which calcium deposits were detected by alizarin red staining from both 15 weeks (Figure 3A) and 20 weeks PMSCs (Figure 3B). Adipogenic differentiation was also achieved for all three layers, as seen with oil red O staining and the formation of round adipocytes for both gestational age PMSCs (Figure 4A and 4B).
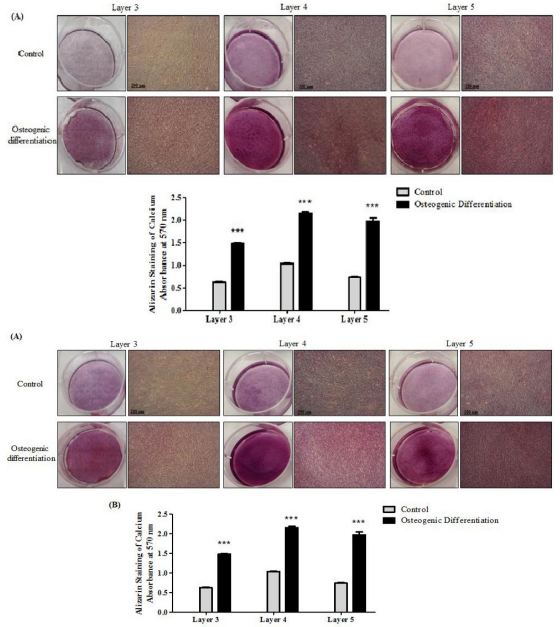
Figure 3: PMSCs from different gestational ages differentiate towards the osteogenic lineage. PMSCs isolated from all three layers showed osteogenic differentiation when cultured in osteogenic medium, as measured by the accumulation of calcium deposits shown with alizarin red staining in cells isolated from (A) 15 weeks and (B) 20 weeks placenta samples compared to untreated control at 3 weeks (10x magnification). The lower panel shows alizarin red staining quantification. Data are representative of three independent experiments from one placenta per gestational age. (Two-way ANOVA followed by a Bonferroni’s multiple comparison test, ***=P<0.001).
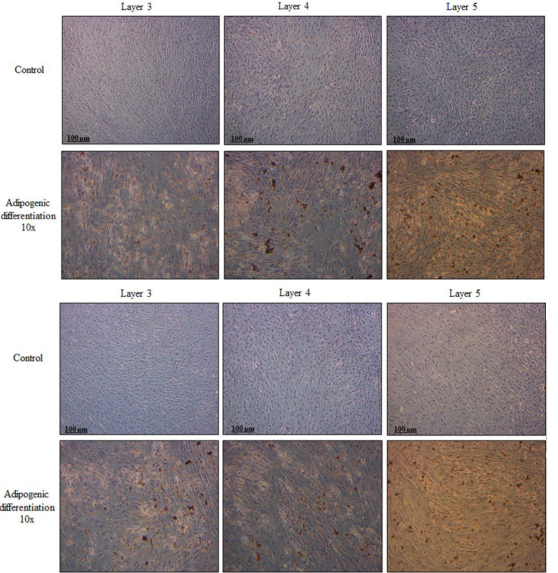
Figure 4: PMSCs isolated from different gestational ages differentiate towards the adipogenic lineage. Cells isolated from 15 week (A) and 20 week (B) placentae, were cultured in adipogenic medium or left untreated (control) for 21 days. All three layers showed the ability to differentiate into adipocytes shown by the accumulation of lipid droplets within the cells with the oil red O staining. Results are representative of three independent experiments from one placenta per gestational age.
Conclusion
PMSCs were isolated based on density gradient separation and growth adherence to tissue-culture plastic. Cultured PMSCs were positive for the mesenchymal markers CD73 and CD105 (>95%) and did not express the hematopoietic marker c-kit (<10%).
Moreover, PMSCs isolated from the chorionic villi of preterm placental tissue (at 15 weeks and 20 weeks) differentiated into osteoblasts and adipocytes; confirming multipotent differentiation of PMSCs. Thus, PMSCs represent an ethical, readily available, and promising cell type for the development of tissue regeneration therapy, due to early ontogeny and multipotent potential, for future experimental and clinical applications.
Acknowledgment
Thanks and appreciation to Dr. Victor K.M. Han (Departments of Anatomy and Cell Biology, and Paediatrics, Schulich School of Medicine and Dentistry; Children's Health Research Institute, Lawson Health Research Institute; University of Western Ontario, London, Ontario, Canada) for his continuous supervision, support, and guidance. Data from this paper was submitted as part of a PhD thesis [22].
This work was supported by CIHR grant 111024.
References
- Till JE, McCulloch A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961; 14: 213-222.
- Phinney Building a Consensus Regarding the Nature and Origin of Mesenchymal Stem Cells. J Cell Biochem Suppl. 2002; 38: 7-12.
- Wagner W, Ho AD. Mesenchymal Stem Cell Preparations- Comparing Apples and Oranges. Stem Cell Rev. 2007; 3: 239-248.
- Friedenstein AJ, Petrakova KV, Kurolesova AI, et Heterotropic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6: 230-247.
- Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem Gene Therapy. 2002; 9: 642-647.
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi- lineage cells from human adipose tissue and bone Cells Tissues Organs. 2003; 174: 101-109.
- Miura M, Gronthos S, Zhao M, et al. SHED stem cells from human exfoliated deciduous teeth. PNAS. 2003; 100: 5807-
- Mareschi K, Biasin E, Piacibello W, et al. Isolation of human mesenchymal stem cells bone marrow versus umbilical cord Hematologica. 2001; 86: 099-1100.
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000; 2: 477-488.
- Maria Ester Bernardo, Franco Locatelli, Willem E. Fibbe. Mesenchymal stromal cells a novel treatment modality for tissue Ann N Y Acad Sci. 2009; 1176: 101-117.
- Sensebe L, Bourin Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009; 87: S49-S53.
- Forbes K, Westwood M. IGF axis and placental function a mini Horm Res. 2008; 69: 129-137.
- Anker PS, Scherjon Sa, Van der Keur CK, et Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells. 2004; 22: 1338-1345.
- Fukuchi Y, Nakajima H, Sugiyama D, et Human Placenta- Derived Cells Have Mesenchymal Stem/Progenitor Cell Potential. Stem Cells. 2004; 22: 649-658.
- Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta comparison with human bone marrow mesenchymal stem Cell Biol Int. 2006; 30: 681- 687.
- Ornella Parolini, Francesco Alviano, Gian Paolo Bagnara, et al. Concise review isolation and characterization of cells from human term placenta outcome of the first international Workshop on Placenta Derived Stem Stem Cells. 2008; 26: 300-311.
- Pieternella S In 't Anker, Sicco A Scherjon, Carin Kleijburg- van der Keur, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004; 22: 1338-1345.
- Kaufmann P, Mayhew TM, Charnock-Jones DS, et Aspects of human fetoplacental vasculogenesis and angiogenesis. II Changes during normal pregnancy. Placenta. 2004; 25: 114- 126.
- Poloni A, Rosini V, Mondini E, et al. Characterization and expansion of mesenchymal progenitor cells from first- trimester chorionic villi of human Cytotherapy. 2008; 10: 690-697.
- Worton RG, McCulloch EA, Till JE. Physical separation of hemopoietic stem cells from cells forming colonies in J Cell Physiol. 1969; 74: 171-182.
- Aboalola D, Han VKM. Insulin-like growth factor binding protein-6 alters skeletal muscle differentiation of human mesenchymal stem Stem Cells International. 2017.
- Aboalola D. Building muscle the role of insulin-like growth factor binding protein-6 and insulin-like growth factors in the differentiation of placental mesenchymal stem cells into skeletal Electronic Thesis and Dissertation Repository. 2017; 4557.