Hyperganglionosis in Pneumatosis Cystoides Intestinalis- A Clinicopathological Review in Adults
Author'(s): Suresh Tharmaradinam MD1, Selliah Kanthan MBBS, FRCS, FRCSC2, and Rani Kanthan MBBS, MS, FRCS, FRCPC, FCAP, M.Ed1
1Dept. of Pathology and Laboratory Medicine, University of Saskatchewan, Canada.
2Division of General Surgery, University of Saskatchewan, Saskatoon, Canada.
*Correspondence:
Dr. S. Tharmaradinam and Dr. R. Kanthan, Dept. of Pathology & Laboratory Medicine, Royal University Hospital, 103, Hospital Drive, Saskatoon, Saskatchewan, S7N 0W8, Tel: 306 655 0695, 306 655 2158, Fax: 306 655 2223.
Received: 29 November 2020 Accepted: 23 December 2020
Citation: Tharmaradinam S, Kanthan S, Kanthan R. Hyperganglionosis in Pneumatosis Cystoides Intestinalis- A Clinicopathological Review in Adults. Gastroint Hepatol Dig Dis. 2020; 3(2): 1-7.
Abstract
Purpose/Background: Pneumatosis cystoides intestinalis, a rare entity, is not an isolated diagnosis but a finding that suggests an underlying process whose pathogenesis is not well understood. In this case series, we explore a novel histopathological finding of hyperganglionosis in pneumatosis cystoides intestinalis [PCI] as a cause-vs-effect phenomenon.
Methods: In a previously published index case of PCI we discovered hyperganglionosis as an associated finding. This discovery led to a twenty-year retrospective search of the Laboratory Information Service [LIS] in our surgical pathology laboratory that identified a total of twenty three cases with reported finding of PCI of which seven cases were excluded due to lack of availability of histological slides and /or blocks. In the remaining sixteen cases all the relevant histopathological slides were reviewed to confirm the diagnosis of PCI. One representative block in each case was subjected to immunohistochemical staining with antibodies to S100, Calretinin and CD68 for further evaluation of hyperganglionosis with review to their clinical context.
Results: This study reports on sixteen cases of PCI that have been studied in detail with their additional stains and their clinicopathological review. All cases upon review confirmed the presence of the diagnostic pathological finding of multiple, varied size and shapes of non-communicating cysts of PCI either mucosal/ submucosal intramuscular and/or subserosal. Additionally, prominent, enlarged hypertrophic ganglions associated with hypertrophic nerve fibers were seen in association with these cysts as highlighted by S100 and Calretinin. CD68 stained slides outlined the histiocytes and giant cells surrounding the cysts of PCI as expected.
Conclusion: The exact pathogenesis of non-communicating air-filled cysts within the bowel wall remains poorly understood especially in cases with no evidence of perforation /obstruction and /or ischemic changes/. Many theories have been proposed to explain the presence of intramural gas that include the mechanical theory of mucosal injury, bacterial theory of gas production, counterperfusion-saturation theory and the pulmonary gas theory. We propose a neuronal theory with a detailed discussion of dysgenetic ganglion cells with abnormal peristalsis resulting reversal airflow’ with intramural accumulation of intraluminal air or that these hypertrophied ganglions and nerves could be the resultant effect of the forced intramural expansion by the cysts; thus, reminiscent of the debate of which came first -the chicken or the egg.
Keywords
Introduction
We prefer the terminology pneumatosis cystoides intestinalis [PCI] or pneumatosis intestinalis [PI] in contrast to pneumatosis cystoides coli [PCC]/pneumatosis coli [PC] as pneumatosis-the finding of intestinal intramural air can be seen in both, the large intestine [colon] and the small intestine. PCI a rare entity, seen in infants, children and adults is not an isolated diagnosis but, a radiological and/or pathological finding to describe pockets of gas within the intestinal walls that suggests an underlying process [1-3].
The aim of this study was to explore the novel histopathological finding of hyperganglionosis associated with the cysts found in pneumatosis cystoides intestinalis [PCI] in adults.
Material and Methods
A twenty three year [1996-2019] retrospective complex search query of the Laboratory Information Service [LIS] in our surgical pathology laboratory was conducted for all cases above the age of 18 years with ‘pneumatosis’ in their diagnostic text. The pathological reports of all cases were appraised and only those cases with the diagnosis of pneumatosis were included for this study. Review of the database identified twenty three potentially eligible patients of which seven cases were excluded due to lack of availability of histological slides and or blocks. A detailed histopathological review of the hematoxylin and eosin (H&E) stained slides of the remaining sixteen cases was undertaken to confirm the presence of PI. One representative formalin fixed paraffin embedded tissue block in each case was subjected to additional immunohistochemical staining with antibodies to S100, Calretinin and CD68. This was performed using a standard streptavidin -biotin method after antigen retrieval with adequate positive and negative controls. The stained slides were reviewed for staining patterns and analyzed independently by two pathologists. A clinicopathological correlation with chart review was also undertaken including database elements of patient demographics, primary surgery undertaken, perioperative studies, location and extent of disease involvement and documented co-morbidities together with their radiological findings.
Results
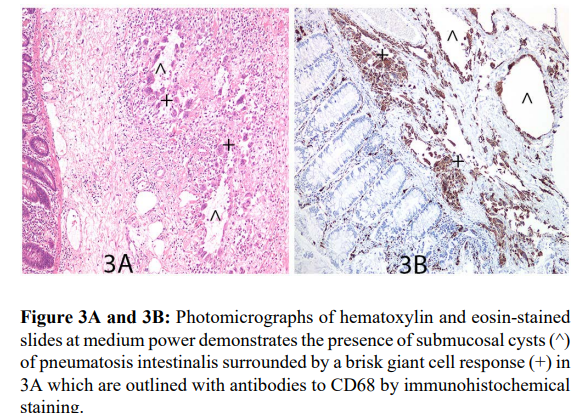
Detailed pathological review of all sixteen cases included in this study confirmed the diagnostic pathological finding of the presence of mucosal/submucosal/ intramuscular and/or subserosal non-communicating air filled cysts within the intestinal wall in keeping with PCI as seen in Figure 1A-1D. The distribution of the cysts was primarily in the submucosa in most cases while some cases demonstrated transmural distribution of the air filled cysts. Additionally, a variety of prominent dysgenetic, enlarged hypertrophic ganglions associated with hypertrophic nerve fibers were seen in association with these cysts. These were beautifully highlighted by S100 and Calretinin with no staining of the adjacent cysts as seen in Figures 2A-2D. Though this was observed in all cases, in cases with only mucosal biopsies available for study, the number and density of hyperganglionosis seen were low due to the limited material available for analysis. CD68 stained slides as seen in Figure 3A and 3B outlined the histiocytes and giant cells surrounding the cysts of PCI as expected.
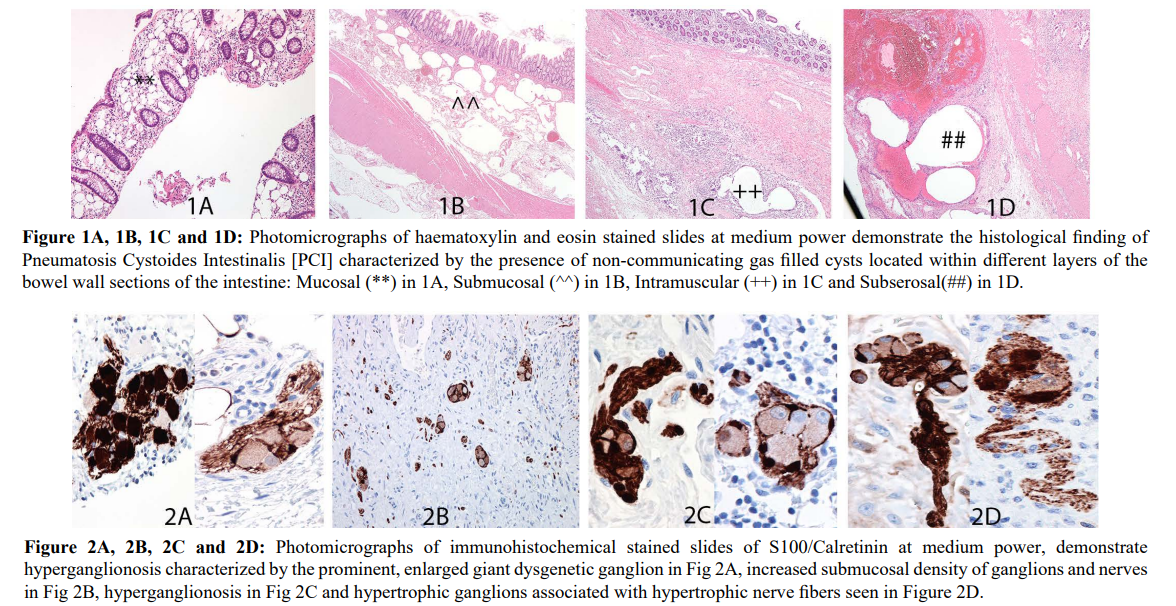
These cases of PCI were commonly observed in adults over the age of 50 years though one case of PI was seen in a 22 year old woman who had severe graft versus host disease with complications of ischemic bowel resulting in a subtotal colectomy. There was no sex predilection with equal prevalence among males and females [8 cases each]. The resected specimens in which PI was seen included sigmoid colons [4 cases], small bowel ileal/jejunal resections [2cases], Ileocecal -right hemicolectomies [5 cases with appendix 1case] and four endoscopic surveillance mucosal biopsies of the hepatic flexure, transverse colon, right colon and left colon. In many of the ileocecal resections PCI was seen in sections of the small bowel (terminal ileum) and large bowel (cecum/ascending colon).
The majority of the resections was associated with states of sepsis, past history of perforation, obstruction, volvulus and suspected carcinoma. The small bowel resections were associated with diverticulae and small bowel obstruction with perforation. The appendix was part of a completion right hemicolectomy for goblet cell carcinoid/adenocarcinoma of the appendix. The endoscopic surveillance mucosal biopsies were associated with past history of cancer, Crohn’s and investigation of polyps. Two of the sixteen patients had associated respiratory comorbidities; one patient with obstructive sleep apnea and another had severe chronic obstructive pulmonary disease [COPD]. PCI was classified as primary idiopathic in 3 cases (cases marked with an asterix**) representing 18%; as they occur in patients with no other relevant significant past or concurrent pathology. These are summarized in Table 1. Interestingly, years following the diagnosis of PCC, one patient subsequently developed obstructive sleep apnea while a second patient developed marked obesity.
Discussion
Pneumatosis intestinalis is an uncommon condition characterized by the presence of multiple gases filled non communicating cysts within the wall of the intestines, better known in veterinary medicine than human medicine as it is common in pigs. It was first described as early as 1730 by Duvernoy during a cadaver dissection [1,4]. The pathogenesis of PCI is not well understood and seems to be associated with a wide variety of benign [5-6] and life threatening conditions in adults and infants. Therefore, the associated cause of PCI can be divided into primary idiopathic up to 15% and those related to secondary causes up to 85% [1,7]. The distribution was approximately the same in our study with 3 cases (~18%) of primary idiopathic PCI and 13 cases of secondary PCI. A study of 919 cases in 1979 found a prevalence of 42% for ileal localization, and 36% for the colon, and the remaining 22% of the cases in which both the small and large intestine were involved [1,8] with almost uniform sparing of the rectum [1,9]. In
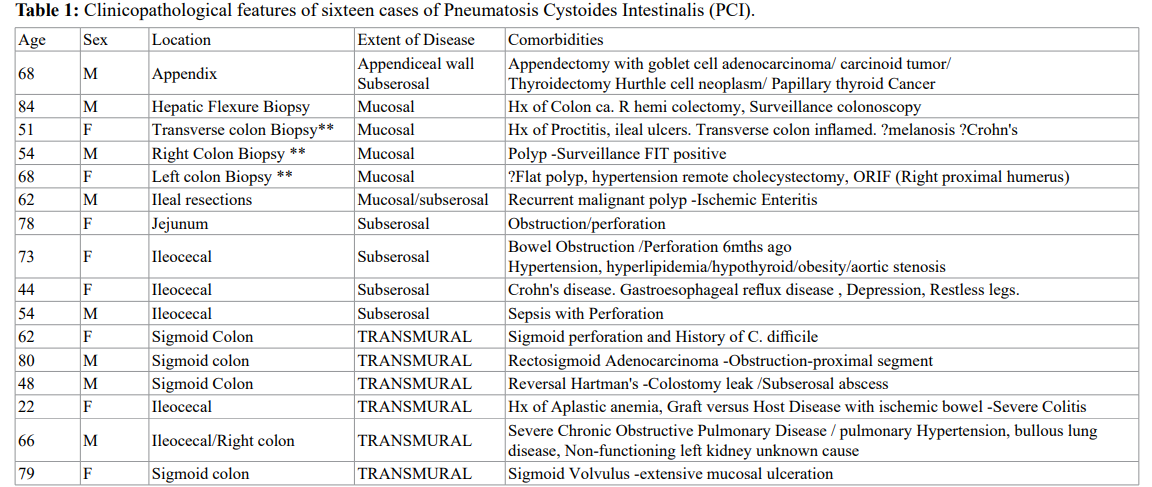
This is a composite table that summarizes the clinicopathological features of sixteen cases of adult pneumatosis intestinalis including age, sex, anatomical site, enteric location, and individual comorbidities in their clinical context. Cases marked with an asterix**denote the presence of primary idiopathic PCI seen in patients with no other relevant significant past or concurrent pathology.
our limited study, we encountered a dominant prevalence of PCI in the ileocecal and sigmoid colon.
The more commonly prevalent secondary causes of pneumatosis includes patients with gastrointestinal infections, ischemia and obstructions along with chronic obstructive pulmonary disease, connective tissue disorders, organ transplantation, leukemia and various state of immunodeficiency that can also present with PCI [1,3-4,10-11]. Thus, it is important to recognize that pneumatosis intestinalis is a clinical sign with histological findings but is in itself not a diagnosis [12]. Radiological imaging can further confound the issue with suggestions of perforation and or malignancy [1,13]. Additionally, in contrast to resected specimens where the diagnosis is relatively straightforward, in mucosal biopsies however, identification of PCI is far more challenging as they are frequently described as polyps by the endoscopists causing a pathological discordant finding of pseudopolyps: further complicated as they can be mistaken for fat cells and therefore are also referred to as pseudolipomatosis on microscopic examination. The presence of giant cells in the mucosa and or submucosa associated with variable inflammation and cyst disarray can lead to diagnostic interpretative confusion with Crohn’s disease [11].
Although the exact etiopathogenesis of PCI remains unclear, PI is attributed to many contributing factors. There are four popular proposed theories to explain the presence of intestinal intramural air filled cysts:
- Mechanical theory of mucosal injury/defects of the bowel with resultant disruption of the gut microbiome that allows for intraluminal air to dissect into the intestinal Wall.
- Bacterial theory of the invasion of gas forming bacterial organisms from within the lumen to within the wall that lead to intramural gas production.
- Pulmonary gas theory-of gas ruptured from the lung parenchyma dissecting into the mediastinum that travels through the retroperitoneum and perivascular tissue planes to be deposited in the wall of the bowel and..
- Counterperfusion-saturation theory wherein the nitrogen and hydrogen diffusion gradients across the intestinal lumen and vessels are disrupted leading to deposition of gas within the luminal wall [1,3,7-9,14].
Recently, we published a case report of PCI wherein based on the identification of hyperganglionosis and hypertrophied nerve fibers seen in association with the intramural cysts we proposed a neuronal theory to explain the pathogenesis of PCI with these findings representing either contributing to the cause or occurring as an effect to the presence of intramural air [1]. We shall now explore this in detail in the following text.
Neuronal theory
The gastroenteric nervous system is derived from the vagal and sacral neural crest cells that delineate from the neural tube and undergo extensive migration and proliferation to colonize the entire length of the gut resulting in its intrinsic innervation [15]. Typically, neurons are overproduced, and the neuronal network undergoes extensive programmed cell death (apoptosis), to as many as 50% [15]. Cell death is an essential evolutionary physiological process that matches the number of neurons to the size of their targeted field [15]. The submucosal nerve plexus develops from primitive neuroblasts derived from neural crest under the influence of Homeobox genes. Neuroblasts migrate through the mesenchyme surrounding the developing gut. They are primarily derived from the vagal source and, thus, migration predominantly occurs in a cranio-caudal direction. There is a smaller contribution from the sacral source as well. The myenteric plexus is cited first, which then moves dorsoventrally to form the submucosal plexus. The precise mechanism of control of this migration is unknown. If there is a defective mechanism present, one would expect that the number of ganglia would be reduced. When a physical barrier to the neuroblasts cell migration is present, the migratory stimulus is hampered which leads to the production of an area with more ganglia than normal resulting in neuroblasts-cell collections at the edge of this obstruction producing a segment with increased number of giant ganglia, usually within the proximal portion [16]. It has been demonstrated that apoptosis occurs in the vagal neuronal crest cells as they migrate towards the developing foregut with inhibition of cell death that leads to excess cells within the pathway leading to the increased numbers of precursor cells with resultant hyperganglionosis [16].
A ganglion cell is considered as any part of a ganglion cell body (with or without visible nucleus) [17]. The definition of a ‘hyperganglion’ /hyperganglionosis is not well established. One criterion is the concept of ganglion size, which refers to the number of ganglion cells in a histological cross section of an individual submucosal ganglion [17]. Most recently studies have reported “giant submucosal ganglia” as more than 8 ganglion cells per ganglion in 15 – micrometer- thick frozen section, that has been stained immunohistochemically for LDH (lactate dehydrogenase) to highlight mature and immature ganglion cells [17]. A “ganglion cell” is considered any part of the ganglion cell body with or without a visible nucleus. Hyperganglionosis is also referred to giant ganglia 2-3 times larger than normal ganglia with hyperplasia of the submucosal/intermuscular plexus or the presence of isolated ganglion cells in the lamina propria mucosae or the muscularis mucosae; however, the exact parameter for this diagnosis remains controversial [15,18]. In the absence of reliable baseline data for normal enteric neuronal density, the diagnosis of hypo and hyperganglionosis remains entirely subjective [19]. Counting of ganglion cells within normal and abnormal large bowel, suggests that hypoganglionosis and hyperganglionosis occur when the numbers of ganglia are 2 standard deviation below or above the mean, respectively [16,19]. However, this lacks definition as to how they identify a ganglion cell as to whether they include the cytoplasmic fragment in the count or only use whole cells and is subject to interobserver variability [16]. Diagnosis of hyperganglionosis may be established solely in the presence of hypertrophy and hyperplasia of the intermuscular and submucosal plexuses [18]. The entire evaluation is fraught with difficulty both in the process and more so in its interpretation. For example, it is well known and reported that in resected specimens from patients with rectal carcinomas, the tumour-free resection margins contained giant ganglion [16]. Furthermore, ganglia containing more than seven ganglion cells have been described in over half of subjects without any identifiable pathological abnormalities [16]. Although hypertrophy and hyperplasia are two distinct processes, they frequently occur together and are used interchangeably to report hyperganglionosis [18]. Adaptive response by gastrointestinal (GI) nervous system secondary to pathological states such as inflammatory, infectious, obstruction, neoplastic/malignant, or degenerative is recognized as neuronal plasticity [20-22]. Neuroplasticity within the gastroenteric microenvironment leads to structural abnormalities ranging from trophic responses of nerve re-arrangement such as hypertrophy and hyperplasia to atrophic responses of degeneration and loss of enteric ganglion cells [23]. Thus, there are multiple factors leading to challenges in diagnosis, including inappropriate extrapolation, lack of appropriate controls, interobserver variability and changing sands in diagnostic criteria.
Enteric neurons undergo postnatal homeostatic apoptosis and replacement by stem cells. Stimuli shown to drive postnatal enteric neurogenesis include tissue injury, whereas neurogenesis in response to obstruction/distension alone has not been documented. Inherent models suggests enteric neural progenitors persistence after ganglia has formed can lead to hyperganglionosis [17]. Homeobox Enx (Hox11L1) is thought to control the position of the enteric neurons, and, when deleted can produce effects similar to migration defects such as increase in the absolute number of ganglia in the myenteric plexus of the colon [16]. Further unequivocal submucosal ganglion cell hyperplasia is often encountered in humans with hamartomatous genetic disorders, such as multiple endocrine neoplasia type 2B (MEN 2B), neurofibromatosis type 1, and PTEN-hamartoma syndromes. Although, the onset of hyperplasia in these syndromes is difficult to determine, significant growth of the lesions can occur postnatally, suggesting an acquired neurogenic component and possibly the involvement of downstream molecular pathways [17]. Mice conditionally expressing RET (C618F) mutation display C cell hyperplasia and hyperganglionosis of the enteric nervous system indicating that RET (C618F) confers gain of function phenotypes. This mouse line may serve as a novel biological platform to investigate pathogenetic mechanism involved in the development of enteric hyperganglionosis [24].
Neuroplastic changes in the enteric nervous system (ENS) are observed in physiological states, such as development and aging, or may be secondary to various pathological conditions such as enteric neuropathies of intestinal or extra-intestinal diseases [20,23,25]. Nearly all chronic GI disorders leading to chronic tissue and neuro-inflammation exhibit structural and functional neuroplasticity. Present review underlines that neuronal plasticity spans all gastrointestinal organs and exhibits common phenotypes and mechanisms [20,23,25]. Response to injury or associated upstream distension, inflammation, strictures due to necrotizing enterocolitis or Crohn disease, congenital intestinal atresia, volvulus, anorectal malformations, intussusception, and smooth muscle hypertrophy could promote submucosal ganglion cell hyperplasia [17]. Autoimmune injury of either the myenteric ganglia or the smooth muscle is also implicated as causes of hyperganglionosis [26]. A study to evaluate the relationship of hyperganglionosis to obstruction by placing ligature around colon of mice, demonstrated that just 20% obstruction was severe enough to induce hyperganglionosis [21].
Currently there is no data available in the literature with regards to the relationship between ganglion hypertrophy and its association to pneumatosis cystoides intestinalis. We propose a new novel neuronal based pathogenetic theory in the context of our findings of abnormal hypertrophied ganglion cells accompanied occasionally by hypertrophic nerve bundles as seen in the mucosa, submucosa and the muscularis propria. Neuroplasticity is associated with aberrant motility, secretion and sensation [25]. Evidence suggests neuroplasticity affects gastrointestinal function, during inflammation and, in many cases, long after healing, where the nervous system fails to reset back to normal [25]. Gastrointestinal dysfunction, specifically sensory-motor and secretory impairment are attributed to up or down regulation of receptors due to altered synthesis, content and release of neurotransmitters [23]. We suggest that an underlying dysgenesis of the ganglion cells is upregulated in the given inflammatory/ischemic microenvironment that results in abnormal peristalsis that causes ‘reversal airflow’ with intramural accumulation of intraluminal air. On the contrary it is quite possible that the hypertrophy of the ganglion cells may occur as a result of the forced expanded mucosa of non-communicating cysts rather than be a causative factor reminiscent of the chicken and the egg scenario. In our study, most of the cases are secondary PCI and thus we favor the latter proposal of the hyperganglionosis occurring as an adaptive visceral neuroplasticity response of the enteric nervous system to the abnormal distended intramural gas cysts. Whether these represent intestinal neuronal dysplasia like submucosal ganglion cell hyperplasia as seen in the proximal margins of Hirschsprung disease resections or they are akin to intestinal neuronal dysplasia Type B as seen in adults, a controversial entity is open for discussion [17,27,28]. Additionally, as this was a retrospective study, the acetylcholinesterase activity in these hypertrophied fibers were not addressed [29,30]. In summary, this study has only addressed structure, density, form and quantity, thus leaving the functionality of this histological finding of hyperganglionosis unanswered. Furthermore increased number and/or abnormalities of the mucosal neuroendocrine cells may play a role in the development of giant submucosal ganglia as postulated in patients with chronic constipation [31]. This was not studied in our limited case cohort. In this context, a dedicated study of a larger series of primary idiopathic and secondary PCI inclusive of functionality assays of ACTH and review of mucosal/ submucosal neuroendocrine cells needs to be undertaken for continued exploration of this novel neuronal theory.
Conclusion
Pneumatosis cystoides intestinalis [PCI] remains a challenging diagnosis. It is important to recognize PCI as a clinical and/or radiological sign and is in itself not a diagnosis [12]. The exact pathogenesis of non-communicating air-filled cysts within the bowel wall remains poorly understood with many proposed theories that include: the mechanical theory of mucosal injury, the bacterial theory of gas production, the counterperfusion-saturation theory and the pulmonary gas theory. In this manuscript, we explore in detail, the neuronal theory of dysgenetic ganglion cells with either causing abnormal peristalsis resulting in ‘reversal airflow’ with intramural accumulation of intraluminal air or being the resultant effect of forced mucosal expansion of cysts. This therefore represents either as a cause and/or effect of PCI in the context of the enteric neuronal landscape that needs continued future exploration.
References
- Tharmaradinam S, Kanthan S, Kanthan Pneumatosis cystoides intestinalis and hyperganglionosis Cause or effect. A review Case Reports Pathol Res Pract. 2020; 216: 152879.
- Soyer P, Martin-Grivaud S, Boudiaf M, et Linear or Bubbly A Pictorial Review of CT Features of Intestinal Pneumatosis in Adults. Journal De Radiologie. 2008; 89: 1907-1920.
- Shawn D St Peter, Abbas MA, Kelly KA. The Spectrum of pneumatosis Arch Surg. 2003; 138: 68-75.
- Braumann C, Menenakos C, Jacobi Pneumatosis intestinalis a pitfall for surgeons. Scand J Surg. 2005; 94: 47- 50.
- Kumar B, Bhawani R, Mokta J, et al. A Benign Case of Medical Pneumatosis Intestinalis in Elderly. Journal of the Indian Academy of Geriatrics. 2017; 13: 23-26.
- Devgun P, Hassan H. Pneumatosis Cystoides Intestinalis A Rare Benign Cause of Pneumoperitoneum. Case Reports in 2013.
- Iida A, Naito H, Tsukahara K, et al. Pneumatosis cystoides intestinalis presenting as pneumoperitoneum in a patient with chronic obstructive pulmonary disease a case report. J Med Case Rep. 2017; 11.
- Azzaroli F, Turco L, Ceroni L, et al. Pneumatosis cystoides World J Gastroenterol. 2011; 17: 4932-4936.
- Marshak RH, Lindner AE, Maklansky Pneumatosis cystoides coli. Gastrointest Radiol. 1977; 2: 85-89.
- Doumit M, Saloojee N, Seppala Pneumatosis intestinalis in a patient with chronicbronchiectasis. Can J Gastroenterol. 2008; 22: 847-850.
- Koreishi A, Lauwers GY, Misdraji Pneumatosis intestinalis a challenging biopsy diagnosis. Am J Surg Pathol. 2007; 31: 1469-1475.
- Donovan S, Cernigliaro J, Dawson Pneumatosis intestinalis a case report and approach to management. Medicine. 2011; 571387.
- Sakurai Y, Hikichi M, Isogaki J, et al. Pneumatosis cystoides intestinalis associated with massive free air mimicking perforated diffuse peritonitis. World J Gastroenterol. 2008; 14: 6753-6756.
- Vecchio E, Jamot S, Ferreira J. Pneumatosis Coli Formation via Counterperfusion Supersaturation in a Patient with Severe Case Reports in Gastrointestinal Medicine. 2018.
- Wallace AS, Barlow AJ, Navaratne L, et Inhibition of Cell Death Results in Hyperganglionosis Implications for Enteric Nervous System Development. Neurogastroenterology & Motility. 2009; 21: 768-E49.
- Lumb PD, Moore L. Back to the Drawing Board Intestinal Neuronal Dysplasia Type B Not a Histological Entity Yet. Virchows Archiv-An International Journal of 1998; 432: 99-102.
- Kapur RP, Reyes-Mugica M. Intestinal Neuronal Dysplasia Type B An Updated Review of a Problematic Diagnosis. Archives of Pathology & Laboratory Medicine. 2019; 143: 235-243.
- Athow AC, Filipe MI, Drake Hyperganglionosis mimicking Hirschsprung’s disease. Arch Dis Child. 1991; 66: 1300-1303.
- Virpi V Intestinal Neuronal Density in Childhood A Baseline for the Objective Assessment of Hypo- and Hyperganglionosis. Pediatric Pathology. 2009; 13: 225-237.
- Demir IE, Schäfer KH, Tieftrunk E, et Neural plasticity in the gastrointestinal tract chronic inflammation neurotrophic signals and hypersensitivity. Acta Neuropathol. 2013; 125: 491.
- Gálvez Y, Škába R, Vajtrová R, et al. Evidence of Secondary Neuronal Intestinal Dysplasia in a Rat Model of Chronic Intestinal Journal of Investigative Surgery. 2004; 17: 31-39.
- Škába R, Frantlova M, Horak J. Intestinal neuronal dysplasia. Eur J Gastroenterol 2006; 18: 699-701.
- Vasina V, Barbara G, Talamonti L, et Enteric neuroplasticity evoked by inflammation. Autonomic Neuroscience. 2006; 126-127.
- Okamoto M, Yoshioka Y, Maeda K, et Enteric neuroplasticity evoked by inflammation. Autonomic Neuroscience. 2006; 264-272.
- Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal Nat Rev Gastroenterol Hepatol. 2014; 11: 611-627.
- Vanderwinden J, Wedel T, Delaet M, et al. P0996 Juvenile fatal intestinal pseudo-obstruction caused by diffuse enteric leiomyositis and ganglionitis in a familial form of constipation and hyperganglionosis. Journal of Pediatric Gastroenterology and 2004: S436.
- Swaminathan M, Oron AP, Chatterjee S, et Intestinal Neuronal Dysplasia-Like Submucosal Ganglion Cell Hyperplasia at the Proximal Margins of Hirschsprung Disease Resections. Pediatric and Developmental Pathology. 2015; 18: 466-476.
- Vougas V, Vardas K, Christou C, et al. Intestinal Neuronal Dysplasia Type B in Adults A Controversial Entity. Case Reports in Gastroenterology. 2014; 8: 7-12.
- Kapur RP, Ambartsumyan L, Smith Are We Underdiagnosing Hirschsprung Disease. Pediatric and Developmental Pathology. 2020; 23: 60-71.
- Kobayashi H, Hirakawa H, Puri P. What Are the Diagnostic Criteria for Intestinal Neuronal Dysplasia. Pediatr Surg Int. 1995; 10: 459-464.
- Kobayashi A, Yokota H, Kobayashi H, et Mucosal Neuroendocrine Cell Abnormalities in Patients with Chronic Constipation. Asian Journal of Surgery. 2004; 27: 197-201.