Is There A Role for Liposomal Bupivacaine as Part of a Multimodal Strategy Inclusive of Intrathecal Morphine for Post-Cesarean Analgesia? A Retrospective Chart Review Study
Author'(s): Antonio Gonzalez Fiol MD1*, Anna-Maria Eid MD1, Mohamed Yassin Elgamal MD1, Hollie Matlin MD2,Kristen L. Fardelmann, MD1, David Yanez, PhD, MS1, Angelique Garay, DNAP, CRNA II, ACNP1 and Aymen Alian MD1
1Department of Anesthesiology, Yale School of Medicine, New Haven,Connecticut, US.
2Department of Anesthesiology, Hospital for Special Surgery, New York, New York.
*Correspondence:
Antonio Gonzalez Fiol, MD, Department of Anesthesiology, Yale School of Medicine, New Haven, Connecticut, US, Tel: 413-386-9415; Fax: 203-785-6664.
Received: 17 August 2020; Accepted: 10 September 2020
Citation: Antonio Gonzalez Fiol, Anna-Maria Eid, Mohamed Yassin Elgamal, et al. Is There A Role for Liposomal Bupivacaine as Part of a Multimodal Strategy Inclusive of Intrathecal Morphine for Post-Cesarean Analgesia? A Retrospective Chart Review Study. Anesth Pain Res. 2020; 4(2): 1-4.
Abstract
Background: The search for the ideal postoperative analgesia is crucial given the current opioid epidemic in the United States. Liposomal bupivacaine for transverse abdominis plane block, inclusive of intrathecal morphine may improve analgesia for more than 24 h. We assessed opioid consumption after cesarean delivery and the time to first narcotic.
Methods: We conducted a single-center retrospective chart review of cesarean deliveries. All patients received a spinal anesthetic with 100 mcg of intrathecal morphine and postoperative multimodal analgesia. A total of 82 patients received the transverse abdominis plane block while 140 patients did not. We tested the adjusted hazards using Cox regression, adjusting for BMI and number of cesarean delivery.
Results: The estimated mean time to first opioid request in the block versus no block group was ~30 hours and 15 hours respectively (p=0.002). A Cox regression analysis comparing the two groups, estimated the risk of receiving a first narcotic in the block group to be one-half of those who did not receive a transverse abdominis plane block
(HR = 0.48, 95% CI = [0.36, 0.65], p< 0.001).
Conclusion: Our study demonstrated that patients who received transverse abdominis plane block had less opioid use in the first 72 hours with a mean opioid-sparing time of 30 hours, twice the time of the other group. This may be explained by the use of liposomal bupivacaine in our blocks which confers a prolonged bupivacaine release and may play a role in longer narcotic free intervals and decreased total postoperative narcotic consumption.
Keywords
Introduction
Cesarean delivery (CD) is the most common surgery performed in the United States and may comprise the first opioid exposure to women of reproductive age [1,2]. The continuous search for the ideal postoperative multimodal analgesia strategy has not been as crucial as it is now given the current opioid epidemic in the United States [3]. Thus far, intrathecal morphine (ITM) remains the gold standard for postoperative analgesia after CD [4]. Given the longitudinal effects of inadequate acute postoperative pain management (e.g., postpartum depression, chronic pain), multimodal analgesia, and other local anesthetic techniques for post-cesarean delivery, such as the transverse abdominis plane (TAP) block have been studied [5-10]. The latter has demonstrated short-lived pain management, providing limited (less than 24 hours) analgesia [6,7,10].
The TAP block offers analgesia by blocking the sensory nerves of the anterior abdominal wall located in the neurofascial plane between the internal oblique and the transversus abdominis muscle [11,12]. When utilized in combination with intrathecal morphine, the role of the TAP block for post-cesarean delivery analgesia remains uncertain [11,13]. The stance of the Society of Obstetric Anesthesia and Perinatology Enhanced Recovery After Cesarean (ERAC) regarding the use of regional blocks for post CD is that it should remain reserved for patients who have not received intrathecal morphine [14]. The consensus statement also states that TAP blocks do not provide significant improvement when given in addition to neuraxial morphine. This is possibly due to the fact that most data published did not use liposomal bupivacaine. Thus, the rate-limiting step regarding the analgesic benefits provided by this block may be related to the local anesthetic utilized to perform the procedure. The majority of previous studies have been performed with bupivacaine [10,15] or ropivacaine [6,9,11]. Liposomal bupivacaine, with its DepoFoam formulation and sustained release, may provide up to 72 hours of postoperative analgesia and therefore, may provide benefit in opioid minimization inclusive of intrathecal morphine [16,17].
This study was designed to evaluate the impact of the introduction of TAP blocks as part of our institutional multimodal strategy. The primary aim of this retrospective study was to assess opioid consumption during the first 72 hours after cesarean delivery. Secondary aims included pain scores 24-48 hours postoperatively, and the time to first narcotic postoperatively. We hypothesize that TAP blocks with liposomal bupivacaine would reduce opioid consumption when used as part of a multimodal analgesia regimen inclusive of ITM over a 24-72-time frame.
Materials and Methods
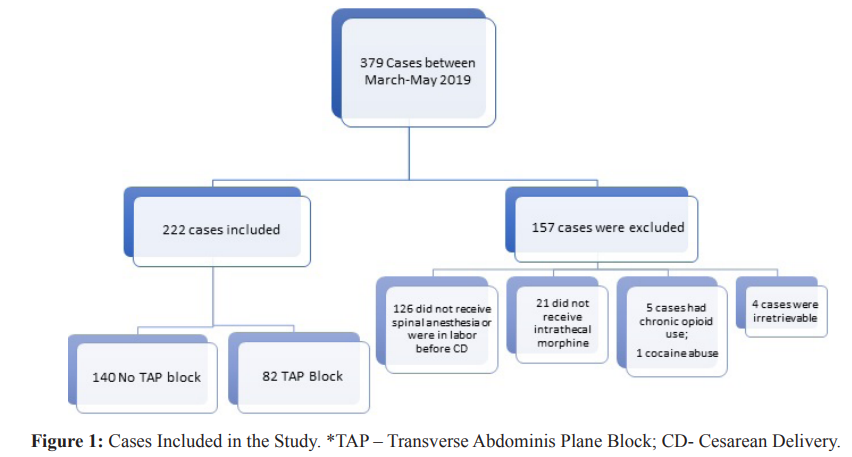
After Institutional Review Board (IRB) approval, with a waiver of written informed consent, we performed a single-center retrospective chart review of all cesarean deliveries at our institution from March 1st to May 31st, 2019. The use of TAP block was introduced in September 2018 as part of our institution multimodal analgesia plan, with the ultimate goal to improve post cesarean delivery analgesia. Given the current literature in TAP after CD, we decided to use liposomal bupivacaine, which was already been used by our regional anesthesia team. We considered the first six months as an introductory phase and decided to collect data after this time. These three months were selected to evaluate patient opioid consumption patterns after the introduction of TAP block as part of our multimodal analgesia strategy. To evaluate the impact of our blocks after its introduction, we added incisional and visceral pain scores into our postoperative anesthesia note. This note is performed by residents 24-48 h after delivery. In addition to asking for pruritus, ability to ambulate, residents asked the patients to score incisional pain, asking “how would you describe your pain from 0-10, 0 been no pain and 10 the worst pain of your life at the incisional area”. For visceral pain, patients would be asked, “can you score the cramping or gnawing feeling in your abdomen from 0-10, 0 been no pain and 10 the worst pain of your life”. In congruence with the Health Insurance Portability and Accountability Act of 1996, data were deidentified. Electronic medical records were utilized to collect patient data. Data collected included age, height, weight, BMI, number of previous CDs, indication for CD, previous surgical history, opioid use in a 72-hour admission period, time to first opioid request, patient’s incisional and visceral visual analog scores (VAS) on a scale of 1-10.
During the defined study period, patients were consented to receive TAP block at the provider's discretion (depending on their level of comfort performing the block). A total of 379 met inclusion criteria. Inclusion criteria included any patient receiving intrathecal bupivacaine 0.75% (1.6 ml), fentanyl 10 mcg, and 100 mcg of preservative-free morphine. Exclusion criteria included cesarean deliveries performed after experiencing labor, history of recent or chronic opioid use, or additional intraoperative interventions (e.g., hysterectomy), exclusion of intrathecal morphine, and allergies to acetaminophen and non-steroidal anti-inflammatory drugs.
Adjuvant analgesics, ketorolac (30 mg) and intravenous acetaminophen (1000mg) were administered during closure of fascia. After wound closure and dressing, bilateral TAP blocks were performed. A linear ultrasound probe (SonoSite M-Turbo, Bothell, WA, USA) was placed immediately above the iliac crest at the midaxillary line and then moved slightly cephalad and laterally. The external oblique (EO), internal oblique (IO), and transversus abdominis muscles (TAM) were identified. A 4-inch 20-gauge Stimuplex insulated needle (B. Braun Medical, Bethlem, PA) was advanced utilizing an in-plane technique into the neurofascial plane between the IO and TAM. A 1 mL normal saline injection was utilized to confirm the proper hydro-dissection of the fascial sheet between the IO and TAM. After negative aspiration, 20 ml of bupivacaine 0.25% (5 ml aliquots at a time), 10 ml of liposomal bupivacaine (133 mg), and 5 ml of saline were injected bilaterally.
Postoperative analgesia consisted of intravenous ketorolac 30 mg every 6 h times 3 doses, transitioning to PO ibuprofen 800 mg every 8 h on postoperative day 1. In addition, the use of acetaminophen 650 mg PO was initiated every 6 h. For breakthrough pain, oxycodone 5, 10, and 15 mg was provided for pain scores of 1-3, 4-6, and 7-10, respectively. Our opioid consumption results were reported as mg of morphine equivalents (MMEQ), utilizing the conversion ratios calculator from the Center for Disease Control. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily dose-a.pdf
Statistical Analyses
We summarize baseline patient characteristics using mean and standard deviations (SD), for quantitative characteristics and frequencies, with percentages, for categorical characteristics. We tested for statistical differences for quantitative characteristics using Welch’s t-test for unadjusted mean comparisons, and chi- square tests of homogeneity for categorical characteristics. We used the log-rank test to test whether the hazard curves for the time to first narcotic use differed between the TAP groups; we tested whether the adjusted hazards (HR) differed using Cox regression. For morphine consumption, we tested whether there were mean differences between the TAP groups, and for mean differences over time (24, 48, 72 hours) by fitting a generalized estimations equations (GEE) regression and allowed for effect modification between TAP block groups and time. GEE accounts for within- patient repeated measures correlation and also uses Huber- White standard error (SE) estimates. These estimates and the corresponding statistical inference tend to be robust to violations of standard modeling assumptions (e.g., variance and correlation misspecification). We tested for mean differences between the TAP groups for visceral and incisional pain scores using linearregression with Huber-White SE estimates. The analyses adjusted for BMI and number of CD. All hypothesis tests, p-values, and confidence intervals are two-sided. All analyses were conducted using the Stata (version 16.0) statistical package.
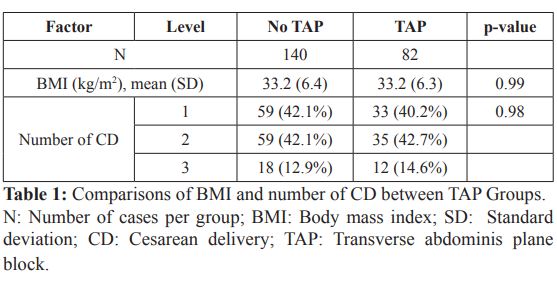
Baseline characteristics
There were 82 women who received TAP block (TAP group) and 140 women that did not (no TAP). The estimated mean BMI for both the TAP and no TAP groups were 33.2 kg/m2, their estimated standard deviations were virtually the same (6.4 kg/m2 vs 6.3 kg/m2). The Welch test comparing mean BMI did not suggest a difference (p-value = 0.99). The frequency distributions for the number of CD were also quite similar between the two groups (chi-square p-value = 0.98) (Table 1).
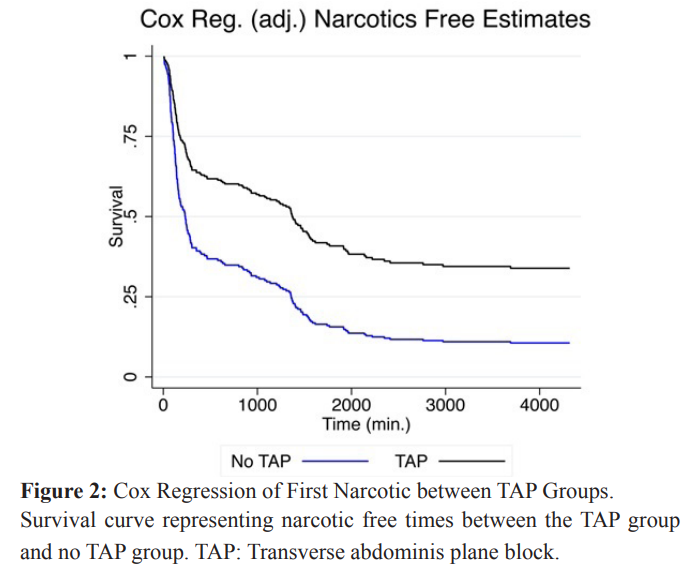
Study outcomes
We compared time to first narcotic using a log-rank test (unadjusted) and Cox regression, adjusting for patient BMI and the number of CD. We present only the adjusted results for conciseness. The estimated risk of receiving a first narcotic for patients that received a TAP block was approximately one-half compared to patients that did not receive a TAP block (HR = 0.48, 95% CI=[0.36, 0.65], p-value < 0.001) comparing patients when adjusted to BMI and number of CD. An almost negligible contribution was noted from these two variables, with an unadjusted HR = 0.484, 95% CI [0.361- 0.650]. Figure 2 presents the two TAP block groups respective “survival” curves over time, for the event of receiving a first narcotic. From the figure, one sees that patients who did not receive a TAP block had their first narcotics sooner. Their survival curve, in blue, dropped more quickly than for patients that did receive a TAP block (black curve). Times to first narcotic were noted to be 30 h (1814.73 min) and 15 h (878.11 min), for patients that received a TAP versus no TAP, respectively (p <0.001). By the end of the study, patients who received a TAP block were less likely to receive narcotics compared to patients who did not receive a TAP block (66.1% vs. 89.4%).
For MMEQs, we estimated differences between patients who received a TAP block compared to patients who did not receive a TAP block at each time. The mean differences were: -57.9 mg at 24 hours, -65.4 mg at 48 hours, and -70.3 mg at 72 hours, indicating that patients receiving a TAP block received less morphine than patients who did not receive a TAP block. The estimated differences at the three times, however, were not statistically different from each other, or time was not an effect modifier for the association between morphine equivalent consumption and TAP block (p-value = 0.314).
Table 2 displays patients’ morphine equivalent consumption over time for patients who received a TAP block and patients who did not receive a TAP block. The results reveal a significant increasing trend in morphine equivalent consumption with respect to time (p-value < 0.001). The mean estimates are adjusted for average BMI (at 33.2 kg/m2) and CD and are estimated for patients having one CD. This group comprised of approximately 41% of the sample data.
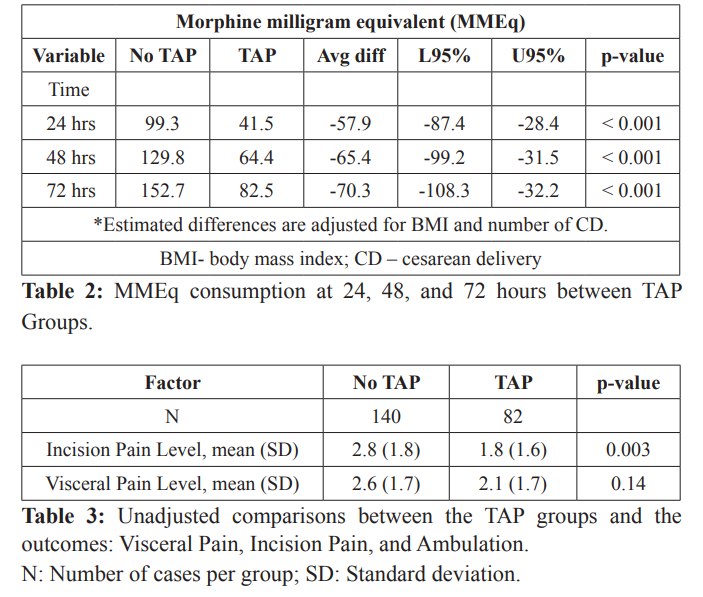
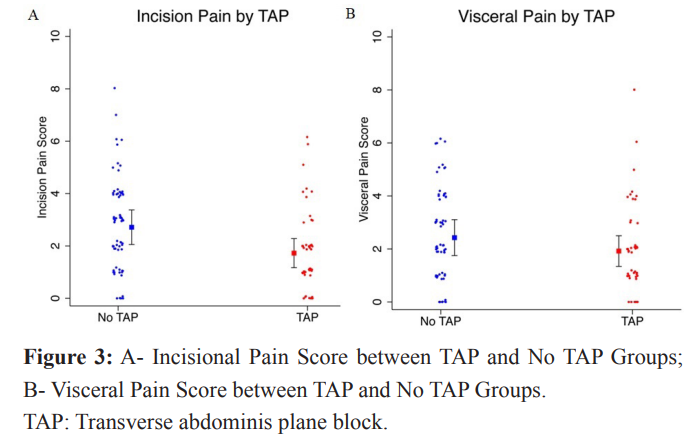
For the incisional pain score outcome, patients who received a TAP block had a mean pain score approximately one point lower than patients who did not have a TAP block (diff = -1.0, 95% CI=[- 1.6, -0.3], p-value =0.003), adjusting for BMI and number of CDs. For visceral pain, patients who received a TAP block had slightly lower, but not statistically significant, mean scores compared to patients who did not receive a TAP block. The mean visceral pain difference was -0.51 (95% CI= [-1.2, 0.2]). However, the magnitudes of these differences were typical of what one might expect if there were no true difference (p-values > 0.10).
Discussion
It has been estimated that a range of 0.12-0.33% of parturients will become long-time opioid consumers after cesarean delivery [1,18]. With an estimated 1.3 million CD being performed annually in the United States, this could potentially result in 3,333 new long-term opioid users [1]. These alarming numbers should prompt the use of opioid sparing alternatives.
Since postpartum opioid consumption is likely related to the number of pills prescribed at the time of patient discharge, newer strategies aiming to reduce the amount of opioid prescribed at discharge have been evaluated [19]. In one study by Osmundson et al. [20], patients discharge prescriptions were tailored to their consumption patterns during admission. Other strategies to reduce patient opioid consumption has eliminated the routine use of opioids after CD [2,21]. Postpartum opioid consumption is likely related to the number of pills prescribed at the time of patient discharge [16,17].
Hence, non-opioid strategies, such as the use of TAP blocks could potentially allow hospitals to adhere with the recommendations from the Center for Disease Control (CDC), that suggests limiting opioid prescription for seven days, ideally ≤ 3 days to reduce potential unintentional chronic opioid use [22].
The addition of TAP blocks has been shown to decrease opioid consumption after cesarean delivery when intrathecal morphine is omitted, but little to no benefit when this drug is utilized [6,10,11,12,15,23,24]. In addition to the inclusion or exclusion of intrathecal morphine, the technique utilized may impact the effectiveness of the block. The use of a landmark technique may result in suboptimal needle placement in 75% of cases [8]. When performing ultrasound-guided TAP block, the use of a lateral (mid- axillary) technique is most commonly described [6,11], whereas the posterior approach has been infrequently described for post- cesarean delivery analgesia [8,12,25]. The posterior technique, described by Faiz et al. [25], might confer better analgesia and decreased opioid consumption by up to 36 h. Of note, intrathecal morphine was omitted in their study. Most of the studies published thus far utilized ropivacaine [6,10,11,23] or bupivacaine [10,15], whereas in our study and that of Baker et al. [16], liposomal bupivacaine was utilized.
The DepoFoam formulation and sustained release of bupivacaine that characterizes liposomal bupivacaine has been shown to prolong its analgesic effects. Previous studies utilizing this formulation have demonstrated up to 72 h of analgesia for abdominal wall surgery, total knee arthroplasty and CD [7,16,17,26]. Our study reflects the prolonged analgesic benefits of liposomal bupivacaine with an average decrease in opioid consumption of 57.9%, 65.4%, and 70.3% at 24, 48, and 72 h, respectively. These results agree with those of Baker et al. [16], who demonstrated a decrease in opioid consumption up to 72 h when comparing TAP block with liposomal bupivacaine versus no TAP after spinal anesthesia inclusive of intrathecal morphine for cesarean delivery.
In addition, our results revealed a statistically significant difference in the time to first narcotic request in patients that received intrathecal morphine with TAP versus no block of 30 h (1814.73 min) and 15 h (878.11 min), respectively (p <0.001). In contrast to our study, the results by Lee et al. [11], showed no statistical difference in the median time (~ 18 h) to first opioid request when comparing patients that received intrathecal morphine with or without TAP block. In the study by Faiz et al. [25], in which the use of intrathecal morphine was omitted, the time to first opioid request was 6h and 13h for the lateral and posterior TAP technique, respectively. The posterior approach described in their study accomplished a similar time to the first opioid request as in our intrathecal morphine group without TAP block. These results suggest that the choice of local anesthetic, and perhaps the technique, when performing a TAP block may improve the analgesic benefit beyond the 11-29 hour that intrathecal morphine may provide [27].
Given that the TAP blocks involve blocking the neural afferents between the internal oblique and the transversus abdominis muscle that supply the anterior abdominal wall, this block is likely to reduce somatic rather than visceral pain. Our study revealed a statistically significant decrease in incisional pain scores between the two groups, but none when it came to the visceral pain scores in the first 24 - 48 h. The retrospective nature of our study did not allow for the evaluation of visual analog scores (VAS) at set interval times; they were obtained within 24 to 48h. Although we did not evaluate for early (<24 h) VAS, our results are in accordance with most studies attributing no benefit to TAP blocks in terms of visceral VAS after 12 h. In contrast, our study revealed at least >12 h of incisional pain relief, an analgesic benefit that has not been noted in previous studies evaluating TAP blocks inclusive of intrathecal morphine after CD. Patients who received a TAP block reported less incisional VAS scores compared to those who did not receive it [11,13,15]. There was, however, no statistically significant decrease in visceral VAS scores between the two groups, which speaks for the nature of the block.
Beside opioid consumption reduction, early mobilization is a paramount aspect of enhanced recovery after cesarean delivery. Jarraya et al. [9], demonstrated that the use of TAP block and not intrathecal morphine was conducive to earlier mobilization after cesarean delivery. Patients in the TAP group had a significantly earlier time to mobilization (6.9 hr) compared to those with spinal morphine (9.4 hours) (p-value=0.024) and more rapid mobilization (7.4 hr vs. 12.4 hr, p-value=0.001). Similarly, the study of Baker et al. [16], who utilized liposomal bupivacaine also demonstrated early mobilization in the patients that received intrathecal morphine and TAP blocks.
This study’s main limitation is in its nature of being retrospective, data concerning the VAS scores and ambulation were missing for some patients. We did not have set times for obtaining the VAS scores which could have diluted the results. That is, if evaluated close to 24 h, the incisional and visceral pain may have been reported to be lower than if the same patient would have been evaluated at 36 h. It is our practice to evaluate patients between 24-48 h, with the great majority of patients being evaluated before 36 h after delivery. When the TAP block was offered as part of our multimodal analgesic approach, we may have captured patients more willing or conscious about reducing their opioid consumption, as this was the goal emphasized when obtaining consent for the performance of the block. The lack of blinding from the procedure may have impact the patients answers. Despite researchers not been blinded, all the data except for incisional and visceral pain scores at 24-36 were recovered from our electronic medical record system. Another limitation is that the TAP blocks were performed by residents which may have impacted the consistency of the blocks’ efficacy amongst the cohort. Nonetheless, the residents were supervised by an attending and local anesthetic spread was confirmed via ultrasound.
Conclusion
In conclusion, the use of TAP blocks with liposomal bupivacaine and multimodal analgesia inclusive of intrathecal morphine resulted in a significant decrease in opioid consumption up to 72 hours post-cesarean delivery. Patients with TAP blocks experienced prolonged opioid-free periods and a longer interval to their first narcotic request. Furthermore, the use of liposomal bupivacaine resulted in a significant decrease in incisional pain scores. Our results suggest that liposomal bupivacaine may be used as partof a multimodal approach, with opioid reductions beyond those offered by intrathecal morphine. Given the retrospective nature of our study, generalizations cannot be drawn. Further randomized controlled studies are needed to identify the role of liposomal bupivacaine after CD.
References
1.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016; 215: 353.
2.Lakhi N, Tricorico G, Kanninen T, et al. Post-Cesarean outpatient opioid consumption and perception of pain control following implementation of a restrictive opioid prescription protocol. Am J Obstet Gynecol. 2019; 1: 100049.
3.Kharasch ED, Avram MJ, Clark JD. Rational Perioperative Opioid Management in the Era of the Opioid Crisis. Anesthesiology. 2020; 132: 603-605.
4.Kanazi GE, Aouad MT, Abdallah FW, et al. The Analgesic Efficacy of Subarachnoid Morphine in Comparison with Ultrasound-Guided Transversus Abdominis Plane Block After Cesarean Delivery: A Randomized Controlled Trial. Anesth Analg. 2010; 111: 475-481.
5.Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008; 140: 87-94.
6.Costello JF, Moore AR, Wieczorek PM, et al. The Transversus Abdominis Plane Block, When Used as Part of a Multimodal Regimen Inclusive of Intrathecal Morphine, Does Not Improve Analgesia After Cesarean Delivery. Region Anesth Pain Med. 2009; 34: 586-589.
7.Fayezizadeh M, Majumder A, Neupane R, et al. Efficacy of transversus abdominis plane block with liposomal bupivacaine during open abdominal wall reconstruction. Am J Surg. 2016; 212: 399-405.
8.Patel SD, Sharawi NE, Sultan P. Local anaesthetic techniques for post caesarean delivery analgesia. Int J Obstet Anesth. 2019; 40: 62-77.
9.Jarraya A, Zghal J, Abidi S, et al. Subarachnoid morphine versus TAP blocks for enhanced recovery after caesarean section delivery: A randomized controlled trial. Anaesth Crit Care Pain Med. 2016; 35: 391-393.
10.Champaneria R, Shah L, Wilson MJ, et al. Clinical effectiveness of transversus abdominis plane (TAP) blocks for pain relief after caesarean section: a meta-analysis. Int J Obstet Anesth. 2016; 28: 45-60.
11.Lee AJ, Palte HD, Chehade JMA, et al. Ultrasound-guided bilateral transversus abdominis plane blocks in conjunction with intrathecal morphine for postcesarean analgesia. J Clin Anesth. 2013; 25: 475- 482.
12.Hebbard P. TAP block nomenclature. Anaesthesia. 2015; 70:112–113.
13.Abdallah FW, Halpern SH, Margarido CB. Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anaesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012; 109: 679-687.
14.Bollag L, Tiouririne M, Lim G, et al. Society of Obstetric Anesthesia and Perinatorlogy (SOAP) Enghanced Recover After Cesarean (ERAC) Consensus Statement. 2019.
15.McMorrow RCN, Mhuircheartaigh RJN, Ahmed KA, et al. Comparison of transversus abdominis plane block vs spinal morphine for pain relief after Caesarean section. Br J Anaesth. 2011; 106: 706-712.
16.Baker BW, Villadiego LG, Lake YN, et al. Transversus abdominis plane block with liposomal bupivacaine for pain control after cesarean delivery: a retrospective chart review. J Pain Res. 2018; 11: 3109-3116.
17.Hu D, Onel E, Singla N, et al. Pharmacokinetic Profile of Liposome Bupivacaine Injection Following a Single Administration at the Surgical Site. Clin Drug Invest. 2013; 33: 109-115.
18.Peahl AF, Dalton VK, Montgomery JR, et al. Rates of New Persistent Opioid Use After Vaginal or Cesarean Birth Among US Women. Jama Netw Open. 2019; 2: 197863.
19.Bateman BT, Cole NM, Maeda A, et al. Patterns of Opioid Prescription and Use After Cesarean Delivery. Obstet Gynecol. 2017; 130: 29–35.
20.Osmundson SS, Raymond BL, Kook BT, et al. Individualized Compared With Standard Postdischarge Oxycodone Prescribing After Cesarean Birth. Obstet Gynecol. 2018; 132: 624–630.
21.Holland E, Bateman BT, Cole N, et al. Evaluation of a Quality Improvement Intervention That Eliminated Routine Use of Opioids After Cesarean Delivery. Obstet Gynecol. 2019; 133: 91–97.
22.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017; 66: 265–269.
23.Singh S, Dhir S, Marmai K, et al. Efficacy of ultrasound-guided transversus abdominis plane blocks for post-cesarean delivery analgesia: a double-blind, dose-comparison, placebo-controlled randomized trial. Int J Obstet Anesth. 2013; 22: 188-193.
24.Ng SC, Habib AS, Sodha S, et al. High-dose versus low-dose local anaesthetic for transversus abdominis plane block post- Caesarean delivery analgesia: a meta-analysis. Brit J Anaesth. 2018; 120: 252–263.
25.Faiz SHR, Alebouyeh MR, Derakhshan P, et al. Comparison of ultrasound-guided posterior transversus abdominis plane block and lateral transversus abdominis plane block for postoperative pain management in patients undergoing cesarean section: a randomized double-blind clinical trial study. J Pain Res. 2017; 11: 5–9.
26.Mont MA, Beaver WB, Dysart SH, et al. Local Infiltration Analgesia with Liposomal Bupivacaine Improves Pain Scores and Reduces Opioid Use After Total Knee Arthroplasty: Results of a Randomized Controlled Trial. J Arthroplasty. 2018; 33: 90– 96.
27.Sultan P, Halpern SH, Pushpanathan E, et al. The Effect of Intrathecal Morphine Dose on Outcomes After Elective Cesarean Delivery. Anesth Analg. 2016; 123: 154–164.