Not All Regulators of Apoptosis are Equally Affected by Compensation Following BCL-2 Suppression by Antisense Oligonucleotides: A Review
Author'(s): Marvin Rubenstein, PhD*
Research program administered by the Hektoen Institute for Medicine; Departments of Biochemistry and Urology, Rush University Medical Center, Chicago, IL.,60612, U.S.A.
*Correspondence:
Dr. Marvin Rubenstein, Principle Urology Investigator, Hektoen Institute for Medicine, 1070 Cobblestone Ct., Northbrook, IL 60062, U.S.A., Tel: 847-291-7410.
Received: 12 June 2019; Accepted: 05 July 2019
Citation: Marvin Rubenstein. Not All Regulators of Apoptosis are Equally Affected by Compensation Following BCL-2 Suppression by Antisense Oligonucleotides: A Review. Cancer Sci Res. 2019; 2(3); 1-9.
Abstract
Almost 25 years ago we first employed antisense oligonucleotides (oligos) against human derived prostatic LNCaP cells using both in vitro [1] and in vivo [2] models; initially targeting the epidermal growth factor receptor, and its ligand, transforming growth factor-alpha. Similar studies and results were found when we treated in vitro and in vivo breast cancer models [3]. Clinically, oligos have been employed to treat cancer patients (including those with prostate tumors), targeting apoptosis inhibitors (bcl-2, clusterin) in attempts to restore tumor chemo- [4] or radio-sensitivity [5].
Yet, in spite of these patient trials and additional advances in early detection of prostate cancer, its treatment has not greatly improved in recent years. In the last few years gene therapy and newly discovered immune checkpoint blockade has given some indication that they could provide some additional improvement, particularly when administered following surgery, chemotherapy or irradiation.
As the role of the immune system in cancer treatment becomes better understood (and augmented), some oligos have been found to alter the immune response towards prostate tumors, in previously un-thought of ways. Using oligos (either mono- or bispecific) targeting bcl-2 we found that certain antisense oligos have secondary conformations based on intra strand sequences which permit complementary base pair binding within the oligo, that can induce interferon [6], enhance cell surface antigen expression [prostate specific membrane antigen (PSMA)] [6], and potentially increase tumor recognition and targeting by the immune system.
However, our studies also indicate that gene therapy employing oligos directed towards bcl-2 (in LNCaP cells) frequently are compensated for by altered regulation of apoptosis, increased androgen sensitivity, enhanced oncogene activity [7-9] and chromosomal instability as indicated by enhanced expression of fusion protein TMPRS22 [10] and fusion transcription protein FLI-1 [11]. All compensation mechanisms noted here suggest increased proliferation, chromosomal instability and greater mitotic activity, producing a more aggressive tumor, or selection of a subset of such cells. This is confirmed by finding greatly increased expression of proliferation factor KI-67 [11] and mitosis related cyclin D1 [11] after treatment directed at bcl-2.
We now believe that immunologic recognition can be an additional pathway for compensation following suppressive bcl-2 treatment and suggest that this type of gene therapy could also influence proteins associated with immune checkpoint blockade, altering its efficacy. Immune checkpoint blockade therapy has become the “standard of care” treatment for melanoma and is now being evaluated (and sometimes recommended for first line treatment) against kidney, lung, hematologic cancers as well as solid tumors (including those of the prostate). Melanoma has a long history of response to immunotherapy, first using in vitro cultivated lymphokine activated killer (LAK) T cells and then employing similarly cultivated and expanded tumor infiltrating lymphocytes (TIL). The addition of additional cytokines (or their cellular production) enhanced the technique. More recently melanoma became the first tumor to be extensively treated by monoclonal agents in checkpoint blockade, where its prolonged survival and even produced some cures in a small percentage of patients. Studies are now in progress to identify those patients most susceptible for this treatment (those having greater tumor surface expression of PD-1) and even ways to alter susceptibility through evaluation of the patient’s colon microbiota (with possible alteration using fecal transplantation).
In a continuation of these studies, and also to see whether compensation has additional effects on the immune response, we found that PD-1 and its ligand PD-Ll were significantly enhanced in their expression following treatment with both mono- and bispecific oligos directed towards bcl-2. Similarly, the prognostic indicator cyclin derived kinase-12 (CDK-12), which identifies a subpopulation of prostate tumors susceptible to immunotherapy was similarly enhanced. The lack of CDK-12 expression in untreated LNCaP cells suggests that this human derived tumor would be susceptible to immunotherapy for in vivo studies. The fact that treatment enhanced both the expression of PD-1 and PD-L1 targets, in addition to CDK-12 shows the complexity of immune therapy using checkpoint inhibitors, which is far from understood. The greatly enhanced expression of CDK-12 would suggest that the LNCaP cells would no longer be susceptible to checkpoint inhibition at the PD-1/PD-L1 level even though these proteins (and particularly PD-1) present a better target. The huge increase in CDK-12 found in the treatment’s groups would probably overwhelm such recognition.
To identify a compensatory response to evade apoptosis in the presence of bcl-2 suppression, levels of mRNA encoding non-targeted bax, caspase-3, clusterin and VDAC1were next evaluated. We found that specific suppression of the apoptosis inhibitor bcl-2 in LNCaP cells does not affect (non-targeted) bax expression nor (non-targeted) clusterin (non-targeted). Non-targeted caspase-3 expression was suppressed while non-targeted VDAC1 appeared to increase. This suggested that tumor cell variants develop which resist apoptosis through diminished expression of promoters, while other mechanisms which facilitate apoptosis may be enhanced. This study suggests that compensatory changes in the regulation of apoptosis can vary or be limited to apoptosis promoters (caspase-3), since the expression of the non-targeted apoptosis inhibitor clusterin is not affected and there is suggestion that the activator of apoptosis VDCA1 could be enhanced. Should bcl-2 suppression be clinically employed with antisense oligos it may require maintenance (or replacement) of caspase-3 activity.
Keywords
Introduction
Antisense oligonucleotides (oligos) have been employed in both in vivo and in vitro prostate cancer models employing LNCaP and PC-3 cell lines [2]. Similar studies have employed the MCF-7 breast cancer cell line [3]. Clinical trials employing oligos have targeted protein growth factors, androgens, receptors for stimulating factors, inhibitors of apoptosis (bcl-2 and clusterin) and oncogenes. Some (OGX-011 developed by Oncogenex Pharmaceuticals) have been studied in clinical trials while others (OGX-225) reached preclinical development [12]. Oligos act through a variety of mechanisms and provide a specific and relatively non-toxic [13] method for translational arrest by degradation of annealed mRNA: oligo duplexes by RNase H, protein binding and DNA triplex formation [1].
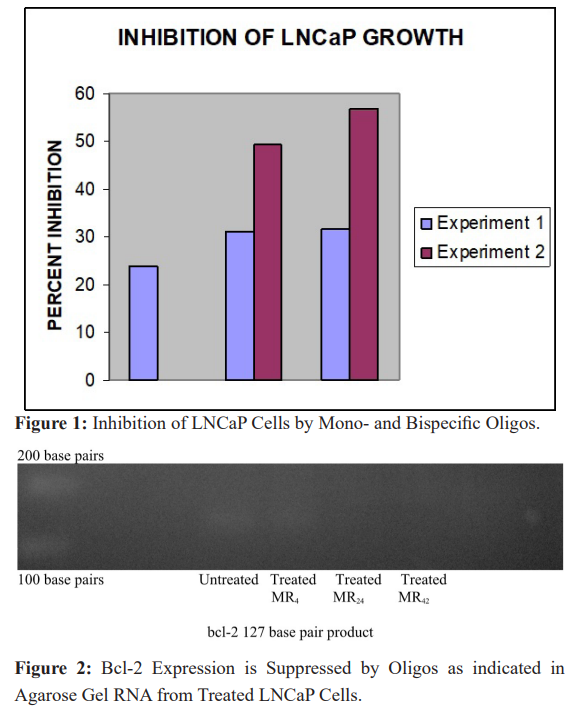
In attempts to increase the efficacy of oligos our lab has been evaluating bispecific derivatives [14] with more than one binding site on a single DNA strand in order to suppress the expression of two different gene products. In LNCaP cells we found that mono- specific oligos targeting bcl-2 and bispecifics targeting both bcl-2 and the epidermal growth factor receptor (EGFR) have comparable activities in suppressing bcl-2 expression as determined by both in vitro growth (Figure 1) and bcl-2 expression (Figure 2) [15]. Therefore, the presence of a second binding site does not diminish activity of the other.
In theory gene therapy should be specific and avoid non-specific effects. However, in practice this is not so, and the non-specific effects can compromise the initial attempt to control tumor growth. Since these approaches are now in clinical practice, and new trials are constantly being initiated, the recognition of side effects becomes increasingly important to enhance efficacy of this innovative therapy. In the LNCaP model, cells treated with mono- or bispecific oligos targeting bcl-2 were extracted for RNA and evaluated for protein expression employing RT-PCR. In addition to the compensatory mechanisms already mentioned, some of our more notable findings were the inhibition of bcl-2 with antisense oligos suppressed the apoptotic promoter caspase-3 (Figure 3) [16] in an attempt to restore apoptosis following bcl-2 suppression; and enhanced androgen sensitivity via increased expression of the androgen receptor (AR), and AR variants ARv7 and ARv9[17]; AR co-activators p300, interleukin-6 (IL-6); oncogene viral myelocytomatosis (v-MYC) [18]; and signal transducer and activator of transcription3 (STAT3) proteins [19].
This suggested that following bcl-2 suppressive therapy, there could be selective pressure for a more rapidly growing aggressive (androgen-sensitive, oncogene driven) phenotype, as suggested, following the discovery of enhanced expression of both KI-67 (a proliferation marker) and cyclin D1 (a regulator of mitosis). Recognition of this transition is important if either gene therapy or immune checkpoint blockade were to be employed.
Immune checkpoint blockade therapy has become the “standard of care” treatment for melanoma and is now being evaluated for kidney, lung, hematologic cancers as well as solid tumors (including those of the prostate). Melanoma has a long history of attempts at immunotherapy first using lymphokine activated killer (LAK) T cells, later with more potent tumor infiltrating lymphocytes (TIL). Proliferation (in vitro) and overall potency of these cells were then further enhanced by cytokine activation (using IL-2 and GM-CSF) when these were either added directly into the culture media or genetically introduced or activated in these cultivated cells. More recently melanoma was the first tumor to be extensively treated by monoclonals directed at either CTLA-4 or PD-1/PD-L1 as agents for immune checkpoint blockade [20].
Prostate cancer has some similarities to melanoma. Each can remain dormant for long periods of time, are frequently treated by irradiation and express tumor associated antigens (gp100 by melanoma and prostate specific antigen [PSA], prostate specific membrane antigen [PSMA], prostatic acid phosphatase [PAP] and prostate cancer antigen-3 [PCA-3] by the prostate). Irradiated tumors shed antigens and provide recognition targets for activated T cells (unless inhibited by Treg suppressor cells). Immune checkpoint blockade works by several mechanisms which include targeting suppressive proteins (like CTLA-4) which interferes with the stimulatory interaction of T lymphocyte CD28 and B7 on antigen presenting cells. The result is continued anti-tumor immunologic responsiveness and prevention of tumor induced anergy and T cell tolerance. Such therapy could be employed following or in combination with gene therapy (including the use of antisense oligos). In addition to targeting the inhibitory signaling of T-cell CTLA-4, newer agents (also monoclonals) target the programmed death protein (PD-1) expressed by tumors, and its ligand (PD-L1) found on lymphocytes. When (or if) expressed, PD-1 acts as a tumor cloacking device, inhibiting specific cytotoxic lymphoid activity. If immune blockade therapy were to enter clinical trials for prostate cancer, these targets could be influenced by prior gene therapy and affect the ultimate outcome, therefore changes in expression must be taken into account and since immune checkpoint inhibitors only work in a small percentage of patients, prognostic indicators for their success much be identified, and ways to manipulate them found to increase the number of responsive patients.
Three FDA approved agents were initially approved to treat melanoma (Yervoy, Keytruda and Optiva). The first was directed against CTLA-4 (for which James Allison was awarded the 2018 Nobel Prize) and the latter two directed against the PD-1/ PD-L1 loop. Since their initial approval they have similarly been approved for a variety of solid and hematologic tumors as well. Against some cancers they are even being employed as the first and preferred agents to employ (i.e. melanoma).
Since oligo directed suppression of bcl-2 seems to alter so many metabolic pathways, most of which seem to indicate a transition to or selection of a more aggressive tumor type, we believe that immune regulation could be yet another pathway to evade therapy (particularly that directed towards bcl-2) through compensation. To test this theory in the current investigation we once again used RT-PCR to evaluate the immune regulatory markers for programmed death-1 (PD-1) and its ligand (PD-L1), in addition to a prognostic indicator (CDK-12) which is reported to identify prostate cancers susceptible (or not) to anti PD-1 immunotherapy
[21] and found all to be significantly enhanced following bcl-2 suppression. Compensatory effects identified with these proteins are important since the PD-1/PD-L1 pathway is now recognized as a target for monoclonal antibody directed immunotherapy used to treat various solid tumors, particularly melanoma and lung tumors.
We employed RT-PCR in these experiments to determine alterations in gene expression. Although more sophisticated techniques are available, we find this method both sensitive enough to identify those genes involved with compensation and able to identify non-targeted genes (like KI-67, and the cyclins) [11] which are particularly affected and could provide combination targets for bcl-2 suppressive therapy. If compensation for bcl-2 suppression results in an increasingly aggressive, androgen sensitive and more rapidly proliferating tumor, increased expression of the proliferation marker KI-67 (or possibly the mitosis regulating cyclin D1) would most likely be the result of this process and could identify another target further intervention. Such proteins could even provide the second target on bispecific oligos; a role currently filled by a binding site for EGFR in these studies.
Cell Culture
LNCaP cells (American Type Culture Collection, Manassas, VA, USA) were grown in RPMI 1640 supplemented with 10% bovine serum, 1% L-glutamine and 1% penicillin/streptomycin in a 5% CO2 incubator. Log phase cells were harvested using EDTA/ Trypsin and equally distributed into 75 cm2 flasks (Corning, NY, USA). At intervals media was either supplemented or replaced with fresh.
Determination of Growth
Four days prior to the addition of oligos 1 x 104 LNCaP cells were added, in a total 200 μl volume of media, to each depression of a
96-well plate and incubated at 37oC in a 5% CO incubator. On the
The purpose of this study was to further evaluate the effects of a single mono- and two bispecific oligos directed against bcl-2 on the apoptotic process employing RT-PCR to measure the expression of targeted bcl-2 and non-specific (compensatory) effects on the non- targeted protein, VDAC1. These proteins were chosen because they regulate apoptosis in different manners; bcl-2 and clusterin are inhibitory, while activated bax and caspase-3 promote the process, and VDAC1 activates the process by releasing apoptotic proteins within the mitochondria through oligomerization and pore formation; while also interacting with inhibitory proteins [22].
From this extension of previous finding we conclude that if gene therapy is to be effective when directed against bcl-2, compensatory changes which compromise the effectiveness of the desired suppression must be identified. Among a mass of heterogeneous tumor cells, those which evade apoptosis are most likely to be selected and altered gene expression could include decreased expression of promoters bax. If compensatory changes are initiated by bcl-2 suppression therapy, to re-establish apoptosis you should at least maintain (or replace) the activity of promoters like caspase-3.
Methods
Oligonucleotides
Oligos (mono- or bispecific) were purchased from Eurofins MWG Operon (Huntsville, AL, USA). Each was phosphorothioated at 5’ and 3’ positions on three terminal bases. Stock solutions were made to a final concentration of 625 μM in sterile Dulbecco phosphate buffered saline (PBS).
Base Sequences
Each oligo contained at least one CAT sequence and targeted the area adjacent to the AUG initiation codon for mRNA encoding the respective targeted protein.
MR4 (mono-specific targeting BCL-2): T-C-T-C-C-C-A-G-C-G-T-G-C-G-C-C-A-T
MR24 (bispecific targeting EGFR/BCL-2): G-A-G-G-G-T-C-G-C- A-T-C-G-C-T-G-C-T-C- T-C-T-C-C-C-A-G-C-G-T-G-C-G-C-C- A-T
MR42 (bispecific targeting BCL-2/EGFR): T-C-T-C-C-C-A-G-C- G-T-G-C-G-C-C-A-T-G-A-G-G-G-T-C-G-C-A-T-C-G-C-T-G-C- T-C day of transfection, the following solutions were prepared:
- 1 μl of buffer containing either oligo or a diluent was added to 50 μl of OPTI-MEM (Invitrogen , Carlsbad, CA, USA) and gently mixed. One dilution was made for each well.
- 1 μl of lipofectin (Life Technologies , Gaithersburg, MD, USA) was diluted in 50 μl of OPTI-MEM and mixed gently for 5 minutes at room temperature.
- Oligo dilutions were mixed with 50 μl of lipofectin and gentlymixed for 20 minutes at room temperature.
- 100 μl of the lipofectin and oligo mixture was added to 100 μl of RPMI medium and mixed.
Cells were incubated for 24-48 hours before solutions were aspirated and re-incubated for an additional 48 hrs in 200 μl of media. Cell counts were determined following the addition of WST-1 reagent to each well, and after 2 hours the color intensity was measured by a microplate reader at a wavelength of 450 nm, using a reference of 650 nm. Values obtained were determined after the subtraction of paired blank samples from the experimental wells and were multiplied by a constant to give whole integers for analysis. Microsoft Excel software was utilized to calculate means and standard deviations, and Students t tests were used to determine significance.
Oligo Treatment Prior to PCR
Fours days prior to oligo addition, when cell density approached 75% confluence, 10 ml of fresh media was added. Cells were incubated for an additional 3 days before 5 ml of media was replaced with fresh the day before oligos were added. 100 μl of stock oligos were added to bring the final concentration to 6.25 μM. Incubation proceeded for an additional 24 hours in the presence or absence of mono-specific MR4, or the MR24 and MR42 bispecific oligos.
RNA Extraction
Following treatment, media was removed, a single ml of cold (4oC) RNAzol B was added to each 75cm2 culture flask and the monolayer lysed by repeated passage through a pipette. All procedures were performed at 4oC. The lysate was removed, placed in a centrifuge tube to which 0.2 ml of chloroform was added, and shaken. The mixture stayed on ice for 5 min, was spun at 12,000 x g for 15 min, and the upper aqueous volume removed and placed in a fresh tube. An equal volume of isopropanol was added, the tube shaken, and then allowed to stay at 4oC for 15 min before similar centrifugation to pellet the RNA. The supernatant was removed, the pellet washed in a single ml of 75% ethanol, then spun for 8 min at 7500 x g. The ethanol was pipetted off and the formed pellet air dried at -20oC.
RNA Quantitation
RNA was resuspended in 250 μl of diethylpyrocarbonate (DEPC, inhibitor of RNAse activity) treated water (Invitrogen), and quantitated using a Qubit florometer and Quant-iT RNA assay kit (Invitrogen).
RT-PCR
Extracted RNA was diluted to 40 μg/μl in DEPC treated water. 1 μl of this RNA was added to1 μl of both sense and antisense primers (forward and reverse sequences from Invitrogen) for human actin (used as a control) or 2 μl of combined primers for BCL-2, bax, caspase-3 or clusterin (RealTimePrimers, Elkins Park, PA). From a kit purchased from Invitrogen the following reactants were added for RT-PCR: 25 μl of 2x reaction mixture, 2 μl SuperScript III RT/platinum Taq mix, tracking dye, and MgSO4 (3 μl of a stock concentration of 5mM, used for BCL-2, bax, caspase-3, clusterin and VDAC1 vials only). DEPC treated water was added to yield a final volume of 50 μl. As a control for RT-PCR product production, human actin expression was tested in RNA extracted from HeLa cells which was provided in a kit purchased from Invitrogen (in the reaction mixture, no MgSO4 was included, the difference compensated for by 3 μl of DEPC treated water). RT-PCR was performed for 2 x 25 cycles using the F54 program in a Sprint PCR Thermocycler
Primers
Bcl-2
Forward primer sequence: 5’ GAG ACA GCC AGG AGA AAT CA 3’
Reverse primer sequence: 5’ CCT GTG GAT GAC TGA GTA CC 3’ PCR product produced was 127 base pairs in length.
Caspase-3
Forward primer sequence: 5’ CCC CTG GAT CTA CCA GCA TA 3’ Reverse primer sequence: 5’ TGT CTC TGC TCA GGC TCAAA 3’ PCR product produced was 262 base pairs in length.
Bax
Forward primer sequence: 5’ GCT GGA CAT TGG ACT TCC TC 3’ Reverse primer sequence: 5’ CTC AGC CCA TCT TCT TCC AG 3’ PCR product produced was 168 base pairs in length.
Clusterin
Forward primer sequence: 5’ GGA GGA GTG AGA TGT GGA TG 3’
Reverse primer sequence: 5’ATG CAG GAG CAA TTC TGT TC 3’ PCR product produced was 221 base pairs in length.
VDAC1
Forward primer sequence: 5’ATT TCG ACA TTG CTG GGC CT 3’
Reverse primer sequence: 5’ AAA TGG AGC CGC CA ACT CT 3’ PCR product produced was 195 base pairs in length.
Detection and Quantitation of Product
Agarose Gel Electrophoresis
1.5% agarose gels were prepared in a 50 ml volume of TBE buffer (1x solution: 0.089 M Tris borate and 0.002M EDTA, pH 8.3), containing 3 μl of ethidium bromide (10 mg/ml in 1x Tris borate buffer) in a Fisher Biotest electrophoresis system. Samples were run for 2 hours at a constant voltage of 70 using a BioRad 1000/500 power supply source. To locate the amplified PCR product, 3 μl of a molecular marker which contained a sequence of bases in 100 base pair increments (Invitrogen) as well as 2 μl of a sucrose based bromphenol blue tracking dye were run in each gel. For actin product localization, the tracking dye was included in each sample run; for all other products the tracking dye was run separately.
Quantitation
Gels were visualized under UV light and photographed using a Canon 800 digital camera. Photographs were converted to black and white format and bands quantitated using Mipav software provided by the National Institute of Health (NIH). Means and standard deviations were compared using student t tests to determine significance.
Results
Cell Culture Experiments
LNCaP cells were incubated with MR4, MR24 and MR42 and compared to lipofectin containing controls (Figure 1). In an initial experiment (Experiment 1) each oligo significantly inhibited the growth of LNCaP cells: MR4 by 23.8% (p = 0.0004); MR24 by 31.2% (p < 0.001); and MR42 by 31.7% (p < 0.001).
In a repeat experiment (Experiment 2), employing only the bispecifics, LNCaP cells were similarly incubated and compared to lipofectin containing controls. MR24, and MR42 produced significant respective inhibitions of 49.5% (p < 0.001) and 56.8% (p < 0.001), and were at least as effective as the mono-specific MR4 directed only towards BCL-2 in the inhibition of in vitro cell growth (Experiment 1).
RT-PCR Experiments
In a series of control experiments (data not shown) to validate RNA extraction and RT-PCR procedures, the expression of human actin in HeLa cells was identified [19].
BCL-2 Expression
LNCaP cells incubated for 24 hours in the presence of 6.25 μM of oligos suppressed BCL-2 expression, and support the finding of comparable biologic activity in both mono- and bispecific oligos measured in the in vitro cell growth inhibition experiments. When photographs of the identified product bands were scanned on agarose gels and quantitated using Mipav software, in a series of runs, the greatest expression of BCL-2 was always found in untreated LNCaP cells. Those treated with oligos, whether mono- or bispecific, produced bands which indicated obvious (to the naked eye) suppression. For each oligo evaluated, the greatest amount of suppression measured approached 100%, for the mono-specific MR4; and for the bispecifics MR24 and MR42, 86 and 100%, respectively. Suppression was found in both repeat PCR runs with BCL-2 primers, as well as in repetitive agarose gel quantifications. Figure 2 presents a BCL-2 product band in the expected 127 base pair region which in this run was inhibited 23% by treatment with the mono-specific MR4, and 86% and 74%, respectively by bispecifics MR24 and MR42, as measured by Mipav software.
Caspase-3 Expression
Comparable amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against BCL-2 (and EGFR in the bispecifics) were then evaluated by RT-PCR using primers directed against caspase-3. A representative band for caspase-3 is presented in Figure 4 and appears below the marker representing 300 base pairs in the expected 262 base pair region.
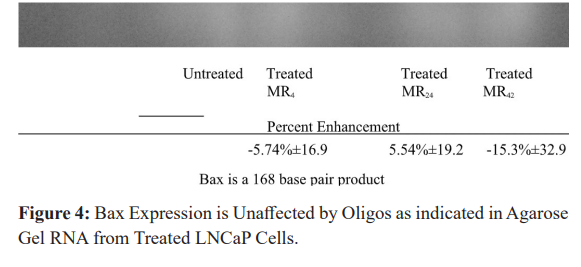
When background intensity was subtracted, the relative intensity of the bands corresponding to caspase-3 representing cells treated with MR4, MR24 and MR42 compared to controls were -35.8%± 12.5 (P = 0.0002), -40.3% ± 16.6 P = 0.0006) and -43.5% ±26.3 (P = 0.006). These results were pooled from both duplicate PCR runs and gels, and indicate similar (significant) suppression of caspase-3 activity is produced by both mono- and bispecific oligo types.
While gene therapy is often aimed at suppressing bcl-2, for re- establishment of apoptosis caspase-3 expression is essential. These experiments identify a mechanism for tumors to select variants which (again) evade apoptosis through the diminished expression of an apoptosis promoter (caspase-3). It also identifies caspase-3 as a necessary gene for expression (or replacement therapy) when oligos target bcl-2 for suppression.
Bax Expression
Comparable amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against bcl-2 (and EGFR in the bispecifics) was then evaluated by RT-PCR using primers directed against bax. A representative band for bax is presented in Figure 4 and appears below the marker representing 200 base pairs in the expected 168 base pair region. When background intensity was subtracted, the relative intensity of the bands corresponding to bax representing cells treated with MR4, MR24 and MR42 compared to controls were -5.74% ± 16.9,5.54% ±19.2, and -15.34% ± 32.9 (mean ± SD). These results were pooled from both duplicate PCR runs and gels, indicating no significant differences in bax expression, compared to that seen with bcl-2.
Clusterin Expression
Comparable amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against bcl-2 (and EGFR in the bispecifics) was then evaluated by RT-PCR using primers directed against clusterin. A representative band for clusterin is presented in Figure 5 and appears where expected between the markers representing 200 and 300 base pairs, as a 221 base pair product.
When background intensity was subtracted, the relative intensity of the bands corresponding to clusterin representing cells treated with MR4, MR24 and MR42 compared to controls were 8.3% ± 14.5, 9.0% ±17.3, and -14.1% ± 22.6 (mean ± SD). These results were pooled from both duplicate PCR runs and gels, indicating (like bax) there are no significant differences in clusterin expression, compared to that seen with caspase-3.
VDAC1 Expression
Comparable amounts of extracted RNA from LNCaP cells treated with either mono- or bispecific oligos directed against bcl-2 (and EGFR in the bispecifics) was then evaluated by RT-PCR using primers directed against clusterin. A representative band for clusterin is presented in Figure 6 and appears where just below the 200 base pair markers, as a 195 base pair product.
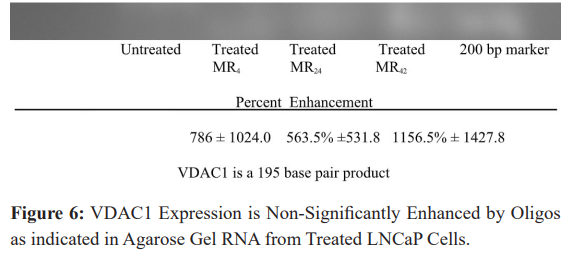
When background intensity was subtracted, the relative intensity of the bands corresponding to clusterin representing cells treated with MR4, MR24 and MR42 compared to controls were 786 ± 1024.0, 563.5% ±531.8, and 1156.5% ± 1427.8 (mean ± SD). These results were pooled from both duplicate PCR runs and gels, indicating there are no significant differences in VDAC1 expression, but a strong indication that an enhancement is indeed produced. Wide variation in results account for the insignificant results, but to the eye enhancement is obvious.
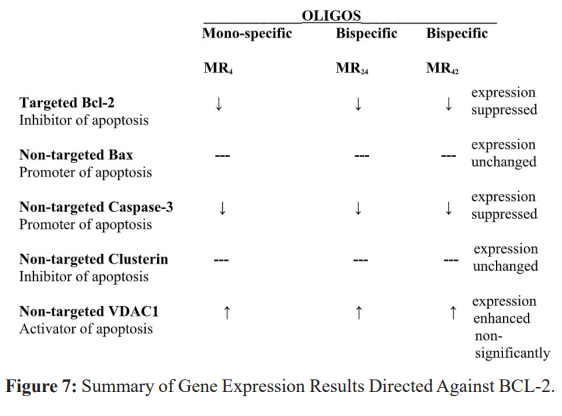
The results of bcl-2 suppression on the regulators of apoptosis are presented in Figure 7. Only Caspase-3 is significantly altered, and suggests that its function as a promoter of apoptosis should be either maintained or replaced when such therapy is directed towards bcl-2.
Discussion
Gene therapy is often promoted as a highly specific and deliverable treatment to control aberrant gene expression by tumor cells (particularly when growth factors, their receptors, apoptosis inhibitors or oncogenes are excessively transcribed). However, it’s now apparent that it’s not as specific as previously believed.
Antisense oligos consist of nucleotide bases synthesized complimentary in sequence to mRNA. When hybridized to mRNA, they produce a translational arrest of the targeted gene’s mRNA expression. This method is an effective, relatively non- toxic and inexpensive form of therapy and various types of antisense RNA have been constructed for this purpose. These include the phosphorothioated oligos used in these evaluations and other formulation including 2’-MOE-RNA, morpholinos, siRNA, miRNA etc. Modifications to the oligo backbone and base structure are used to prevent nuclease degradation, increase systemic half- life and to enhance distribution and delivery, particularly across the blood: brain barrier. Some of these derivatives have been evaluated in clinical trials, but all oligos employed were directed against single gene transcriptional (mRNA) products. In contrast, the oligos discussed in this paper included both mono- and bispecific forms, each having a base sequence complementary to and directed against mRNA encoding the apoptosis inhibitor bcl-2, (bispecifics included an additional site directed against EGFR). We evaluate bispecific oligos because it would be naïve to believe targeting a single mRNA would be sufficient to produce a clinical response in most tumors, and activity at one site does not affect binding at a second [15]. Furthermore, we have shown that both mono- and bispecific oligos have comparable activity when suppressing bcl-2 [15]. Therefore, administration of a single oligo having two mRNA targets could have an additional suppressive benefit, particularly if EGFR is excessively produced (when using these bispecifics).
While it’s understandable that unrelated genes which share sequence homology would also be susceptible to antisense oligos, when directed towards that common sequence, what is not expected are the effects on non-targeted genes having unrelated sequences; many of which control additional growth regulating pathways, and as we now believe regulate the immune response, as well.
In addition to unanticipated effects upon unrelated genes either having or not containing related sequences; we have also shown that certain complementary stretches of base sequences can occur within the oligo itself which can also produce unanticipated effects affecting the immune system. Complementary intra strand binding has been shown to increase the expression of interferon which may have a role in the expression of cell surface antigens and differentiation proteins, like PSA or PSMA. In an early evaluation of bispecifics we reported the enhanced expression of PSMA [6] when oligos were directed against bcl-2. The unique capacity to produce such changes by these bispecfics (and not a similarly directed monospecific) is attributable to an unusual double strand conformation present in bispecifics and interferon induction (an enhancer of surface antigen expression, like PMSA) [6]. Such expression could enable better recognition and targeting by cytotoxic T cells toward cell surface PSMA [6]; to a greater extent than secretory PSA or PAP.
Tumors are a mass of genetically unstable heterogeneous cells capable of both rapid mutation and selection. As noted above, compensation for suppression of bcl-2 also leads to greater chromosomal instability and gene fusion involving TMPRS22 [10] and fusion transcription protein FLI-1 [11]. All this leads to greater proliferation and mitosis as indicated by KI-67 and cyclin D1 [11]. Therefore, just as bacterial and viral agents develop resistance to chemotherapeutics, tumors cells have a similar capability. Initial evaluation of protein expression associated with compensation regulating the traditional mode of apoptosis (mitochondrial mediated) focused on the bcl-2, bax, bad, clusterin etc. However, more recent work evaluated proteins associated with tumor cell destruction, via apoptosis, mediated by a secondary route for activation, involving direct signal transduction. This is a process of initiating apoptosis through the binding of activating proteins (ligands, like PD-L1) on immune cells to cell surface receptors (like tumor cell PD-1). When ligands bind to these receptors, they activate a destructive cascade of protein interactions which lead to cell death. These receptors are structurally similar to the tumor necrosis factor receptor (CD95) and also regulate the immune system’s cytotoxic T cell response.
We conclude that the increase in CDK-12 expression following treatment would suggest a resistance to immunotherapy, not present in the untreated LNCaP cells; and follow the pattern that compensation for antisense oligos directed towards bcl-2 make tumors more aggressive, androgen sensitive and proliferative. However, one would have thought that the increased PD-1 expression that results from oligos directed against bcl-2 would have made the tumor cell a better target for immunotherapy. That PD-1 its ligand PD-L1 and CDK-12, were all significantly enhanced following bcl-2 suppression suggest that immunoregulation, is an additional pathway for compensatory based resistance. It is also obvious that, there is much to learn regarding the non-specific effects of gene therapy in general and antisense oligos in particular. As demonstrated here, findings can at times be counter-intuitive unless the massive increase in CDK-12 expression in these same cells could overwhelm the apparently advantage of increased PD-1 expression.
Clinically, gene therapy protocols are being administered which suppress apoptotic inhibitory proteins bcl-2 and clusterin. Several include antisense oligos meant to restore apoptosis associated with chemotherapy [4] and radiation treatment [5,24], and one is administered against prostate cancer [23].
Within a tumor mass, cells are individually heterogenous, and those which evade growth regulation or programmed cell death (apoptosis) are selected. In addition, as their DNA becomes increasingly unstable, variants tend to accumulate additional adaptations (mutations) which further contribute to malignancy, dissemination, resistance to apoptosis, and as a result of suppressed bcl-2 expression we find the potential for increased androgen sensitivity mediated via its receptor. Selection of cells which resist apoptosis is no different than the process by which hormone sensitive prostate tumor cells, in the absence of androgen, are selected (and establish themselves) as insensitive variants.
This year (2019) the American Cancer Society (ACS) estimated that in spite of early detection, screening for prostate specific antigen (PSA) and effective treatments for localized disease, in the United States there are 31,620 expected deaths from prostate cancer with 174,650 newly diagnosed cases [24]. New types of treatment, including gene therapy and translational inhibition must be developed and employed (probably in combination with traditional androgen ablation).
Acknowledgments
The Cellular Biology laboratory at the Hektoen Institute is supported, in part, by the Blum Kovler Foundation, Lawn Manor Beth Jacob Hebrew Congregation, the Max Goldenberg Foundation, the Pritzker Traubert Family Foundation, the Janet and Maurice Rubenstein Memorial Foundations, the Sternfeld Family Foundation, and the Plitt Charitable Trust.
References
- Rubenstein M, Dunea G, Guinan Growth factor deprivation therapy utilizing antisense oligonucleotides. Drug News and Perspectives. 1994; 7: 517-524.
- Rubenstein M, Mirochnik Y, Chow P, et al. Antisense oligonucleotide intralesional therapy of human PC-3 prostate tumors carried in athymic nude mice. J. Surg. Oncol. 1996; 62: 194-200.
- Rubenstein M, Mirochnik Y, Chou P, et al. Growth factor deprivation therapy of hormone insensitive prostate and breast cancers utilizing antisense Meth. Find. Clin. Pharmacol. 1998; 20: 825-831.
- Yamanaka K, Miyake H, Zangemeister-wittke U, et al. Novel bispecific antisense oligonucleotides inhibiting both bcl-2 and Bcl-xL expression induce apoptosis and enhance chemosensitivity in human androgen-independent prostate cancer cells. 2005; 4: 1689-1698.
- Yip KW, Mocanu JD, Au PY, et al. Combination bcl-2 antisense and radiation therapy for nasopharyngeal cancer. Clin. Cancer Res. 2005; 11: 8131-8144.
- Rubenstein M, Hollowell CMP, Guinan P. Bispecific oligonucleotides may induce interferon in LNCaP cells enhancing surface antigen expression Effect of intra strand base pair complementarity. 2011; 25: 61-67.
- Rubenstein M. Suppression of bcl-2 by antisense oligonucleotides is compensated through increased activity of the androgen receptor and co-activators but not 5-alpha reductase. Cancer Research and Oncology: Open access. 2016; 2.
- Rubenstein M, Hollowell CMP, Guinan P. Following inhibition of BCL-2 by antisense oligonucleotides compensatory suppression of apoptosis involves the direct signal transduction pathway of LNCaP Online Journal of Apoptosis. 2015; 4: 1-10.
- Rubenstein M, Hollowell CMP, Guinan P. Oligonucleotide suppression of bcl-2 in LNCaP cells is compensated by increased androgen sensitivity, p53 and oncogene activity, and suppressed caspase-3. Med. Oncol. 2013; 30: 599.
- Rubenstein Expression of TMPRSS22, androgen receptor and its co-activators are increased following suppression of bcl-2 by antisense oligonucleotides But the TMPRSS22 fusion partner ERG is not detectable in the compensatory response. Cancer Research and Oncology Open access. 2016; 2.
- Rubenstein M. Mechanisms by Which Non-targeted Genes Compensate for Specific Gene Therapy Directed Towards Bcl-2 in a Prostate Cancer Model. eBook: Recent Advances in Prostate Cancer. 2017; 2.
- Oncogenex.ca
- Rubenstein M, Mirochnik Y, Ray V, et al. Lack of toxicity associated with the systematic administration of antisense oligonucleotides for treatment of rats bearing LNCaP prostate tumors. Med. Oncol. 1997; 14: 131-136.
- Rubenstein M, Tsui P, Guinan P. Bispecific antisense oligonucleotides with multiple bindings sites for the treatment of prostate tumors and their applicability to combination therapy. Meth. Find. Clin. Pharmacol. 2006; 28: 515-518.
- Rubenstein M, Guinan Bispecific antisense oligonucleotides have activity comparable to monospecifics in inhibiting expression of Bcl-2 in LNCaP Cells. In. 2010; 24: 489-493.
- Rubenstein M, Hollowell CMP, Guinan Inhibition of bcl-2 by antisense oligonucleotides is followed by a compensatory suppression of caspase-3 in LNCaP cells. Eur. J. Clin. Med. Oncol. 2011; 3: 1-6.
- Rubenstein M. Suppression of bcl-2 by antisense oligonucleotides is compensated through increased activity of the androgen receptor variants ARv7 and ARv9 and several co-activators. Oncol. Res. Rev. 2018; 1: 1-7.
- Rubenstein M, Hollowell CMP, Guinan In LNCaP cells enhanced expression of both androgen receptor and co-stimulatory protein p300 compensate for antisense oligonucleotide suppression of bcl-2 Ther. Adv. Urology. 2011; 3: 243-250.
- Rubenstein M, Hollowell CMP, Guinan P. Additional compensatory mechanisms -altering antisense oligonucleotide suppression of Bcl-2: Effects upon AKT-1 and -3. 2014;28: 867-870.
- http://www.washingtonpost.com/business/studies-merck- drug-keytruda-effective-against-3-cancers/2015/04/19/01d- 94cfc-e6b4-11e4-8581-633c536add4b_story.html
- Furlow B. CDK-12 loss a biomarker for prostate cancer response to immunotherapy. Cancer and Genetics, News, Prostate Cancer. 2018.
- Shoshan-Barmatz V, Maldonado EN, Krelin VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress. 2017; 1: 11-36.
- Mu Z, Hachem P, Pollack A. Antisense BCL-2 sensitizes prostate cancer cells to The Prostate. 2015; 65: 331- 340.
- American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society. 2019.