Observational Study of the Efficacy of Immediate-Release Metformin in People with Prediabetes in Russia
Author'(s): Ulrike Hostalek1*and Svetlana Mustafina2
1Global Medical Affairs, Merck Healthcare KGaA, Darmstadt, Germany.
2Research Institute of Therapy and Preventive Medicine - a branch of Federal Research Center Institute of Cytology and Genetics, Novosibirsk, Russia.
*Correspondence:
Dr. Ulrike Hostalek, Merck KGaA, Frankfurterstr. 250, 64293 Darmstadt, Germany, Tel: 06151/723106.
Received: 22 May 2020 Accepted: 29 June 2020
Citation: Ulrike Hostalek, Svetlana Mustafina. Observational Study of the Efficacy of Immediate-Release Metformin in People with Prediabetes in Russia. Diabetes Complications. 2020; 4(2); 1-2.
Abstract
Aim: To study the effects of metformin in prediabetes.
Methods: This was an observational, non-interventional, uncontrolled study of the effects of the immediate-release formulation of metformin in 551 people with pre-diabetes, according to diagnostic criteria from the American Diabetes Association (ADA).
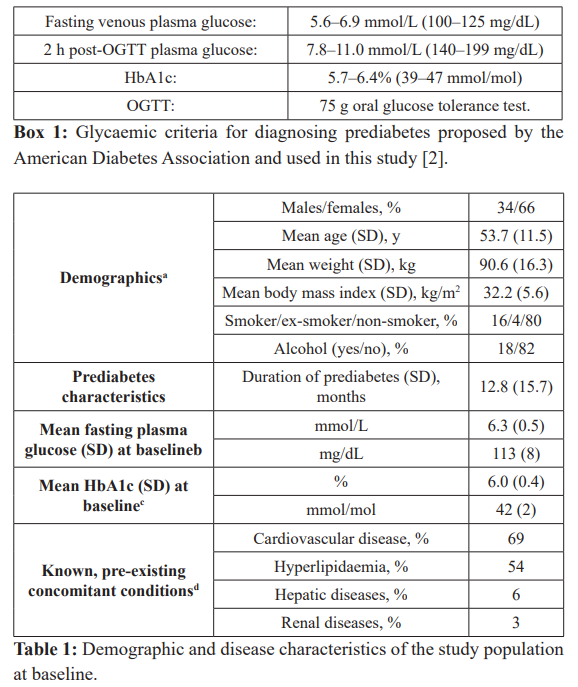
Results: The study population was predominantly female (66%), and on average was middle-aged (mean age 54 [SD 12] years) and obese (mean body mass index 32 kg/m2 [SD 6]) at baseline. Treatment with metformin for 12 weeks was associated with a mean reduction in fasting plasma glucose (FPG) of –0.8 mmol/L (95%CI –0.8 to –0.9) from a baseline value of 6.3 mmol/L (SD 0.5). FPG was normalised, according to ADA criteria (<5.6 mmol/L) in 86% of the population, at 12 weeks (the primary endpoint of the study). Reductions from baseline were also seen for HbA1c (by –0.4% [95% CI -0.3 to -0.5]) and post-load glucose following an oral glucose tolerance test (by –2.0 mmol/L [95%CI -1.5 to -2.4]) at 12 weeks. These parameters were normalised according to ADA criteria in a majority of patients where data were available, although measurements were available from only 189 and 51 patients, respectively. A logistic regression analysis suggested significant influence of age and starting dose of metformin on the likelihood of normalisation of FPG. Adherence to metformin was high (>75% in 93% of the population). Metformin was well tolerated, with common finding of mild-to-moderate gastrointestinal side-effects, as expected.
Conclusion: These real-world data add further evidence in support for a role for metformin in the management ofpeople with prediabetes.
Keywords
Introduction
Type 2 diabetes is associated with a markedly increased risk of adverse cardiovascular outcomes, compared with people without this condition [1]. The diagnosis of type 2 diabetes is categorical, and made when measurements of plasma glucose exceed a predetermined level [2]. However, the risk of cardiovascular events increases continuously with increasing plasma glucose [3,4], and people who have indices of glycaemia in the high-normal range are at increased cardiovascular risk, compared with people with lower levels of plasma glucose [5-8]. Moreover, up to 10% of these individuals will progress to developing type 2 diabetes each year [9]. Accordingly, the presence of elevated, but non-diabetic, levels of plasma glucose has been termed “prediabetes” [10].
The mainstay of treatment of prediabetes, to avoid conversion to type 2 diabetes, is lifestyle intervention, including increased physical activity, improvement of the diet to promote weight loss and smoking cessation [11-13]. Treatment with metformin has also been shown to reduce the risk of conversion from prediabetes to clinical diabetes, principally in the Diabetes Prevention Program (DPP) and its post-trial follow-up [14,15], and also in other studies (reviewed elsewhere [16]). Guidelines for diabetes prevention support a role for metformin, especially in obese (BMI ≥35 kg/m2) and younger (<60 y) subjects [12,13], and metformin is indicated for the prevention of diabetes in many countries [16].
The prevalence of prediabetes in Russia, is high compared with most other countries [17] and was estimated to be 19% in a study published in 2016 [18]. The effects of metformin on glycaemia in people with prediabetes in Russia are therefore of clinical interest. We evaluated the effects of the commonly used immediate-release formulation of metformin on glycaemic control in an observational study in a population of 551 people with prediabetes in Russia.
Methods
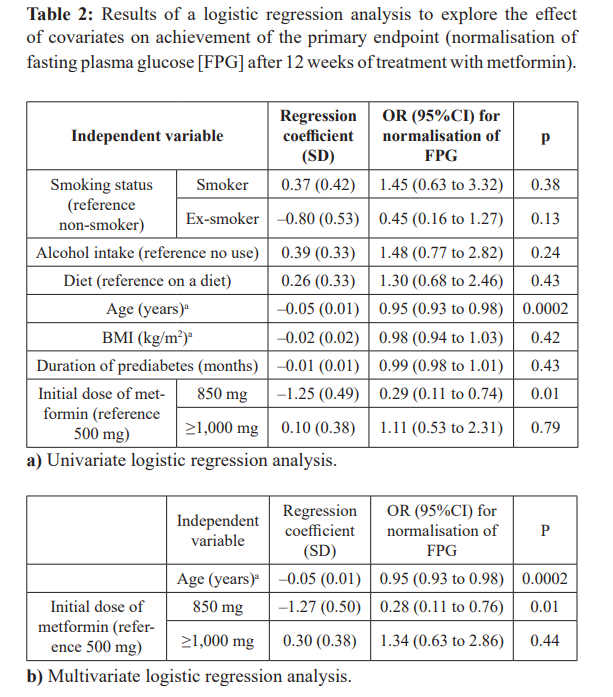
This was a non-interventional, open, non-randomised uncontrolled, observational study conducted in 20 centres in Russia. Eligible subjects were naïve to pharmacologic anti-diabetes treatments and had prediabetes according to commonly-used glycaemic criteria proposed by the American Diabetes Association (ADA), based on measurement of fasting venous plasma glucose (FPG), post-load glucose or HbA1c (Box 1) [2]. Subjects received immediate-release metformin (Glucophage®) orally and in two daily doses, at a total dose of 1,000–1,700 mg/day, depending on efficacy and tolerability as assessed by the physician within the usual care setting. Women of childbearing age maintained adequate contraception for the duration of treatment. The duration of treatment analysed was 12 weeks.
The primary endpoint was the percentage of subjects with normalised FBG, defined as a final FBG <5.6 mmol/L (100 mg/ dL). Secondary endpoints were the percentage with normalised HbA1c (<5.7% [39 mmol/mol]), absolute changes in HbA1c, and the percentage of subjects with normalised post-load glucose following a 75 g oral glucose tolerance test (OGTT), where data were available from patients’ routine care notes. Safety was assessed by recording adverse events (AEs), according to standard Medical Dictionary for Regulatory Activities (MedDRA) criteria.
The desired size of the population was estimated based on the expected proportion of patients with normalisation of glycaemia based on previous studies. Data were analysed using descriptive statistics and data are presented as percentages of patients or as means (SD). No formal significance testing was used for measurements of plasma glucose. Patients with missing data were omitted from analysis of individual variables. A logistic regression analysis was undertaken to explore the effects of several demographic and disease characteristics known to influence metabolic parameters on the likelihood of achieving normalised plasma glucose. Adherence was measured by tablet count (number of tablets taken divided by number of tablets prescribed times 100). Categories of adherence to treatment were: “excellent” for >90%, “Good” for 76-90%, “moderate” for 50–75%, and “bad” for <50%.
Patients provided written, informed consent before enrolment. The study was conducted in a manner consistent with Good Clinical Practice. Formal ethical approval of the study was not required, as this was a non-interventional study with no impact on physicians’ usual clinical practice.
Results
Patients
Of 553 patients screened, 551 were enrolled into the study, between February and November 2018; 531 subjects (96%) completed the 12-week treatment period. The most common reason for withdrawal from the study was loss to follow-up (9 patients [1.6%]).
On average, the population was middle-aged, and overweight or obese (Table 1). About two-thirds were female, and about four- fifths stated that they did not use tobacco or alcohol. Prediabetes had been present for about one year, on average. Mean HbA1c and fasting glucose were in the high-normal range at baseline, consistent with the diagnosis of prediabetes (Box 1).
Treatments
All received lifestyle advice before receiving metformin, resulting in 97% reportedly altering their diets and 90% reportedly increasing the amount of exercise they took. The mean daily dose of metformin at study end was 936 mg (SD 260 mg). The range of doses taken was 500–1,700 mg.
Effects on glycaemia
Treatment with metformin was associated with a reduction in FPG of –0.8 mmol/L (14 mg/dL); although no formal significance testing was conducted, the 95% CI for this reduction did not include 1.0, suggesting a significant effect (Figure 1a). FPG was normalised (the study’s primary endpoint) in about 6 patients in 7 (Figure 1b).
Mean HbA1c was reduced by –0.4% (95% CI -0.3 to -0.5), although data at baseline and last visit were available for only 189 patients. HbA1c was normalised at 12 weeks in 100 patients, and not normalised on 89 patients. Mean post-OGTT glucose was reduced by –2.0 mmol/L (95%CI -1.5 to -2.4) [-36 mg/dL (95% CI -27 to -43)], although data were available only for 51 patients. Post-OGTT glucose was normalised in 40 patients, and not normalised in 17 patients.
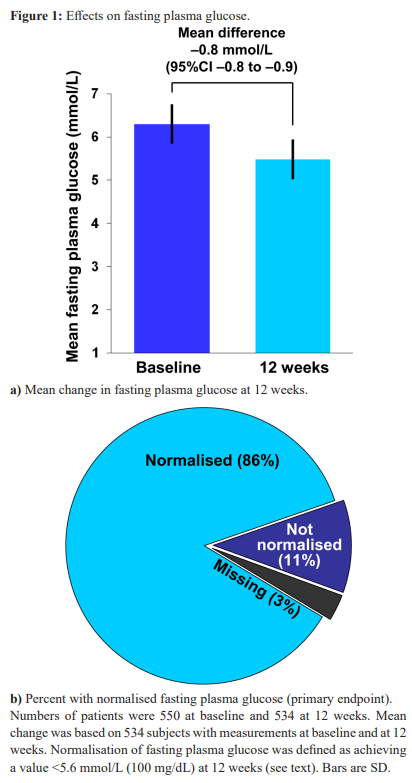
A logistic regression analysis was performed to assess the effects of covariates on the likelihood of achieving normalisation of FBG (Table 2). Increasing age slightly but significantly reduced the likelihood of normalisation of FBG at 12 weeks. An initial dose of 850 mg of metformin was associated with a lower likelihood of achieving normalisation of FBG, compared with an initial dose of 500 mg, while a higher initial dose had no significant effect. These effects were similar in Univariate and multivariate analyses. Smoking, alcohol, diet, BMI and duration of prediabetes were without significant effect.
Changes in medication and adherence to therapy
The mean daily dose of metformin was 936 mg (SD 260), and the median daily dose was 1,000 mg. Information on changes to medication during the study was available for 536 patients (97% of the overall population). Most of the population (82%) did not change their initially prescribed medication. Changes to medication by 12 weeks occurred for 15% of the population, usually an increase in dose. Adherence to metformin therapy was “excellent” (>90%) in 79%, “good” (76–90%) in 14% “moderate” (50–75%) in 3%, and “bad” (<50%) in 1% (data on adherence were unavailable for 19 subjects (3% of the overall population). Accordingly, adherence was “excellent” or “good” in 93% of our population.
Tolerability and safety
Thirteen patients (2%) reported 32 AEs. All were mild to moderate in intensity. Sixteen AEs considered related to treatment occurred in 7 patients (1%). Gastrointestinal symptoms were common (9 treatment-related AE, of which 5 were diarrhoea). One AE (ventricular tachycardia) was reported as a serious AE and was considered to be unrelated to study treatment. No AEs related to changes in vital signs or laboratory parameters were reported.
Discussion
We showed that a treatment with metformin reduced mean FPG substantially, and normalised fasted plasma glucose, according to ADA criteria [2], in a population of 551 subjects with prediabetes in Russia. Tolerability findings included gastrointestinal symptoms, as would be expected with metformin. All of the AE considered related to treatment were mild or moderate in intensity, consistent with the high retention rate of patients in the study.
The reduction in mean FBG (–0.8 mmol/L) and rate of normalisation of FBG (86%) were larger than those seen in an observational evaluation of similar design in subjects with prediabetes in Hungary (–0.55 mmol/L and 43%, respectively), despite similar FBG at baseline (6.3 mmol/L and 6.1 mmol/L, respectively) [19]. The reason for this difference is unclear. The median metformin dose in each study was identical, at 1,000 mg/ day. Previous clinical experience in type 2 diabetes suggests that the efficacy of the two formulations is similar [20]. Differences in usual care for prediabetes between the centres in each study may be relevant, in particular the application of lifestyle intervention, as advised for most patients in our study. Also, these formulations have not been compared directly in people with prediabetes, and comparisons between different trials must be made cautiously. Metformin reduces plasma glucose effectively irrespective of ethnicity [16], and these results are likely to be relevant to other populations, although further studies in specific populations will be useful in future.
As the diagnosis of type 2 diabetes is made according to the level of plasma glucose, a reduction in plasma glucose with metformin may explain part of the reduction in the risk of diabetes observed in the DPP, and other randomised, controlled trials [14,16,21]. Lower fasting glucose predicted reversion from prediabetes to normal glucose tolerance in the DPP [22]. However, an analysis from the DPP showed that a short-term effect of metformin on diabetes accounted for only about one-quarter of its efficacy in preventing diabetes [23].
The finding that increasing age slightly reduced the efficacy of metformin in normalising plasma glucose is consistent with experience form the DPP, where metformin was more effective in younger patients [14]. There was no effect of increasing BMI on glycaemia in our study, however, in contrast to the DPP where metformin was most effective in more obese subjects. The apparent lack of efficacy of metformin 850 mg, compared with 500 mg, in the logistic regression analysis is puzzling, as the efficacy of metformin on plasma glucose in people with type 2 diabetes is dose-related over this range of doses [24].
Metformin was well tolerated in this study. Indeed, metformin was chosen for evaluation in the DPP partly due to its well understood safety profile from decades of therapeutic use in diabetes, as well as its known efficacy in reducing plasma glucose [21]. Starting at a low dose, titrating the dose cautiously, and reducing the dose (perhaps temporarily) have been shown to assist patients in tolerating metformin [25]. The prolonged release formulation, which also normalised fasting glucose in a substantial proportion of patients, is an option for some patients who cannot tolerate the immediate release formulation [26].
Our study has some important limitations, particularly its uncontrolled, observational design. While the reduction on plasma glucose with metformin is consistent with reduced risk of clinical type 2 diabetes, as described above, our study was not long enough to measure rates of development of diabetes. However, recognition of the importance of observational data in the evaluation of pharmaceutical products has increased in recent years, as they reflect real-world usual care practices unconstrained by the more rigid design of a randomised, controlled trial [27]. The proportion of patients without data on HbA1c or post-OGTT glucose was high, reflecting the non-interventional nature of the study, where only data collected during routine care visits were available. Strengths of our study included the relatively broad inclusion criteria that allowed recruitment of a population typical of that seen in routine care, the retention of 96% of our patients, and the high level of compliance with metformin.
Conclusion
Treatment of a population of people with prediabetes with the immediate-release formulation of metformin normalised FBG (according to criteria from the ADA) in 86% of the population, in an observational study conducted under usual care conditions in Russia. Metformin was well tolerated, consistent with previous clinical experience with this treatment. These real-world data add further evidence in support of a role for metformin with the management of people with prediabetes in the routine, usual care clinical setting.
Acknowledgements
The study was funded by Merck Healthcare KGaA. UH is an employee of Merck Healthcare KGaA.
A medical writer (Dr Mike Gwilt, GT Communications, funded by Merck Healthcare KGaA) provided editorial assistance.
References
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes systematic literature review of scientific evidence from across the world in 2007- 2017. Cardiovasc Diabetol. 2018; 17: 83.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020; 43: S14-S31.
- Stratton IM, Adler AI, Neil HA, et Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes UKPDS 35 prospective observational study. BMJ. 2000; 321: 405-412.
- Sarwar N, Gao P, Kondapally Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration and risk of vascular disease a collaborative meta-analysis of 102 prospective studies. Emerging Risk Factors Collaboration Lancet. 2010; 375: 2215-2222.
- Vistisen D, Witte DR, Brunner EJ, et Risk of cardiovascular disease and death in individuals with pre diabetes defined by different criteria: the Whitehall II Study. Diabetes Care. 2018; 41: 899-906.
- Huang Y, Cai X, Mai W, et al. Association between pre diabetes and risk of cardiovascular disease and all cause mortality systematic review and meta analysis. BMJ. 2016; 355: i5953.
- Perreault L, Temprosa M, Mather KJ, et al. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk results from the Diabetes Prevention Program outcomes study. Diabetes Care. 2014; 37: 2622-2631.
- Brannick B, Dagogo Jack S. Prediabetes and Cardiovascular Disease Pathophysiology and Interventions for Prevention and Risk Endocrinol Metab Clin North Am. 2018; 47: 33-50.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78: 305- 312.
- Tabák AG, Herder C, Rathmann W, et Prediabetes a high- risk state for diabetes development. Lancet. 2012; 379: 2279- 2290.
- Cosentino F, Grant PJ, Aboyans V, et 2019 ESC Guidelines on diabetes prediabetes and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020; 41: 255- 323.
- American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020; 43: S32-S36.
- https://www.nice.oruk/guidance/ph38
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346: 393-403.
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015; 3: 866-875.
- Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015; 75: 1071-1094.
- Assaad Khalil SH, Abdelaziz SI, Al Shammary A, et al. Prediabetes management in the Middle East Africa and Russia Current status and call for action. Diab Vasc Dis Res. 2019; 16: 213-226.
- Dedov I, Shestakova M, Benedetti MM, et al. Prevalence of type 2 diabetes mellitus T2DM in the adult Russian population NATION study. Diabetes Res Clin Pract. 2016; 115: 90-95.
- Hostalek U, Zilahi Z. Observational study of the efficacy of prolonged-release metformin in people with Curr Med Res Opin. 2020.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended- release formulation. Clin Ther. 2003; 25: 515-529.
- Aroda VR, Ratner RE. Metformin and type 2 diabetes prevention. Diabetes Spectr. 2018; 31: 336-342.
- Perreault L, Kahn SE, Christophi CA, et al. Regression from prediabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009; 32: 1583-1588.
- Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Diabetes Care. 2003; 26: 977-980.
- Garber AJ, Duncan TG, Goodman AM, et al. Efficacy of metformin in type II diabetes results of a double-blind, placebo-controlled dose-response Am J Med. 1997; 103: 491-497.
- Scarpello JH, Howlett Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008; 5: 157-167.
- Feher MD, Al-Mrayat M, Brake J, et al. Tolerability of prolonged-release metformin Glucophage® SR in individuals intolerant to standard metformin – results from four UK centres. Br J Diabetes Vasc Dis. 2007; 7: 225-228.
- Cohen AT, Goto S, Schreiber K, et al. Why do we need observational studies of everyday patients in the real-life setting. Eur Heart J Suppl. 2015; 17: D2-D8.