Prevalence of Patent Foramen Ovale in Ischemic Stroke Patients Over 60 Years of Age
Author'(s): Lamia Bencherif1*, Olivier Heinzlef2 and Nadia Ajili1
1Neurology Department, Poissy Hospital Center, France.
2Head of Neurology Department, Poissy Hospital Center, France.
*Correspondence:
Lamia Bencherif, Poissy Hospital Center, Neurology Department, 78100 France, E-mail: bencherif5@yahoo.fr.
Received: 11 Apr 2023; Accepted: 23 May 2023; Published: 28 May 2023
Citation: Lamia Bencherif, Olivier Heinzlef, Nadia Ajili. Prevalence of Patent Foramen Ovale in Ischemic Stroke Patients Over 60 Years of Age. Cardiol Vasc Res. 2023; 7(3): 1-7.
Abstract
Introduction: The patent foramen oval (PFO) prevalence is higher in young subjects with cryptogenic stroke compared to the general population, but there is not much information in subjects over 60 years old.
The goal of this study is to estimate the prevalence of PFO in patients over 60 years of age managed at Centre hospitalier intercommunal de Poissy/Saint Germain en Laye (CHIPS) for an acute ischemic stroke (AIS) and compare these results to what has already been reported in the literature.
Methods: It is a monocentric, observational, descriptive and retrospective study of a series of patients aged 60 to 80 years with ischemic stroke managed in the Neurovascular Intensive Care Unit of the CHIPS from September 1, 2020 to December 31, 2020. We excluded from our series AIS of identified non-cardio-embolic cause according to the TOAST classification. We have chosen to favour contrast TTE over TEE in our population of elderly subjects.
Results: Among the 22 patients analysed (mean age of 72.3 years), 7 patients received TEE and 15 patients received contrast TTE for PFO screening.
In our series, we have a significant PFO prevalence of 12.5% in cryptogenic AIS (1 in 8 cryptogenic AIS), which corresponds to the prevalence reported in the literature in subjects over 60 years of age with cryptogenic AIS (13%).
Conclusion: This is a preliminary descriptive study on the prevalence of PFO in elderly subjects with cryptogenic AIS in our center before undertaking further studies on the association between PFO and AIS in patients over 60 years of age, and eventually on the interest of PFO closure in this age group.
Keywords
Introduction
The association between cryptogenic acute ischemic stroke (AIS) and patent foramen ovale (PFO) is clearly established in the literature for subjects under 60 years of age, with a PFO prevalence of 40% compared to 25% in the general population [1-3], but there is not much information in subjects over 60 years old.
The question arises for this age group because a previously asymptomatic PFO can theoretically become significant with age and therefore symptomatic. Indeed, several factors have been associated with PFO enlargement, including dilatation of the heart chambers and the aortic arch, secondary to the decrease in collagen levels in the tissues, and the presence of pulmonary arterial hypertension (PAH) in elderly subjects. Also, the incidence of venous thromboembolic disease, and therefore the risk of paradoxical embolism, is greater in the elderly.
Before randomized studies evaluating the efficacy of PFO- closure in subjects over 60 years, it is necessary to demonstrate the association between PFO and cryptogenic AIS in this age group. A few studies have addressed this issue, but their results are inconsistent [3-6].
The goal of this study is to estimate the prevalence of PFO in patients over 60 years of age managed at Intercommunal Hospital of Poissy/Saint Germain en Laye (CHIPS) and compare these results to what has already been reported in the literature.
Methods
We conducted a monocentric, observational, descriptive and retrospective study of a series of patients aged 60 to 80 years with ischemic stroke managed in the Neurovascular Intensive Care Unit (NICU) of the CHIPS from September 1, 2020 to December 31, 2020 in order to identify the prevalence of PFO in this age group.
An age limit of 80 years was defined because of the difficulties in performing contrast TTE with the Valsalva manoeuvre or TEE in subjects older than 80 years.
We chose to not exclude AIS of cardioembolic origin (classified 3 according to the TOAST classification) [7] in the hypothesis that the PFO could be a competitive etiology with the possible indication of the management of both etiologies (closure and anticoagulation). Indeed, the only randomized trial comparing anticoagulants with PFO closure did not show that anticoagulation significantly reduced the risk of recurrence in PFO (without atrial fibrillation (A-FIB)) [8]. In addition, in the elderly, risk factors for A-FIB related to aging of the electrical tissue can aggravate an existing PFO and make it symptomatic.
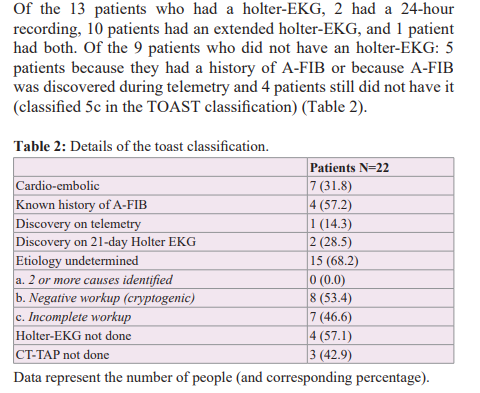
We excluded from our series AIS of identified non-cardio- embolic cause according to the TOAST classification [7] (AIS of atheromatous origin (1), AIS related to small cerebral artery disease (2), AIS with determined cause (4) such as arterial dissections and cancer), transient ischemic attacks (TIA), as well as patients with an initial modified Rankin score (mRS) > 3 [9]. We therefore kept patients with AIS of cardio-embolic origin (A-FIB) (3) and of undetermined origin (5) (those with a negative etiological work-up, i.e. cryptogenic (5b) and finally those with an incomplete etiological work-up (5c)).
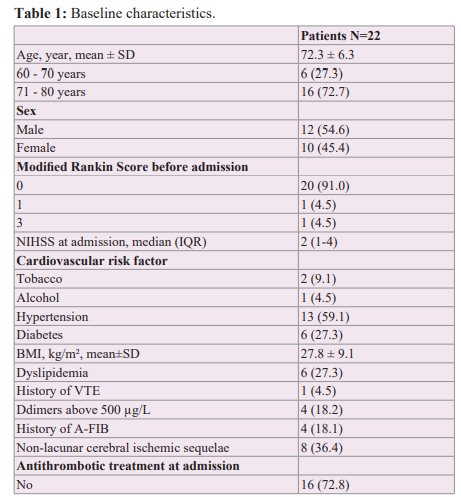
We collected for each of the included patients the following variables (Table 1): age, sex, mRS before admission, NIHSS score on admission, major cardiovascular risk factors (active smoking, chronic ethylism, arterial hypertension, diabetes, BMI and dyslipidemia), history of venous thromboembolic disease (deep vein thrombosis (DVT) and pulmonary embolism (PE)) as well as Ddimer level on admission, the known history of A-FIB, the presence of a non-lacunar ischemic sequela, the use or not of antithrombotic treatment at the time of the occurrence of the AIS, the topography of the AIS, the aetiology retained according to the TOAST classification and finally the RoPE score [10].
The etiological workup was as follows:
- An acute phase brain MRI was used to diagnose AIS and thus exclude TIA and Stroke
- An exploration of the supra-aortic trunks by CT angiography and/or Doppler ultrasound.
- An EKG during the acute phase and 24-48h telemetry during
- A biological assessment with a TSH and D-dimer measurement and a vascular risk factor assessment.
- A 24-hour and/or long-term Holter EKG (between 14 and 21 days)
- A thoraco-abdomino-pelvic CT-scan (CT-TAP)
- Microbubble contrast Transthoracic Echocardiography (TTE) and/or Transoesophageal Echocardiography (TEE)
We have chosen to favour contrast TTE over TEE in our population of elderly subjects. The multidisciplinary consultation meeting between neurologists and cardiologists during the virtual day of the French Neurovascular Society (SFNV) held on January 15, 2021 was in line with this decision. TEE is restrictive in this age group due to its invasive nature and often requires general anaesthesia, which carries a risk. Nevertheless, TEE remains the reference technique, particularly for the morphological detection of Atrial Septal Aneurysm (ASA). The Sono-Vue ® contrast agent presents a low or non-existent risk of side effects in the majority of cases observed clinically, without correlation with the age of the patient. All our patients have been informed of this.
The CT-TAP was performed to identify subdiaphragmatic infarcts and/or neoplasia. The main contraindications were renal failure and allergy to iodinated contrast agents. CT-TAP was not performed if a patient had A-FIB. Holter-EKG was performed after discharge to look for A-FIB if it was not detected by telemetry during hospitalization or if the patient had no known history of A-FIB. Patients' autonomy was reassessed at 3 months according to the modified Rankin score. The mRS at 3 months worse than or equal to 4 were excluded from the statistical analyses due to the impossibility of performing a contrast TTE.
Results
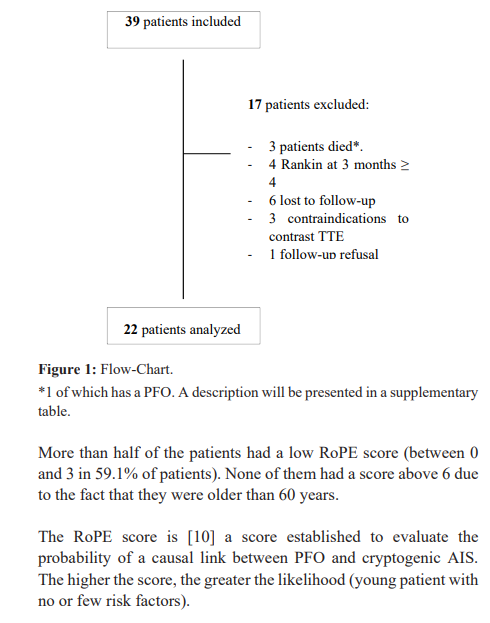
From September 2020 to December 2020, 39 patients met the inclusion criteria. Among these patients, 17 were excluded from the statistical analyses: 3 patients died (acute leukemia, lung cancer, death of unknown origin), 4 patients with mRS at 3 months greater than or equal to 4, 6 patients lost to follow-up, 3 patients with a contraindication to contrast TTE, 1 patient who refused follow-up.
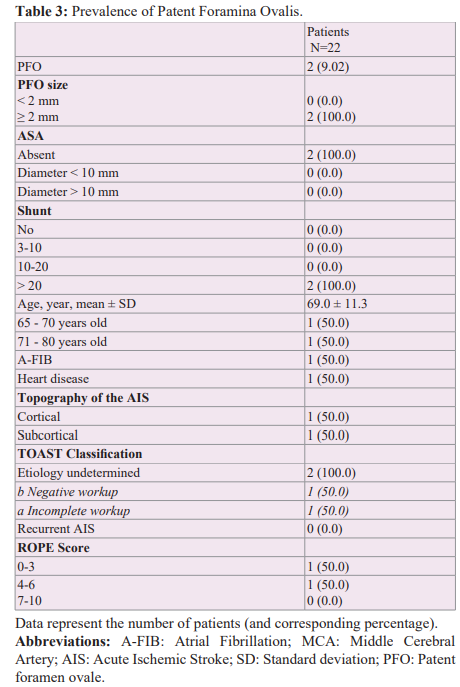
Among the 22 patients (Table 1), the mean age was 72.3 years, with 72.7% between 71 and 80 years. There was a balanced distribution of men and women (54.6% and 45.4% respectively). Most of the patients had a good previous autonomy (91% of the patients had a pre- admission mRS of 0). The patients had relatively few deficits at the time of the cerebral infarction with a relatively low mean admission NIHSS score of 3.8 (minimum NIHSS of 0 and maximum NIHSS of 18). This is because most patients who were severe on arrival were ultimately excluded because they remained disabled with a modified Rankin score ≥ 4 at 3 months and thus unable to perform contrast TTE. The most common cardiovascular risk factors were hypertension (59.1%), diabetes and dyslipidemia (27.3% each). Furthermore, 63.6% of AISs were of cortical topography.

Discussion
The influence of PFO closure (and thus PFO identification) in preventing AIS recurrence was first investigated in three randomized trials in young subjects (Closure 1 [11], PC Trial [12] and Respect [13] conducted in 2012 and 2013), compared to medical treatment. No significant result was found. But three trials published in 2017 and 2018 (Extension of Respect [14] study, Close [8], Reduce [15] and Defense PFO [16] studies) had significant results in favour of closure. The meta-analysis performed by the Sainte-Anne Hospital team in 2018 [17] demonstrated the efficacy of percutaneous PFO closure compared to medical treatment with a 64% reduction in the risk of recurrent AIS [17].
Of these studies, only Defense PFO included patients over 60 years of age (up to 80 years). However, the mean age remained below 60 years (i.e. 51.8 years). These randomized trials therefore do not answer the question about the value of PFO-diagnosis in subjects over 60 years.
Before randomized studies evaluating the efficacy of PFO- closure in subjects over 60 years, it is necessary to demonstrate the association between PFO and cryptogenic AIS in this age group. A few studies have addressed this issue, but their results are inconsistent [3-6].
Overell et al. in 2000 [3], consists of a case-control study with no age limit, evaluating the prevalence of PFO, ASA and PFO + ASA in patients with cryptogenic AIS and in patients with AIS of known cause. A significant association was found that in patients over 55 years of age between cryptogenic AIS and the presence of isolated ASA (3.43 (95% CI, 1.89 to 6.22)) and ASA + PFO (5.09 (95% CI, 1.25 to 20.74)). No significant result was found for the association between AIS and PFO (OR 1.27 (95% CI, 0.80 to 2.01)).
Handke et al. in 2007 [4], performed a study with 503 patients who had undergone TEE, including 372 patients over 55 years of age. They showed a significant association between cryptogenic AIS and PFO in young patients as well as in patients over 55 years of age (OR 2.92; 95% CI, 1.70 to 5.01; P<0.001) and between cryptogenic AIS and the association ASA + PFO (OR 3.88; 95% CI, 1.78 to 8.46; P<0.001). On multivariate analysis adjusted for age, atheroma, ischemic heart disease, and hypertension, the presence of PFO remained independently associated with cryptogenic ASA in both age groups ((OR, 3.00; 95% CI, 1.73 to 5.23; P<0.001) for those >55 years).
M Force et al. in 2008 [5], conducted a study that included 132 patients over 55 years of age, comparing the prevalence of inter- atrial septal defects among patients with cryptogenic AIS/TIA and those with AIS/TIA of known cause. Patients were explored by TEE. Only the combination of PFO + ASA was significantly associated with cryptogenic AIS/TIA (OR, 7.4; 95% CI, 1.4- 38.2), which was also confirmed in the age group above 75 years (OR, 15.0; 95% CI, 1.5-146.7). In contrast to the previous study, the prevalence of isolated PFOs and isolated ASAs was not significantly associated with cryptogenic AIS/TIAs.
Mazzucco et al. in 2018 [6], in a study of 572 patients without age limit explored by micro-bubble transcranial Doppler ultrasound, surprisingly finds a significant association between cryptogenic AIS and wide right-to-left shunt only for patients older than 60 years (OR 1-93, 95% CI 1-32-2-82; p=0-001) and not in subjects younger than 60 years (OR 0-92 (0-39-2-15) p=0-85). The prevalence of large PFO in the elderly is reported to be 13%. A meta-analysis was carried out using the results of the various existing studies on the elderly (including those cited above) and found a significant association between PFO and cryptogenic AIS in the elderly with an OR of 2.51 (95% CI 1.69 - 3.74; p=0-0001). Thus, the association between PFO and cryptogenic AIS seems to persist beyond the age of 60 in some studies. Moreover, the absolute risk of recurrence of AIS in case of PFO seems to increase in subjects over 60 years old who have presented a cryptogenic AIS (2% patients/year vs. 1.3% patients/year in those under 60 years old) [18].
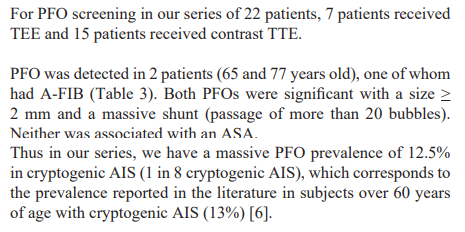
All this leads us to re-discuss the relevance of the age limit of 60 years in randomized controlled trials and the value of closing the PFO. First, it is important to determine what the best strategy is for atrial septal defect screening in the population of elderly patients. Most studies investigating PFO screening have primarily used TEE regardless of age [18]. TEE is more sensitive and remains the reference technique but is more difficult to perform in standard practice in elderly patients (poor tolerance in connection with swallowing disorders, contraindications to general anaesthesia, difficulty in performing a Valsalva manoeuvre). There is also a lack of accessibility in many centres. Some studies have used micro-bubble transcranial Doppler ultrasound [6], which is less invasive but does not allow any assessment of the morphology of the PFO, which is an important element in determining the indication for a possible closure. Mazzuco et al. [6], proposed a systematic review of all studies that have detected PFO in elderly subjects using both techniques. They found that studies that used micro-bubble transcranial Doppler ultrasound in this age group detected a 40-50% higher rate of large PFO compared to studies that used TEE. The main benefit of this technique would be to pre-select patients for TEE if a bubble passage is detected on transcranial Doppler. But the feasibility remains limited in many centres because of the expertise that this technique requires. This is the case in our centre. In our study, PFO was detected mainly by contrast TTE (16 patients vs 6 patients by TEE). According to the same principle, a positive contrast TTE must be completed by a TEE, in particular for a better morphological appreciation of the inter-atrial septum (mainly for identifying ASAs). Among the 22 patients included and analyzed, we detected a PFO in 2 of them: a 60 year old patient assessed by TEE and a 77 year old patient assessed by contrast TTE. For the latter, we could not complete with a TEE because of the patient's refusal. Thus in our series, we found a prevalence of massive PFO of 12.5% (1 in 8 cryptogenic AIS), which corresponds to the prevalence reported in the literature in subjects over 60 years old (13%) [6].
Our study is not a comparative study since we excluded patients with a known cause (apart from a cardio-embolic cause on A-FIB). We cannot conclude any association or causal link between PFO and cryptogenic AIS in our series. Our aim was to make an initial estimate of the presence of PFO in our elderly patients treated at CHIPS before conducting further studies on the subject.
In our series, 59.1% of the patients had a low RoPE score between 0 and 3 and thus a zero probability according to the Kent et al. study [10] of a causal link between the presence of a possible PFO and the occurrence of an AIS. This is consistent with the high mean age of our patients (72.3 years). Nevertheless, we note a significant proportion of patients with a RoPE score between 4 and 6 (40.9%). This could be explained by the exclusion of atheromatous and lacunar causes, which reduced the proportion of patients with many vascular, risk factors.
Among the 22 patients included and analyzed, a PFO was discovered in two patients (Table 4). In both cases the PFO was massive (PFO size > 2 mm, passage of more than 20 bubbles) without ASA, PAH or Eustachian valve. Nevertheless, they presented different profiles.
First, a relatively young profile (65 years) without vascular risk factors with a RoPE score of 6, the highest in our series. The cerebral infarction was of superficial sylvian topography (rolandic), limited in size, with no visible occlusion, and revealed by a transient aphasia on two occasions at 2-minute intervals. Because of the absence of occlusion and an initially low NIHSS of 2 which rapidly regressed, the patient was not thrombolysed and received secondary prevention with Aspirin. The PFO was detected on outpatient TEE. In addition, a complete workup had been performed on this patient with a negative prolonged holter- EKG, a complete biological workup for young subjects (with investigations for antiphospholipid syndrome and autoimmunity), and an CT-angiography of the supra-aortic trunks and the aortic arch which did not reveal atheroma. This patient therefore met the criteria for cryptogenic AIS (5b according to the TOAST classification). He thus presented the criteria for PFO closure, excepted for the age. According to the 2018 SFNV and SFC recommendations (Cerebral infarction and patent foramen ovale), in the absence of identified A-fib and any other potential cause apart from the presence of a PFO, the placement of an implantable holter-EKG such as the Reveal® device should be proposed to patients over 60 years of age before deciding on a possible PFO closure. Nevertheless, in this patient with a borderline age and no risk factor for A-fib, it was decided after discussion with an interventional cardiologist to proceed with PFO closure. Indeed, the risk of recurrence of AIS in case of PFO seems to increase in subjects over 60 years old (2% patients / year vs 1.3% patients / year in those under 60 years old) [18]. Repeat prolonged holter- EKG or Reveal® (more sensitive with 25% of A-FIB detected19) could be done in addition to closure. If A-FIB is finally detected, the difficulty will be to determine if this condition was pre-existing or is secondary to the procedure. Indeed, according to the meta- analysis of randomized trials on the value of closure in patients under 60 years of age, the incidence of post-procedure A-FIB was between 3.65 and 5.61 per 100 patients depending on the devices used [16]. Of note, most of these post-procedure A-FIB were without major consequences in the reported cases. Our patient did not present any recurrence of AIS afterwards.
The second patient had a different profile with a higher age (77 years), cardiovascular risk factors (diabetes, hypertension, obesity, ischemic heart disease) and a subcortical stroke topography without visible occlusion, from which he recovered well. The RoPE score was therefore low (2). The patient also had a known history of paroxysmal A-FIB anticoagulated by vitamin K antagonists with an INR of 2.46 on admission (contraindication to direct oral anticoagulants due to end-stage renal failure on dialysis). The etiological work-up was negative, with a CT-TAP without injection and a biological work-up without any anomaly (in particular, no neoplasia or sign of disseminated intravascular coagulation). There was no clinical or biological evidence of Horton's disease. The PFO was detected by contrast-TTE, as the patient refused TEE. He therefore had two possible aetiologies. He was on fluindione which was continued as secondary prevention of his AIS. Dialysis is a factor influencing INR imbalance. A switch to warfarin, which is more stable and easier to balance, could be discussed but was not done.

It should be noted that the patient had a SARS-COVID-2 infection two weeks before the onset of the AIS without any severity criteria and without requiring oxygen therapy or hospitalization. The Ddimer levels on arrival were low (912 ng/mL), which was not significant enough to indicate venous thromboembolic disease (and therefore a possible paradoxical embolism). A venous doppler ultrasound of the lower limbs was not performed.
A 75-year-old patient, excluded because she died within 15 days, was also found to have a PFO smaller than 2 mm in size and ≥ 20 bubbles without ASA on TEE (RoPE score of 4) with the discovery of A-FIB and atrial disease on telemetry. She presented with a partial right sylvian stroke due to a terminal carotid occlusion and benefited from thrombolysis and thrombectomy with very good clinical recovery. She was put on direct oral anticoagulants. A pacemaker was installed on day 4. She finally died at home of unknown cause (Table 5).
Finally, a 73-year-old patient with no vascular risk factors (RoPE score of 4) was found to have an ASA without PFO on contrast TTE. He presented a subcortical stroke. The prolonged holter- EKG did not show A-FIB. Nevertheless, the patient refused to perform a CT TAP, which did not allow us to formally consider his AIS as cryptogenic.
Previously published studies on the association between ASA alone and cryptogenic AIS in patients over 55 years of age have found discordant results [3-5]: In only one study [3], ASA without PFO was significantly more present in the cryptogenic AIS group than in the AIS group with known cause.
Our study has inherent and known limitations due to the small number of patients and the retrospective, observational and descriptive design.
In addition, we were confronted with difficulties in retrieving some paraclinical evaluations (in particular the contrast-TTE and the prolonged holter-EKG). These are performed in private institutions because of the long waiting time for appointments in our centre. We deplore the fact that 6 patients were lost to follow-up. The current pandemic context has not facilitated patient follow-up.
Conclusion
This is a preliminary descriptive study on the prevalence of PFO in elderly subjects with cryptogenic AIS. This is a first description of the state of affairs before undertaking further studies on the association between PFO and AIS in patients over 60 years of age, and eventually on the interest of PFO closure in this age group.
References
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988; 318: 1148-1152.
- Alsheikh-Ali AA, Thaler DE, Kent Patent foramen ovale in cryptogenic stroke: incidental or pathogenic?. Stroke. 2009; 40: 2349-2355.
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control Neurology. 2000; 55: 1172-1179.
- Handke M, Harloff A, Olschewski M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007; 357: 2262-2268.
- Force M, Massabuau P, Larrue V. Prevalence of atrial septal abnormalities in older patients with cryptogenic ischemic stroke or transient ischemic attack. Clin Neurol Neurosurg. 2008; 110: 779-783.
- Mazzucco S, Li L, Binney L, et al. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. 2018; 17: 609-617.
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24: 35-41.
- Mas JL, Derumeaux G, Guillon B, et Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. New England Journal of Medicine. 2017; 377: 1011-1021.
- Arnold M, Nedeltchev K, Brekenfeld C, et Outcome of acute stroke patients without visible occlusion on early arteriography. Stroke. 2004; 35: 1135-1138.
- Kent DM, Ruthazer R, Weimar C, et An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013; 81: 619-625.
- Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J 2012; 366: 991-999.
- Meier B, Kalesan B, Mattle HP, et Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013; 368: 1083-1091.
- Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic N Engl J Med. 2013; 368: 1092-1100.
- Saver JL, Carroll JD, Thaler DE, et al. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after N Engl J Med. 2017; 377: 1022-1032.
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic New England Journal of Medicine. 2017; 377 : 1033-1042.
- Lee PH, Song JK, Kim JS, et Cryptogenic Stroke and High- Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J Am Coll Cardiol. 2018; 71: 2335-2342.
- Turc G, Calvet D, Guérin P, et al. Closure, Anticoagulation, or Antiplatelet Therapy for Cryptogenic Stroke With Patent Foramen Ovale: Systematic Review of Randomized Trials, Sequential Meta-Analysis, and New Insights From the CLOSE J Am Heart Assoc. 2018; 7.
- Mazzucco S, Li L, Rothwell PM. Prognosis of Cryptogenic Stroke With Patent Foramen Ovale at Older Ages and Implications for Trials: A Population-Based Study and Systematic JAMA Neurol. 2020; 77: 1279-1287.
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. N Engl J Med. 2014; 370: 2478-2486.