Prognostic Factors and Impact of Immunohistochemistry in the Management of Biliary Tract Cancer– A Single Center Experience in Germany
Author'(s): Holger G. Hass M.D1, Ute Smith2, Michael Schäffer Prof., M.D.3, Ulrich WellhäuΒer M.D.4, Hans-Ulrich Markmann M.D.5, and Claudio Denzlinger Prof., M.D.6
1Department of Internal Medicine, Oncology and Rehabilitation, Paracelsus Hospital Scheidegg, Germany.
2Onkologischer Schwerpunkt Stuttgart (OSP), Germany.
3Department of Abdominal, Visceral and Thorax Surgery, Marienhospital Stuttgart, Germany.
4Department of Gastroenterology and Hepatology, Marienhospital Stuttgart, Germany.
5Department of Pathology, Marienhospital Stuttgart, Germany.
6Department of Hematology, Oncology and Palliative Care, Marienhospital Stuttgart, Germany.
*Correspondence:
Holger G. Hass, Department of Internal Oncology and Rehabilitation, Paracelsus Hospital, Kurstrasse 5, 88175 Scheidegg, Germany, Tel: ++49-8381-501-225; Fax: ++49-8381-501-290; E-mail: dr.holger.hass@paracelsus-kliniken.de.
Received: 14 February 2018 Accepted: 08 March 2018
Citation: Holger G. Hass, Ute Smith, Michael Schäffer, et al. Prognostic Factors and Impact of Immunohistochemistry in the Management of Biliary Tract Cancer – A Single Centre Experience in Germany. Gastroint Hepatol Dig Dis. 2018; 1(1): 1-6.
Abstract
Purpose: Cholangiocarcinoma (CC) is characterized by a still unfavorable prognosis due to a late diagnosis and high recurrence rate. In this retrospective study prognostic factors for long-term survival and an immunohistochemical panel of markers (IHC) for distinction of CC and other primary liver malignancies were analyzed.
Materials and Methods: In 208 patients with CC clinical data, tumor characteristics and the primary mode of treatment were analyzed using univariate and multivariate statistics. In 145 cases the immunohistochemical profile of the tumors was established using markers such as CK7, CK20, CA19-9, HepPar-1, AFP, CD34 and CDX2 routinely in comparison to 60 cases of HCC (control group). Significance of marker expression in relation to histological subtype were estimated using SPSS 10.0.
Results: Median overall survival (OS) was 18.8±22.5 months. Multivariate analysis identifies subtype of CC / tumor localisation (dCC vs. iCC or pCC), tumor size (P=0.001), grading (P=0.002) and tumor resection (P<0.0001) as independent prognostic factors for survival. CK7, CK20 and CA19-9 were the most commonly overexpressed markers in CC (86.2%, 55.2% and 50%), whereas expression of these markers in HCC was significantly less frequent (24.3%, 0% and 8.3%, respectively; P<0.001 and P<0.0001).
Conclusions: Tumor localization and stage, surgical resection and tumor biology as expressed by tumor cell differentiation are the most important factors for OS in CC. Best predictive markers for differentiation between CC and HCC were CK7, CK20 and HepPar-1. Using this panel a fast and accurate differentiation was possible in more than 95% of all analyzed liver tumors.
Keywords
Introduction
Cholangiocarcinomas (CC) of the biliary tract are rare tumors compromising only 3-5% of all gastrointestinal cancers. However it is the second most common primary hepatic malignancy next to hepatocellular carcinoma (HCC), accounting for 10-15% of all liver tumors. This neoplasm originates from the intra- or extrahepatic bile duct or gallbladder epithelium [1] and is classified according to the anatomic location in the biliary tract as intrahepatic (iCC) or extrahepatic cholangiocarcinoma (eCC) and gallbladder cancer (GBC). Extrahepatic CC can be classified as perihilar tumors (pCC) or distal cancers (dCC). Perhilar tumors or so-called Klatskin tumors are subclassified using the Bismuth- Corlette classification [2].
Recent epidemiologic studies have shown an increasing incidence of CC in western countries [3]. Established risk factors for ductal cholangiocarcinoma include diseases leading to chronic inflammation of bile ducts such as infections with Clonorchis sinensis, Opisthorchis viverrini (liver flukes) and chronic viral hepatitis, chronic intrahepatic lithiasis, primary sclerosing cholangitis (PSC), congenital diseases (Caroli disease, congenital choledochal cysts) and exposure to the radiopaque medium thorium dioxide (Thorotrast) [4,5]. Especially patients with PSC have an increased risk for cholangiocarcinogenesis and epidemiologic studies have shown a lifetime risk for CC of approximately 1.5% per year of disease [4]. However, in most patients with CC no risk factors for cholangiocarcinogenesis can be identified.
Despite recent progress in various imaging modalities most CCs are diagnosed at an advanced stage due to the fact that early disease is often asymptomatic. Thus, curative treatment options by surgical resection are limited leading to poor prognosis especially in patients with intrahepatic CCs with median survival times of only three to six months [6].
Another pitfall in the management of patients with iCC is the differentiation of these tumors from HCC or other adenocarcinomas of the liver, e.g. metastases of other gastrointestinal cancers. In clinical routine this is a frequent diagnostic dilemma for pathologists. However, accurate diagnosis is crucial because treatment and treatment goals for these tumors may differ. In contrast to iCC hepatocellular carcinoma is chemo-resistant; whereas liver transplantation is a potential therapeutic option in patients with HCC and liver cirrhosis, this option is rarely recommended in patients with iCC [6,7]. Thus, correct classification of these tumors is critically important. In addition to a hematoxylin and eosin (H&E) staining several immunohistochemical markers are used routinely in clinical practice for distinction of liver tumors. However, the utility of each of these markers is limited either by suboptimal sensitivity or by difficulties in interpretation, especially in poorly differentiated tumors or scirrhous hepatocellular carcinoma [8,9].
In this retrospective unicenter study we analyzed the clinical use of different immunohistochemical markers (IHC) for diagnosis and subclassification of primary liver cancer (CC vs. HCC) and elaborated clinical characteristics as potential prognostic markers in our cohort of patients with biliary tract cancer.
Patients and Methods
Patients
Clinical and histopathological data of 208 patients with biliary tract cancer (50.0% males; mean age 72.4 ± 10.9 years) who were admitted to the Marienhospital Stuttgart between January 2006 and February 2016 were analysed in this retrospective study. Additional clinicopathological characteristics of the enrolled patients are summarized in table 1.
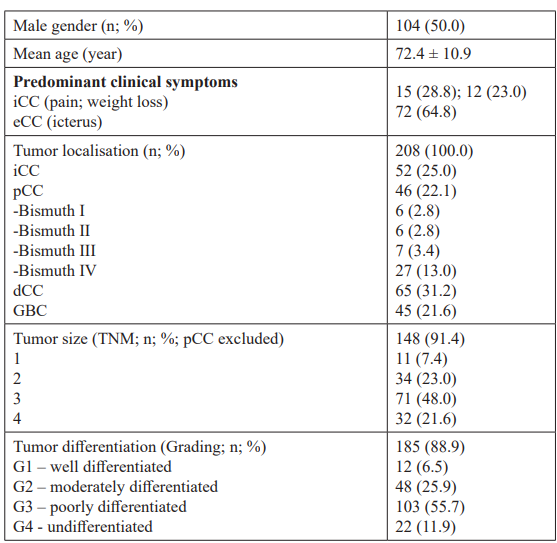
Table 1: Histopathologic findings and clinical data of the study population (n=208).
Histopathological evaluation Clinical and pathological data were obtained from patients including information on tumor type, stage (according to UICC 2009 or Bismuth-Corlette classification), and grade (WHO graduation G1– 4). Hematoxylin-eosin staining was performed to detect features of bile canalicular structure and Mallory hyaline bodies. Additional histochemical staining plus immunohistochemical staining was performed routinely in 145 patients with CC and in a control group of 60 patients with HCC to confirm the histological diagnosis of CC and to exclude other types of liver malignancy, especially of HCC. These studies included antibodies against HepPar-1, AFP, CK7, CK20, CDX2, CA19-9, CD34, and CEA.
Treatment modalities of cholangiocarcinoma (CC)
In 56.3% of all cases (n=117) a surgical tumor resection including lymph node dissection was performed. Type of surgical resection differed by tumor location: atypical or lob-/hemihepatectomy for iCC, bile duct resection with hepatico-jejunostomie and/ or hemihepatectomy for pCC; Whipple operation for dCC and cholecystectomy with or without atypical hepatectomy in cases of GBC. In 19.2% of patients (n=40) with multifocal tumor, metastatic tumor stage or other contraindications for surgical treatment palliative chemotherapies (1-14 sessions; mean 5.8) or transarterial chemoembolizations (TACE; n=4) were performed. In 13.5% (n=28) palliative drainage by stenting was initiated. In 11% (n=23) of the patients only best supportive care (BSC) could be offered due to advanced disease or patients preferences.
Statistical analysis
The association between several histopathological findings such as tumor differentiation and tumor stage and survival rates was analysed using t test and 2-sided Fisher´s test. Clinicopathological factors were analysed for influence on survival by univariate and multivariate methods. Multivariate analysis was performed using Cox proportional hazards method. Only factors found to be significant on univariate analysis were included in the regression model. Median overall survival (OS) was estimated according to the Kaplan–Meier method. Differences in survival were analyzed using a log-rank test. SPSS 10.0 software (Inc., Chicago, IL) was used to test for sensitivity, specify and for predictive values of different immunohistochemical markers as well as of clinical data. P ≤ 0.05 was considered statistically significant.
Results
Clinical symptoms at diagnosis; association of age, tumor localisation (iCC, pCC, dCC, GBC) and treatment modalities with overall survival (OS)
The clinical presentation was dominated by the anatomic location of the tumor. Whereas in most patients with iCC right upper abdominal quadrant pain (28.8%) or weight loss (23.0%) were the leading symptoms, patients with eCC presented initially in 64.8% with painless jaundice (p<0.0001).
Median overall survival (OS) of all patients was 18.8 ± 22.5 months. One year after diagnosis OS was 44.5% and dropped to 25.7% and 20.5% after 3 and 5 years, respectively. In older patients (>70y) OS was significantly lower as compared to younger patients (≤70y) (25.1 ± 27 vs. 12.6 ± 12.6 years; p<0.0001). In male patients there was a trend to better OS as compared to females (71.6 ± 10.9 vs. 73.3 ± 11 years; p=ns).
OS correlated significantly with localisation of CC. Patients with dCC had significant better survival rates in comparison to patients with pCC, iCC or GBC (p=0.0001 up to 0.0004; Figure 1) reasonable to the fact that dCC was diagnosed twice as frequently at an earlier stage (T1/2 = 31.2%) as compared to iCC (T1/2 = 14%). Patients with perihilar tumors localized distal or in the biliary confluence (Bismuth I or II) had also a significantly better OS when compared to perihilar CC with involvement of the right and/or left hepatic bile ducts (Bismuth III/IV; p=0.0025).
After surgical therapy OS was 26.4 ± 24.3 months and significantly higher than in patients without tumor resection or other treatment modalities (9.8 ± 5.8 months with chemotherapy only, 6.5 ± 6.1 months with stenting only, 4 ± 3 months with best supportive care; P<0.0001).
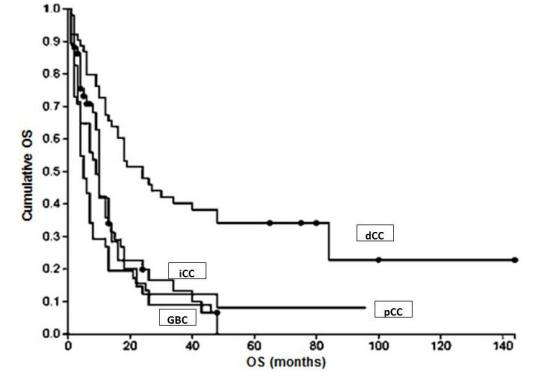
Figure 1: OS in relation to tumor localization.
Association of CC stage (TNM), tumor differentiation (grading), occurrence of metastasis and overall survival (OS) Patients with lower tumor size (T1) survived significantly longer (OS 33.4 ± 26.8 months) compared to patients with T4 tumors (OS 8.7 ± 6.8 months; P<0.0001). After 3 years of observation 72.7% of patients with T1 tumors were still alive whereas all patients with T4 tumors had died. In patients with metastasic disease at the time of diagnosis OS was similarly short as in patients with T4 tumors (8.4 ± 8.8 months in median; 1-year OS 19.3%; Figure 2).
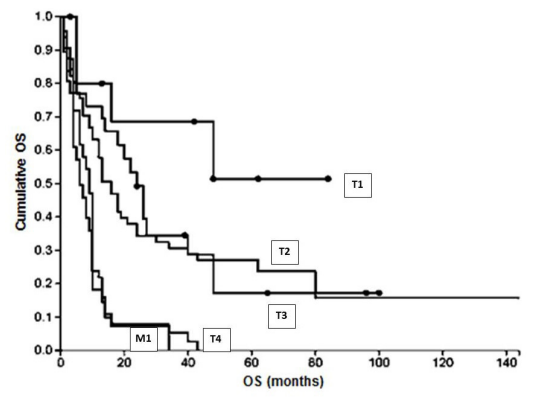
Figure 2: OS in relation to T stage and existence of metastasis at primary diagnosis.
Similarly, tumor T-stage correlates significantly with cell differentiation. In tumors with well or moderate cell differentiation (G1 or G2) disease was diagnosed at an advanced tumor stage (T3 or T4) in 58.2%, whereas in tumors with poorly or undifferentiated tumor cells (G3 or G4) incidence of advanced tumor stage increased to 74.5% (p=0.0018; Figure 3). A non-significant trend between poor tumor cell differentiation and metastatic tumor stage (M1) was observed (32.7% vs. 38.8%; p=ns).
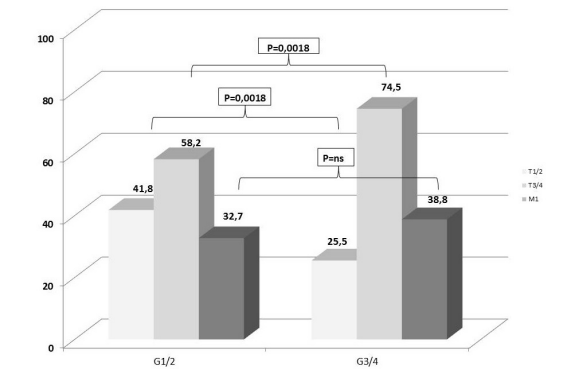
Figure 3: Correlation between tumor stage and tumor cell differentiation (grading).
Multivariate analysis of prognostic factors in HCC
Based on the results, a multivariate analysis was performed. This analysis identified early tumor size (T1/2 stage; P=0.001), G1/2 cell differentiation (P=0.002), performed surgical resection (P<0.0001) and tumor localisation (dCC vs. other localisations), as independent prognostic factors for survival.
Immunophenotypic Profile of intra- and extrahepatic Cholangiocarcinoma
CK7 was the most commonly overexpressed marker in CC. A total of 125 biopsies (86.2%) of CC were positive for this marker using IHC. In 72 cases (49.6%) also a strong reaction for CK20 was documented. These results were significantly different in the control group of hepatocellular carcinoma (23.3% and 0%, respectively; P<0.001 and P<0.0001). Using IHC positivity of carbohydrate antigen 19-9 (CA19-9) was detected in 80 cases of CC (55.2%) and in 5 cases (8.3%) of HCC (P<0.0001).
In comparison to these markers HepPar-1 was the most commonly expressed marker in HCC, positive in 48 cases (80%). AFP staining was positive in 29 HCCs (48.3%; P<0.0001). However, in two patients with combined hepatocellular-cholangiocarcinoma (combined HCC-CC) and >50% of malignant cholangiocytes AFP and HepPar-1 were also positive. CD34 protein, an endothelial marker showed a significant positive reaction in 32 patients (53.3%) with HCC compared to 5 patients (3.5%) with cholangiocarcinoma (P<0.0001). For more details see table 2 and figure 4a-b.
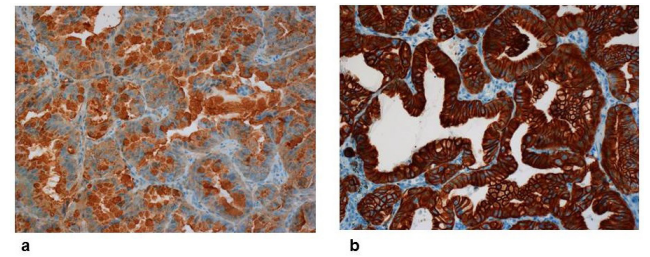
Figure 4: a,b: Immunohistochemistry of intrahepatic cholangiocarcinoma (iCC). Strong reaction of antibodies against a: CA19-9 (x200, above) and b: CK7 (x200, below).
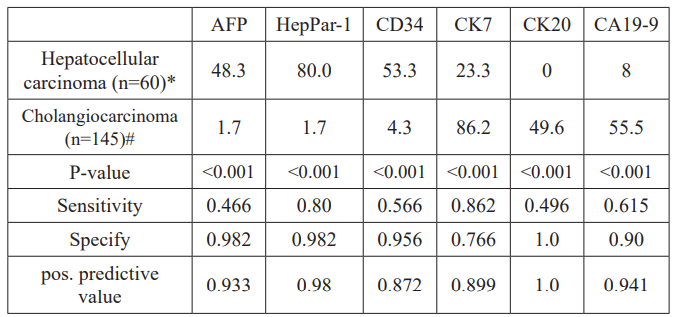
Table 2: Specify and sensitivity of immunohistochemical markers for diagnosis of CC and HCC.
Abbreviations: CEA, polyclonal antibody to carcinoembryonic antigen; HepPar-1, Hepatocyte Paraffin 1; CD34, Cluster of differentiation 34; CK7, cytokeratin 7; CK20, cytokeratin 20; CA19-9, carbohydrate antigen 19-9. Numbers reflect percentages.
In some cases, available tissue was insufficient to perform all stains. Numbers of patients with analyses performed were* 55 for CK19 and CD34, 50 for CA19-9 and CK20, # 130 for CA19-9, 115 for AFP, CD34 and HepPar-1.
Discussion
Prognosis of advanced cholangiocarcinoma is very poor, with median survival time less than 1 year [10], in most cases due to diagnosis at an advanced, unresectable stage. Even in patients who can be resected, risk of recurrence is high.
In the present study, overall survival of 208 patients with CC was analyzed in relation to different risk factors for tumor recurrence and overall survival. In addition, accuracy of an immunohistochemical panel was evaluated to discriminate between CC and HCC. In line with earlier studies OS in patients with CC was disappointingly short with a 1-year OS of 44.5%, dropping to 25.7% and 20.5% after 3 and 5 years, respectively. OS correlated significantly with histopathological and clinical parameters.
Poor tumor cell differentiation is associated with aggressive tumor biology in many human cancers. This is mainly due to early metastasis. Tumor differentiation in CC has already previously been shown to have a significant effect on prognosis [10,11]. This is confirmed by our study demonstrating that prognosis of cancers with low differentiation is poorer than of those with well tumor cell differentiation. Tumor stage has also been shown to be related with prognosis [12,13]. At later stages, risk of metastasis increases due to vascular or lymph vessel infiltration. In advanced tumor stages a complete tumor resection may not be feasible without leaving infiltrated i.e. positive resection margins. In the literature there is considerable evidence that infiltration of resection margins indicates higher recurrence rates and poor prognosis [14-16]. In our retrospective analysis investigation for infiltration of resection margins was not sufficiently possible. Nevertheless we found a significant independent correlation between tumor stage and OS in intrahepatic as well as extrahepatic tumors (Figure 2). OS was also significantly lower in patients with perihilar tumor localization in Bismuth-Corlette III and IV tumors as compared to class I and II tumors probably due to a lower surgical resection rate (20% vs. 50%; p=0.025). Accordingly, Boudjema et al. reported higher rates of inoperability associated with vascular invasion in case of infiltration of the confluence of bile ducts [17]. In contrast to these observations OS in dCC was significantly better as compared to intrahepatic or perihilar CC and to the group of patients with GBC. A possible explanation for these differences could be the fact, that in dCC more cases were diagnosed at an earlier stage. In tumors of the distal bile duct jaundice or pruritus resulting from cholestasis are more commonly reported as early clinical complaints leading to earlier diagnosis, earlier treatment and thus better prognosis. Obvious clinical manifestations such as pain in the right upper abdominal quadrant or weight loss indicate advanced stage of cholangiocarcinoma. In our study, this constellation was seen significantly more often in patients with iCC. DeOliveira et al. [18] reported in a large series of 564 patients with biliary tract cancer only 8% to be localized in the liver in contrast to 25% of the patients in the present study. This discrepancy may be reconciled by the observation that the incidence of iCC is disproportionally increasing [19,20].
By univariate analysis older age (>70y) at diagnosis was a significant prognostic factor for poor survival in our collective, possibly due to a lower rate of surgical tumor resections performed. Advanced age was recently identified as independent prognostic factor in a large meta-analysis including over 4700 patients [21]. In our multivariate analysis tumor stage (TNM), tumor cell differentiation (grading), distal location of tumor (dCC) and surgical therapy but not age, were found to be significant prognostic factors in patients with biliary tract cancer. Not surprisingly, the impact of tumor resection on survival has been confirmed repeatedly in recent studies [22,23]. Inoperable patients derived benefit from systemic treatment with chemotherapy significantly prolonging survival as compared to BSC (9.8 ± 5.8 months vs. 4 ± 3 months; p<0.0001). Even in highly palliative situations stenting of occluded bile ducts may be of benefit not only to control jaundice and risk of cholangitis but also to prolong OS (6.5 ± 6.1 months, P=0.049).
A fast and accurate differentiation is indispensable in primary liver cancers because treatment options differ substantially. In our patients with cholangiocarcinoma CK7 staining showed the highest sensitivity (0.86) but specify was low (0.76) because more than 23.3% of our patients in the control group with HCC showed similar positivity for this marker. Interestingly, CK7 is expressed in hepatic progenitor cells (HPCs) but normally not in hepatocytes. CK7 positive HCCs and combined hepatocellular- cholangiocarcinoma, as suggested by our results may derive from HPCs [24]. Stem cell markers such as CD117, EpCAM and CD56 may be helpful to establish the diagnosis of combined hepatocellular-cholangiocarcinoma in tumors expressing both hepatic markers (e.g. AFP, HepPar-1) and positive cytokeratins [25,26]. Compared to CK7, CK20 is not expressed in HCC and thus may be useful to distinguish CC from HCC. However, in CK20 positive tumors secondary neoplasms of the liver need to be considered because sensitivity to CK20 in CC is low and expression in colorectal cancer is considerably stronger [27].
In summary, our data confirm the disappointing prognosis of CC. Biological parameters such as tumor cell differentiation as well as clinical criteria such as stage of disease or surgical therapy are the most important prognostic factors in CC. These data underline the importance of earlier diagnosis and of treatment in surgical centers with expert knowledge in hepatobiliary diseases. From a practical point of view, the present study confirms also earlier reports on the complexity of making an unequivocal diagnosis of HCC or cholangiocarcinoma. For accurate distinction of primary liver cancer a panel of immunohistochemical markers such as CK7, CK20, HepPar-1 and AFP is helpful in addition to clinical data. Currently novel markers emerge to improve discrimination of primary liver tumors (e.g. Glypican3, Arginase-1) [28,29]. In future clinical routine gene expression profiles may also be helpful for subclassification of HCC, CC and especially combined hepatocellular-cholangiocarcinoma [30].
References
- De Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract N Engl J Med. 1999; 341: 1368-1378.
- Hirohashi K, Uenishi T, Kubo S, et al. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepatogastroenterology. 2002; 49: 326-329.
- Davila JA, El-Serag HB. Cholangiocarcinoma: the “other” liver cancer on the rise: Am J Gastroenterol. 2002; 97: 3199-
- Bergquist A, Ekbom A, Olsson R, et Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002; 36: 321-327.
- Gores Cholangiocarcinoma: Current Concepts and Insights. Hepatology. 2003; 37: 961-969.
- Blechacz B. Cholangiocarcinoma: Current Knowledge and New Gut Liver. 2018; 11: 13-26.
- Llovet JM, Ricci S, Mazzaferro V, et Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359: 378-390.
- Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagn 2012; 7: 149.
- Sugiki T, Yamamoto M, Taka K, et Specific characteristics of scirrhous hepatocellular carcinoma. Hepatogastroenterology. 2009; 56: 1086-1089.
- Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar holangiocarcinoma: analysis of 46 Arch Surg. 2004; 139: 514-523.
- Mao ZY, Guo XC,Su D, et Prognostic Factors of Cholangiocarcinoma After Surgical Resection: A Retrospective Study of 293 Patients. Med Sci Monit. 2015; 21: 2375-2381.
- Singal AG, Rakoski MO, Salgia R, et The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre.Aliment Pharmacol Ther. 2010; 31: 625-633.
- Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus Gut. 2002; 51: 1-9.
- Yeh CN, Hsieh FJ, Chiang KC, et al. Clinical effect of a positive surgical margin after hepatectomy on survival of patients with intrahepatic Drug Des Devel Ther. 2015; 9: 163-174.
- Jarnagin WR, Shoup Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004; 24: 189-199.
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal Ann Surg Oncol. 2011; 18: 651-658.
- Boudjema K, Sulpice L, Garnier S, et A simple system to predict perihilar cholangiocarcinoma resectability. J Gastrointest Surg. 2013; 17: 1247-1256.
- DeOliveira ML, Cunningham SC, Cameron JL, et Cholangiocarcinoma: thirty-one- year experience with 564 patients at a single institution. Ann Surg. 2007; 245: 755-762.
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers 2006; 15: 1198-1203.
- Endo I, Gonen M, Yopp AC, et Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008; 248: 84-96.
- Mavros MN, Economopoulos KP, Alexiou VG, et Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014; 149: 565-574.
- Scheuermann U, Kaths JM, Heise M, et Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma-- a single-center experience. Eur J Surg Oncol. 2013; 39: 593-600.
- Baton O, Azoulay D, Adam DV, et Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007; 204: 250-60.
- Durnez A, Verslype C, Nevens F, et The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006; 49: 138-1351.
- Terada Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol. 2013; 6: 737-48.
- Akiba J, Nakashima O, Hattori S, et al. Clinicopathologic analysis of combined hepatocellular-cholangio- carcinoma according to the latest WHO Am J Surg Pathol. 2013; 37: 496-505.
- Maeda T, Kajiyama K, Adachi E, et The expression of cytokeratins 7, 19, and 20 in primary and metastatic carcinomas of the liver. Mod Pathol. 1996; 9: 901-909.
- Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013; 280: 2471-
- Timek DT, Shi J, Liu H, et al. Arginase-1, HepPar-1, and Glypican-3 are the most effective panel of markers in distinguishing hepatocellular carcinoma from metastatic tumor on fine-needle aspiration specimens. Am J Clin Pathol. 2012; 138: 203-210.
- Wang L, Zang W, Xie D, et al. Comparison of hepatocellular carcinoma (HCC), holangiocarcinoma (CC), and combined HCC-CC (CHC) with each other based on microarray Tumour Biol. 2013; 34: 1679-1684.