Risk Factors of Cervical Cancer in Two Reference Hospitals of Douala: A Case-Control Study
Author'(s): Engbang Ndamba Jean Paul1,2,*, Essome Henri1,2, Mve Koh valère3, Ndjom Nack Jean Paul1and Foumane Pascal3
1Faculty of Medicine and Pharmaceuticals Sciences of Douala,University of Douala, Douala, Cameroon.
2Laquintinie Hospital of Douala, Douala, Cameroon
3Faculty of Medicine of Biomedical Sciences, University of Yaounde I, Yaounde, Cameroon.
*Correspondence:
Engbang Ndamba Jean Paul, Faculty of Medicine and Pharmaceuticals Sciences of Douala, University of Douala,Douala, Cameroon.
Received: 23 May 2020; Accepted: 28 June 2020
Citation: Engbang Ndamba Jean Paul, Essome Henri, Mve Koh Valère, et al. Risk Factors of Cervical Cancer in Two Reference Hospitals of Douala: A Case-Control Study. Cancer Sci Res. 2020; 3(2): 1-6.
Abstract
Background: Cervical cancer is the second most common cancer in women. Few data are available in the city of Douala. Our objective is to determine risk factors for cervical cancer at Laquintinie Hospital and Douala General Hospital.
Patients and Methods: This was a case-control study of patients seen from 02/02/2016 to 31/05/2018 in both sites of the study. The cases were women with histological evidence of cervical cancer, those with no cervical cancer at visual inspection with acetic acid and lugol (IVA-IVL). The odds ratio with 95% confidence interval were used to measure the association between cervical cancer and the variables of interest.
Results: We included 250 cases of cervical cancer and 1000 controls. In cases, 82.8% had metrorrhagia. The most recovered FIGO stages were stages IIIb and Iva with 22.8% and 18.4% respectively. Squamous cell carcinoma accounted for 91.2% of the histological types and 64.0% received concomitant neoadjuvant chemotherapy. The risk factors found were tobacco (OR = 25, P <0.0001), STIs (OR = 13.7, P <0.0001), number of sexual partners ≥2 (OR = 4.4), HIV seropositivity (OR = 8.6, P = 0.0001), age ≥ 50years (3.6), low socio-economic status (OR = 3), sexual intercourse before the age of 18 (OR = 2.8), delivery before age 20 (OR = 2.56), oral contraceptive use over 5 years or more (OR = 2.3).
Conclusion: The study carried out within the framework of our thesis, despite its limitations, constitutes a source of information for scientific research on cervical cancer. It contributes to the improvement of cervical cancer knowledge, allows us to define at-risk populations, and subsequently allows us to set up an early diagnosis algorithm.
Keywords
Introduction
Cervical cancer is considered as a non communicable disease with an estimated global prevalence of 2.3 million women [1]; With an incidence of 528,000 new cases it represents the 4th most common malignant tumor in women worldwide after breast, colorectal and lung cancers [2]. It is also the 4th leading cause of cancer deaths with 266,000 deaths in 2012 [2,3].
However, there is significant inequality in the distribution of incidence across countries. In fact, about 85% of cervical cancers are registered in developing countries, where access to testing and care is totally different from that of developed countries [2,4]. In Europe, this pathology occupies the 9th rank of female cancers in terms of incidence and the 12th rank in terms of mortality. With approximately 3,000 new cases and 1,100 deaths per year, cervical caancer represents the 12th leading cause of cancer and the 10th leading cause of cancer death among women in France [5]. In America, Canada has diagnosed 1,550 new cases in 2017 and in the United States in the African-American population there are 6 new cases per 100,000 population [5,6]. In Africa and mainly in sub-Saharan Africa, cervical cancer has become more and more common in the last two decades with more than 75,000 cases diagnosed in 2013 and nearly 50,000 deaths related to it during the same year [7].
Cervical cancer is a multifactorial disease, the most important of which is Human Papillomavirus (HPV) infection; it is the necessary etiopathogenic factor and more than 90% detected in the diagnosis of this malignant tumor [10,11]. However other cofactors have been found including the precocity of first intercourse, the multiplicity of sexual partners, smoking and alcohol, hormonal contraception and immunosuppression potentiating the oncogene character of Human Papillomavirus [8-11,13-15]. In Cameroon, cervical cancer is the leading women genital cancer, 40.18% of cervical cancers are diagnosed in urban areas [16,17]. Engbang et al. reported from 2005 to 2014, 2559 cases of genital cancer in women with 2078 cases of cervical cancer, giving 82.26% in Cameroon [18]. Given these works in the literature, it seemed important for us to study the risk factors for cervical cancer at Laquintinie Hospital in Douala and Douala General Hospital. In fact, very little data is available on the risk factors for cervical cancer, while knowledge of these will help to improve prevention.
Patients and Methods
This is a descriptive cross-sectional and analytical study. Data collection was done from subjects at the time of cancer screening through the use of a standardized questionnaire. The study population consisted of women aged from 18 years old who were screened for cervical cancer in two references centers, Douala Laquintinie Hospital and Douala General Hospital from March 2010 to May 2013.
The included cervical cancer cases were patients from the selected recruitment centers in our study. Witnesses were also recruited from the centers selected in our study. For each case of cervical cancer, four controls were matched by age (<50 years and ≥50 years) and residence. We defined as controls, women at least 18 years old, sexually active, consenting, consultants in recruitment centers, with IVA (visual inspection after application of acetic acid) and IVL (visual inspection after application of lugol). The recruitment of controls was done on the basis of age-matched (± 5 years) cases. Concerning controls, a sensitization of the women through the messages and communicators sent in different communities (Churches, meetings) of the city of Douala was carried out. Participants were enumerated and recorded daily for mass screening.
After validation of the informed consent, a chart abstraction instrument was elaborated for collecting data from participant files. Information in files was collected by trained providers during systematic screening, and data were extracted retrospectively from those files stored in the two sites by providers from the respective sites. The collection of the data of the various participants followed by the IVA and IVL screening test was done individually in a soundproof room.
One dependent variable of interest was chosen, cervical cancer was defined as a binary variable. Independent variables included: Sociodemographic variables (age, region, social level, level of education, marital status), Clinical variables (hypertension, diabetes, serological status, blood type, STI, family history of cervical cancer, tobacco), Variables in reproductive life (age of first coitus, number of pregnancies, number of deliveries, number of abortions, number of partners, contraception), Family history (history of cervical cancer in the family, history of other cancers in the familly).
For 1:4 allocation ratios the required number of controls were 250, hence 1000 individuals were included in the study. Data analysis was done using statistical package for social sciences software. Data is summarized in form of tables. Univariate logistic regression analysis was used to evaluate the factors significantly associated with cervical cancer. Multiple Logistic Regression was used to calculate the adjusted Odds ratio with 95% CI.
Results
Distribution by age
In case group, the age of cases ranged from 25–82 year with mean age of cases is 53.29 ± 12.71 years. In control group the mean age of the participants was 40.38 ± 12.20 years from 17 to 77 years.
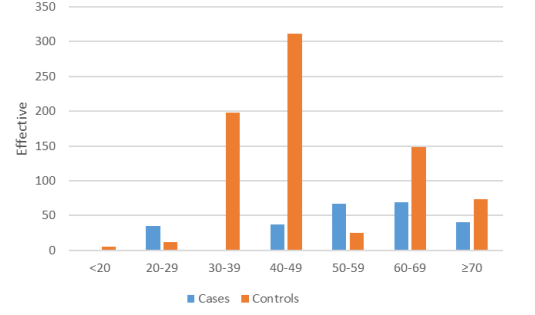
Figure 1: Age distribution of cases and controls.
Univariate Logistic regression analysis
Socio-demographic characteristics of women screened for cervical cancer
As shown in table I, the distribution by age, low socio-economic level and level of education was significant (P <10-3, P = 0.002 and P <0.0001).
sexual factors.
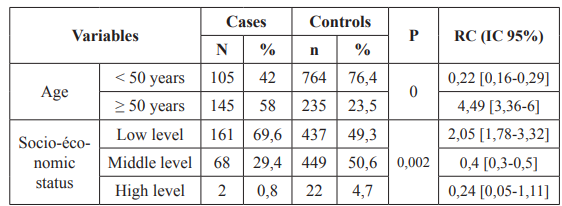
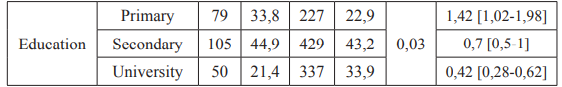
Table 1: Univariate Logistic regression analysis for socio-demographic variables.
Medical and toxicological history in relation to cervical cancer HIV seropositivity, STIs, high blood pressure, smoking, family history of cancer was all significant.
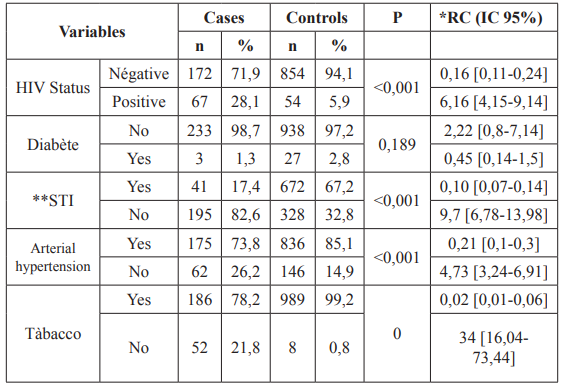
Table 2: Variables of medical and toxicological antecedents in relation with cervical cancer.
Reproductive and sexual factors
Age at first sexual intercourse was significant (P = 0.02), hormonal contraception was significant (P = 0.03), multi sexual partnership was significant (P = 0.03).
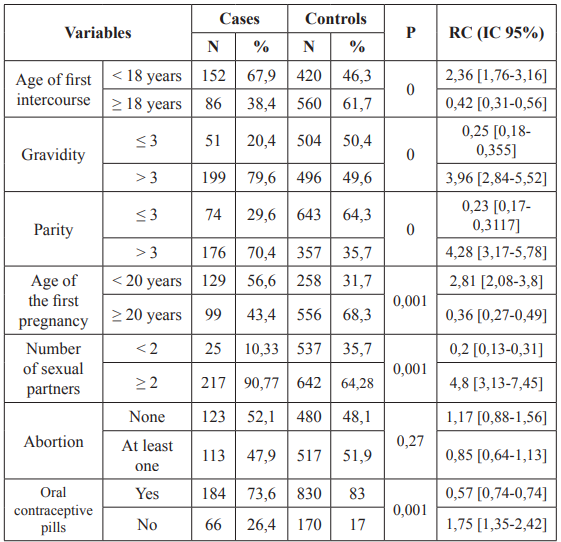
Table 3: Univariate Logistic regression analysis for reproductive and
Multivariate regression
Age at first coitus of <18 years showed high significance (p = < 0.001) with cervical cancer with OR of 5.44 (2.73–10.86). Low economic status was significantly associated with risk of cervical cancer. Age at first pregnancy, consumption of tobacco and other factors also had significant association with risk of cervical cancer (Table 4).
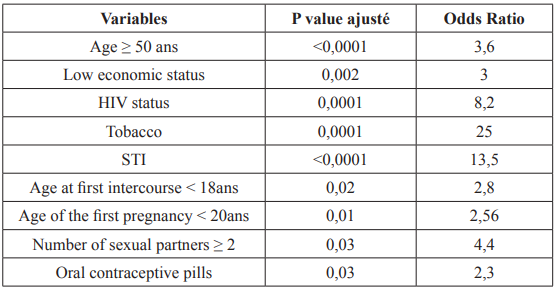
Table 4: Risk factors of cervical cancer.
Discussion
In the study, in case group, the age of cases ranged from 25–82 year with a mean age cases of 53.29 ± 12.71 years. In control group the mean age of the participants was 40.38 ± 12.20 years from 17 to 77 years. These results are similar to those found by Biswas et al. (participants from the age range of 25–70 year), Pragati Sharma et all, whose participants ages range from 35 to 78 years [19,20]. Shields et all included women from 20 to 74 years [21].
In our study the flagship sign was metrorrhagia with a frequency of 82.8% seconded by pelvic pain with a frequency of 46.4%. These data of metrorrhagia corroborate with 94,2 %, 92,2 % and 88, 7% found by Diaki, Sahraoui and Nguyen [22-24] respectively. Dem reported only 19.50% cases with pelvic pains [25]
Regarding the FIGO classification, 22.8% of women were in stage IIIB followed by 18.4% in stage IVA at the time of diagnosis. Results lower than 65,9% at stage IIIB followed by 8,8 % at stage IV found by Diakite in Mali and 65% for stages III and IV found by Tonato Bagnan et al. in 2013 in Benin [22,26]. This result can be explained by the fact that patients at risk do not consult frequently and are therefore screened late.
As for the histological type, squamous cell carcinoma was the most represented with 91.2% and adenocarcinoma with 8.8% which corroborates with 94.2 % and 5.1% respectively found by Ell Diakite in 2019 in Mali, with 81.18% and 12.95% found by Engbang et al. in 2016, in Cameroon and differs from 62% of squamous cell carcinoma found by Tonato Bagnan et al. in 2013 in Benin [18,22,26].
In our study, the average age was 53 ± 12 years. Women over the age of 50 were 3 times more likely to develop cervical cancer (OR= 3.6; 95% CI = [3.34-7.3]) result that agrees with the literature stipulating of this affection being that of elderly women [27]. The disease caused by HPV infection is very slow (around 15 years). Over time, this development can be potentiated by the presence of multiple cofactors. In addition, it can in some cases be slowed down or eradicated by immunity, but reinfections associated with other factors will cause the cycle to resume with late onset of the disease [28].
Regarding socioeconomic level, 64.4% of cases had a low socioeconomic level. In our study, the low socioeconomic level increased the risk of cervical cancer 3 times (OR = 3; 95% CI = [1.51-6.21]). As with many other infectious diseases, poverty is the strongest determinant of the incidence of and mortality from cervical cancer around the world [29]. The human development index and poverty rates have shown a clear association (inverse and direct, respectively) with incidence and mortality, thus explaining about 52% of variability in cervical cancer globally [30,31]. In our context, the low socioeconomic level exposes women to promiscuity, precociousness of sexual intercourse, and sometimes sexual vagrancy which all together potentiates the occurrence of infection. In addition, women of this same level are for the most part not in a position to ensure medical follow-up.
Regarding HIV infection, 28% of the cases were infected. In our study, immunosuppression to HIV increased the risk of cervical cancer by 8 times (OR = 8.6; 95% CI = [3.84-20]). Women immunocompromised with HIV see their immunity weaken over time. As a result, they will be more vulnerable to infections than the general population and not at the same time to eradicate HPV for those who come into contact [29]. Abraham et al. in 2013 in a study done in North America found that women infected with HIV with reference CD4 + T lymphocytes ≥ 350, 200-349 and<200 cells / μL had an increase in incidence of cervical cancer 2.3 times, 3.0 times and 7.7 times respectively compared to women not infected with HIV (P = 0.001) [32].
For STIs, 82.6% of the cases had a history of STIs. In our study, STIs increased the risk of cervical cancer by 13 times (OR = 13.5; 95% CI = [6.62-33.33]). According to the literature, STIs (chlamydia and herpes simplex2) constitute a risk factor because they potentiate HPV infection which over time leads to changes in the mucosa and the occurrence of cancer [33].
Regarding tobacco, 21.8% of cases were smoking. In our study, tobacco increased the risk of developing cervical cancer by 25 times (OR = 25; 95% CI = [7.24-83.33]). According to various studies have shown that smoking is one of the most important risk factors for CIN 3 and invasive cervical cancer [34,35]. Smoking can increase the risk of cervical neoplasia through several mechanisms. One of the mechanisms is the local induction of immune suppression by tobacco metabolites. In addition, chemicals in cigarettes, such as nicotine and its metabolites, can cause DNA damage in squamous cells [36]. Smoking can cause epigenetic changes in the epithelium of the cervix and contribute to the pathogenesis of neoplasia in the cervix and in many other parts of the body [37].
In terms of sexual behavior, at the age of first sexual intercourse, 67.9% of cases had their first sexual intercourse before the age of 18 years and 56.6% of the cases had given birth before the age of 20 years. In our study, the first report before 18 years as well as the first delivery before the age of 20 were risk factors (OR = 2.8; 95% CI = [1.52-5.36]). Sharma et al. Age at first coitus of <18 years showed high significance (p = < 0.001) with cervical cancer with OR of 5.44 (2.73–10.86) [20]. Similar findings were observed in a study by Biswas N et al. which showed OR of 2.7 (0.8–8.9) with p = 0.005 for age at 1st coitus of <18 years [19]. Cervical cancer is extremely rare in women younger than the age of 20. However, many young women become infected with multiple types of human papilloma virus, which then can increase their risk of getting cervical cancer in future. Early age at first intercourse is consistently found to be a risk factor in most of the early epidemiological studies of cervical cancer [19,38]. Cervical cancer risk is double in women who first had sexual intercourse at the age of 14 or less, compared to those who did at 25 or more [38]. This could be explained by the immaturity of the cervix (transition zone) in adolescence and therefore a sensitivity to aggression. Furthermore, the first coitus before 18 years of age will lead to a long sexual life, consequently an increased risk for STIs and a long time of exposure to the carcinogen (HPV) [26].
With regard to hormonal contraception, 26.4% of the cases had used hormonal contraception. In our study, hormonal contraception increased 2 times the risk of occurrence of cervical cancer (OR = 2.3; 95% CI = [1,11-5]). It has been reported by some authors that the use of oral contraceptive method for 5 years or more can double the risk of cancer. However, there is a slight increase in the incidence of invasive cervical cancer in those who used only injectable progesterone for 5 years or more [39]. Moreno et al. affirmed that among women who tested positive for HPV DNA, the risk of cervical cancer increased by 3 times if they have used oral contraceptive pills for 5 years or more. The results of this study showed that the risk of cancer does not depend on the first time use of OC pills [40].
Conclusion
Cervical cancer is a pathology with an infectious basis that affects older women much more and whose diagnosis in our context is made much later. Several factors increasing the occurrence of cervical cancer have been determined, namely age ≥50 years, low socioeconomic level, tobacco, HIV immunosuppression, STIs, multi sexual partnership, age <18 years at first sexual intercourse, oral contraceptive. These factors will then allow us to set up an algorithm allowing us to define the patients at high, medium and low risk. This will allow us to improve the policy of early detection which will guarantee the fall in the incidence of cervical cancer in our country.
Author’s Contributions
Author ENJP designed the study, wrote the protocol and wrote the first draft of the manuscript. Authors EH and NNJP participated in data collection supervision and analysis. Author MKV contributed in literature search. Author FP reviewed the final manuscript. All authors read and approved the final.
References
- Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide sources methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136: 359-386.
- Dernières statistiques mondiales sur le 2012; 3.
- Boumba M, Bouassa R, Prazuck T, et al. Cancer du col de l’utérus en Afrique subsaharienne une maladie associée aux papaillomavirus humains oncogènes, émergente et évitable. Médecine et Santé Tropicales. 2017; 27: 16-22.
- Bosh F, Broker T, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013; 31: 1-31.
- Cancer du col de l’utérus Données par INSTITUT DE VEILLE SANITAIRE. 2017.
- Thun M, Linet MS, Haiman CA, et al. Cancer epidemiology and prevention. Oxford University Press. 2017; 1329.
- De Vuyst H, Alrmany L, Lacey C. The burden of human papillomavirus infections and related diseases in subsaharan Africa. Vaccine. 2013; 31: 32-46.
- Luna J, Plata M, Gonzalez M, et al. Long-term follow-up observation of the safety immunogenicity and effectiveness of GardasilTM in adult PLOS ONE. 2013; 8: e83431.
- Dalstein V, Riethmuller D, Prétet JL, et al. Persistence and load of high-risk HPV are predictors for development of high- grade cervical lesions a longitudinal French cohort Int J Cancer. 2003; 106: 396-403.
- Nyitray AG, Menezes L, Lu B, et al. Genital human papillomavirus HPV concordance in heterosexual couples. J Infect Dis. 2012; 206: 202-211.
- Muñoz N, Bosch FX, Herrero R, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348: 518-257.
- Isautier S. Place de la vaccination antipapillomavirus humains dans la prévention du cancer du col de l’utérus. DE LORRAINE. 2012.
- Denny L, Franceschi S, De Sanjosé S, et al. Human papillomavirus human immunodeficiency virus and immunosuppression. 2012; 30: F168-F174.
- García Closas R, Castellsagué X, Bosch X, et al. The role of diet and nutrition in cervical carcinogenesis a review of recent evidence. Int J Cancer. 2005; 117: 629-637.
- Drolet M, Laprise JF, Boily MC, et al. Potential cost effectiveness of the nonavalent human papillomavirus HPV vaccine. Int J Cancer. 2014; 134: 2264-2268.
- Engbang JP, Koh VM, Nguefack CT, et al. Aspects histo- épidémiologiques des cancers génitaux de la femme dans la région du Littoral Cameroun. Pan Afr Med J. 2015; 21.
- Sando Z, Fouogue JT, Fouelifack FY, et Profil des cancers gynécologiques et mammaires à Yaoundé - Cameroun. Pan Afr Med J. 2014; 17.
- Engbang NJP, Tchente NC, Owona MLJ, et Open Journal of Obstetrics and Gynecology. 2016; 6: 232-239.
- Biswas LN, Manna B, Maiti PK, et al. Sexual risk factors for cervical cancer among rural Indian women a case control study. Int J Epidemiol. 1997; 26: 491-495.
- Sharma P, Pattanshetty A study on risk factors of cervical cancer among patients attending a tertiary care hospital: A case-control study. Clinical Epidemiology and Global Health. 2018; 6: 83-87.
- Shields TS, Brinton LA, Burk R, et A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2004; 13: 1574-1582.
- Diakité A, Koné AS, Diallo YL, et al. Epidemiological and Clinical Profile of Cervix Cancer at Bamako Radiotherapy Center. Open Journal of Obstetrics and 2019; 9: 92-97.
- Sahraoui S, Bouras N, Acharki A, et al. Adénocarcinome du col utérin étude rétrospective de 83 cas Gynécol Obstét 2010; 30: 291-298.
- Nguyen D, de la Rochefordière A, Chauveinc L, et al. Chimioradiothérapie dans les cancers du col utérin localement évolués. Étude rétrospective de 92 patientes traitées à l’institut Curie de 1986 à 1998. Cancer Radiother. 2012; 6: 201-208.
- Dem Les carcinomes épidermoïdes du col utérin à l’Institut du cancer de Dakar. Cahiers Santé. 2008; 18.
- Tonato Bagnan JA, Denakpo JL, Aguida B, et al. Épidémiologie des cancers gynécologiques et mammaires à l’hôpital de la Mère et de l’Enfant-Lagune HOMEL et à la clinique universitaire de gynécologie et d’obstétrique CUGO de Cotonou, Bénin. Bull Cancer Paris. 2013; 100: 141-146.
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer a retrospective cross-sectional worldwide study. Lancet Oncol. 2010; 11: 1048-1056.
- Tranbaloc P. Histoire naturelle des lésions précurseurs du cancer du col utérin. Gynécol Obstétr 2008; 36: 650-655.
- Boffetta P, Kogevinas M, Westerholm P, et al. Exposure to occupational carcinogens and social class differences in cancer occurrence. IARC Sci Publ. 1997; 138: 331-341.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 0, Cancer incidence and mortality worldwide: IARC CancerBase. 2013; No. 11. Lyon, France: International Agency for Research on Cancer.
- Singh GK, Azuine RE, Siahpush Global inequalities in cervical cancer incidence and mortality are linked to deprivation low socioeconomic status and human development. Int J MCH AIDS. 2012; 1: 17-30.
- Abraham AG, Strickler HD, Jing Y, et al. Invasive cervical cancer risk among HIV-infected women A North American multi-cohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013; 62: 405-413.
- Almonte M, Albero G, Molano M, et al. Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine. 2008; 26: L16-L36.
- Kim J, Kim BK, Lee CH, et al. Human papillomavirus genotypes and cofactors causing cervical intraepithelial neoplasia and cervical cancer in Korean International Journal of Gynecological Cancer. 2012; 22: 1570-1576.
- Fonseca Moutinho JA. Smoking and cervical cancer. ISRN Obstetrics and Gynecology. 2011; 847684.
- Roura E, Castellsagué X, Pawlita M, et Smoking as a major risk factor for cervical cancer and pre-cancer Results from the EPIC cohort. International Journal of Cancer. 2014; 135: 453-466.
- Ma Y, Collins S, Young LS, et al. Smoking initiation is followed by the early acquisition of epigenetic change in cervical epithelium A longitudinal study. British Journal of Cancer. 2011; 104: 1500-1504.
- International Collaboration of Epidemiological Studies oCervical Cancer. Cervical Carcinoma and Sexual Behaviour: Collaborative Reanalysis of Individual Data on 15,461 Women with Cervical Carcinoma and 29,164 Women without Cervical Carcinoma from 21 Epidemiological Studies. Cancer Epidemiology, Biomarkers & Prevention. 2009; 18: 1060-1069.
- Cancer ICESC, Appleby P, Beral V, et al. Cervical cancer and hormonal contraceptives Collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007; 370: 1609-1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection The IARC multicentric case- control study. Lancet. 2002; 359: 1085-1092.