Risk Factors of Prostate Cancer in Douala (Cameroon): A Case-Control Study
Author'(s): Jean Paul Ndamba Engbang1,2*, Theodore Beyeme Sala1,2, Marina Ebane Abane1, Armel Essomba3 and Marcelin Ngowe Ngowe1,4
1Faculty of Medicine and Pharmaceutical Sciences of Douala, University of Douala, Douala, Cameroon.
2Laquintinie Hospital of Douala, Douala, Cameroon.
3Douala general Hospital.
4Faculty of Medicine and biomedical Sciences, University of
*Correspondence:
Jean Paul Ndamba Engbang, Faculty of Medicine and Pharmaceutical Sciences of Douala, University of Douala, Douala, Cameroon.
Received: 22 January 2021; Accepted: 15 February 2021
Citation: Engbang JPN, Sala TB, Abane ME, et al. Risk Factors of Prostate Cancer in Douala (Cameroon): A Case-Control Study. Cancer Sci Res. 2021; 4(1): 1-7.
Abstract
Background: Generally, cancers are among the principal causes of Morbidity and Mortality given their increase rate, with prostate cancer being the most frequent neoplasm in men and the second leading cause of cancer death after bronchopulmonary cancers. We decided to carry out this study to highlight its risk factors and create awareness on the need for regular check-ups to avoid complications of late diagnosis. The aim of this study is to assess the impact of Prostate Cancer risk recognition, and therapeutic attitudes towards the patients.
Material and Methods: We conducted a cross sectional and analytical study in the surgical and oncology consultation units of the Douala general hospital (HGD) and Douala laquintinie hospital (HLD) within a period of four months. Patients diagnosed of Prostate cancer were eligible to our study. The patients responded to a questionnaire containing data on socio-demographic conditions and lifestyle data. Risk factor exposure and complications of late diagnosis. Anthropometric parameters were measured and recorded. Data was analyzed using SPSS 17.0 software. Determinants were identified using multiple logistic regressions. Statistical significance was set at P< 0.05.
Results: Two Hundred and Fifty patients were surveyed during this period. The median age was 62.5 ± 9.51, with ages ranging from 43-60years. The age group with the largest number of patients that is 89 cases with 35.6% was those from 60-69 years. Age greater than 50years was also known to be associated with Prostate Cancer. (CI=0.11-0.24, OR: 6.12, p value=<0.0001). Other factors significantly associated with Prostate Cancer were, Hypertension (CI=5.49-10.6, OR: 7.43, p value=<0.0001), Diabetes (CI=3.22-9.12, OR: 4,1, p value=<0.0001), Tobacco (CI 3.93-7.26, OR 5.33, p value= 0.001). Also, the consumption of Carbohydrates (CI= 1.86-4.19, OR 2.78, p value=<0.0001) and sexual activity less than 5 times a month (CI: 2.67-4.85, OR: 3.6) were significantly associated with Prostate Cancer.
Conclusions: Many factors are significantly associated with prostate cancer (Age˃50 years, hypertension, diabetes, tobacco and carbohydrates consumption, sexual activity less than 5 times a month). Therefore, early diagnosis and multiple check-ups are necessary. Especially as men grow older given that age is a great risk factor.
Keywords
Introduction
Generally, cancers are among the principal causes of Morbidity and Mortality given their increase rate, with prostate cancer (PCa) being the most frequent neoplasm in men and the second leading cause of cancer death after bronchopulmonary cancers [1,2]. The incidence of prostate cancer varies worldwide, with the highest rates found in the United States, Canada, and Scandinavia, and the lowest rates found in China and other parts of Asia [3,4]. These differences are caused by genetic susceptibility, exposure to unknown external risk factors or differences in health care and cancer registration, or even a combination of these factors [4]. Unlike the West, the survival rate in Africa does not seem to exceed 50%, for example in Uganda the 5-year survival rate was estimated at 47.2% [5]. Many risk factors are mentioned for this cancer, including age, which is the main risk factor and largely reflects cell DNA damage accumulating over time, the damage which could result from biological processes or from exposure to other risk factors [6]. Other risk factors include race, with Black men exposed to a higher risk of prostate cancer than White men, and Family history [6,7]. Other risk factors are emerging, such as diet, lifestyle, environmental and, especially, genetic factors. [8]. Screening is testing to find cancer in people before they have symptoms [9]. For some types of cancer, screening can help find cancers at an early stage, when they are likely to be easier to treat [10]. When diagnosis is not done early enough, some complications may occur, such as Urinary incontinence and Erectile dysfunction. An evolution of this disease without treatment could lead to metastasis which often affects the bones and causes spinal cord compression, paralysis and fractures amongst other things. In Cameroon, the urogenital apparatus is the target of 6.5% of all malignant neoplasms, prostate cancer accounts for the clear majority (72%) of these cancers, with 6.32% of patients diagnosed before the age of 50 years [11,12]. Many other studies have been carried out on prostate cancer, but we did not find anyone on its risk factors [13]. For this reason, we decided to carry out this study on its risk factors and to propose ways by which it could be diagnosed early.
Materiel and Methods
We carried out a case-control (prospective and retrospective) study in Douala, a city found in the Littoral Region and the economic capital of Cameroon. The study was done in two hospitals in that city, Laquintinie and General Hospitals of Douala. The study in perspective and retrospective aspects. It concerned all men aged 18 and over who had consulted in our study sites for the past five years, i.e. from January 1, 2012 to December 31, 2017 for the retrospective study and from February 2018 to May 2018 for the prospective study. Concerning cases, it was male patients living in Douala presenting symptoms of Prostate Cancer: and controls patients with no physical or biological symptoms of Prostate Cancer. For each case of prostate cancer, four controls were matched by age and residence. The recruitment of controls was done on the basis of age-matched (± 5 years) cases. Concerning controls, a sensitization of the men through the messages and communicators sent in different communities (Churches, meetings) of the city of Douala was carried out. The study participants were approached by the primary investigator. The study purpose, procedure, risks and benefits were explained to the patient in a language they best understand. An information notice about the study was given to the participant’s or guardian. Participants could ask questions and give their consent if the agrees to participate in the study. After obtaining informed consent, the primary investigator: we went through the medical records of the patients who met the inclusion criteria. We got information from the files. For those who had incomplete files, we called the patients to complete the information. We discussed with the patients who came for consultation in the Urology and Oncology units with Prostate Cancer Disease or Not. The choice of patients here was random but was those who met the inclusion criteria. These patients responded questions in the questionnaire. One dependent variable of interest was chosen, prostate cancer was defined as a binary variable. Independent variables included: Sociodemographic and anthropometric variables (age, sex; residence; weight, height, profession, marital status, origin), Clinical variables (urinary Incontinence, erectile dysfunction, spinal cord compression, fractures), Risk factor exposure data (tobacco, alcohol; medical history, family history, Obesity, meal constituent, number of sexual partners). Data was recorded and processed using EPI info and EXCEL after which it was analysed using SPSS version 20. Quantitative variables (risk factors) were represented as means and standard deviation while qualitative variables were presented as frequencies and percentages. In bivariate analysis, the qualitative variables was compared using the chi 2 test and Fisher’s exact test was used in cases of dichotomous variables. Finally, a multi-varied analysis was done to study the factors associated with prostate cancer, the objective of this analysis is to obtain, from the set of variables, a final model that holds the largest number explanatory variables that are significant in the explanation of the dependent variable. For this purpose, we performed a conditional logistic regression because it is a case-control study where the matching was done on the age (4 witnesses for a case). Quantitative variables was compared using the Student t-test. Statistical significance was set at a P value <0.05.
Results
Distribution according to age
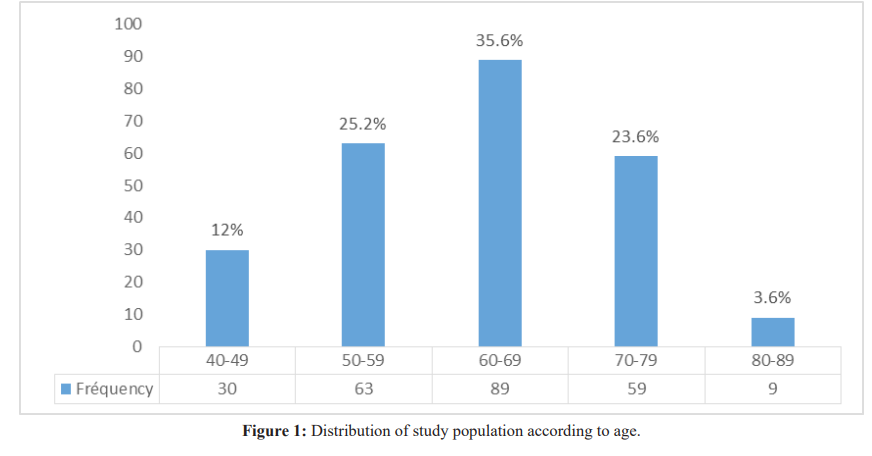
The mean age was 62.5 ± 9.51with extremes from 43 to 80years. The predominant age group was 60-69years (89 cases) representing 35.6% of the study population. The age group with the lowest population was 80-89 years (9cases) which represented 3.6%, as seen in figure 1.

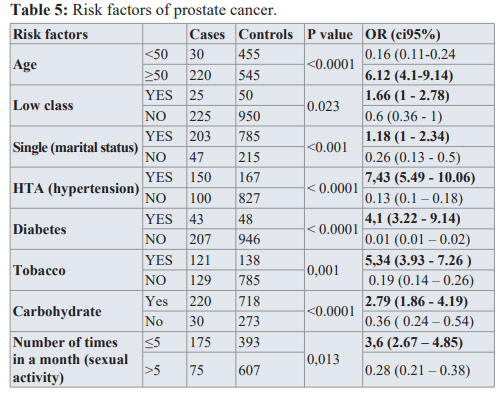
Being more than 50 years of age has a significant increase in Being Hypertensive (OR=7.19, CI=5.27-9,6, p< 0.0001), Diabetic (OR=3.42, CI=2.22-5.26, p< 0.0001) and consuming tobacco (OR=5.34, CI=3.93-7.25, p<0.001) had a great effect on developing prostate Cancer. the risk of having prostate Cancer (OR=6.12, CI=4.1-9.14, p value=<0.0001).
Here we grouped Middle and upper class as high for the social status, but the social status of the patients had a minimal effect on them having prostate cancer. (OR=1.88, CI=1.08-3.28, p = 0.138).
For the marital status we also grouped all those who lived under ‘marital conditions’ (living as married, divorced, separated) under one group. Being single has a significant increase in the risk of having prostate cancer (OR=5.89, CI=2.3-14.6, p value= < 0.0001), as shown in table 1.
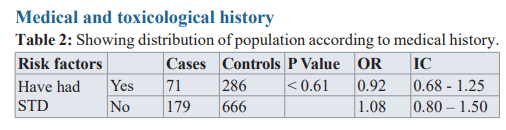
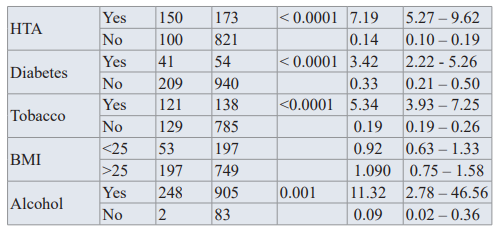
Being Hypertensive (OR=7.19, CI=5.27-9,6, p< 0.0001), Diabetic (OR=3.42, CI=2.22-5.26, p< 0.0001) and consuming tobacco (OR=5.34, CI=3.93-7.25, p<0.001) had a great effect on developing prostate Cancer.
The BMI and past medical history of a sexually transmitted disease had little or no impact, as shown in table 2.
Diet
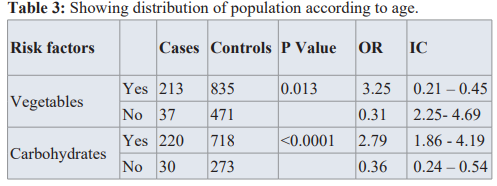
Heavy consumption of carbohydrates could increase the risk of prostate cancer, (OR=2.79, CI=1.86-4.19, p value=<0.0001).
Consuming vegetables and fatty foods had no impact.
Sexual history
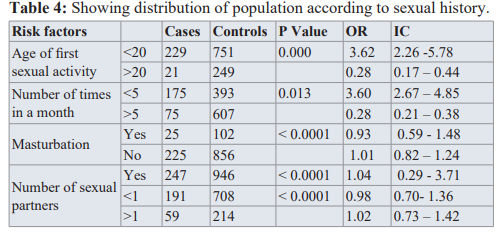
We realized that having your first sexual activity before the age of 20years increased the risk of developing prostate cancer (OR=15.8, IC=7.16-33.03, p value= 0.14).
Also having at most 1 sexual partner and masturbation could reduce the risk of prostate cancer. While having sexual activity less than 5 times a month could increase the risk for prostate cancer by 3. (OR=3.60, CI=2.67-4.85, p value=0.013).
Risk factors: Multivariate regression
Table 5 shows that there is a significant association between age and prostate cancer (P < 0.0001). Being more than 50 years is times as likely to develop breast cancer as those being under 50 years (OR = 6.12; CI = [4.1 – 9.14]). Some factor like hypertension, diabetes, tobacco and carbohydrate consumption, and sexual activity (less than 5 times a month) were significantly associated with prostate cancer. Low economic status is associated and being single were also associated (but not significantly) with prostate cancer.
Discussion
The mean age of diagnosis was 62.54 ± 9.58 years, with extremes ranging from 43 to 80 years. 37.2% (93cases) of the patients were between 40-59, 9% (9cases) where above 80years. The age group, 60-79 years had the highest number of patients, 146 cases (58.4%).
We studied that patients aged 50 years and above had 6 times risk of developing Prostate Cancer (OR:6.12, CI:4.1-9.14, p<0.0001. A study carried out by Strangle Berger et al. also had age as a risk factor, studying those patients greater than 60 years had 2 times risk of developing prostate cancer. (OR: 2.01, CI: 3.14-8.34, p<0.0001) [14]. These cases bring up the question of the age of onset of screening, particularly in African subjects. The population of African origin according to several authors is more affected by prostate cancer and the average age at diagnosis seems lower both in Africa and among African-Americans unlike Caucasians [15- 17]. It has been shown that men of African descent are 2.5 times more likely to develop prostate cancer than Caucasians themselves are more at risk than Asians. One of the causes would be attributed to the specific genetic variations within these groups, notably the high prevalence among Africans of the gene SRD5A1 (steroid-5- alpha-reductase, alpha polypeptide 1 (3-oxo-5 alpha-steroid delta 4 in fact, this gene forms the enzyme Steroid 5-alpha-reductase (EC 1.3.99.5) catalyzing the conversion of testosterone to the more potent androgen, dihydrotestosterone (DHT) [18,19].
With 35.6% of patients older than 60 years, it should be agreed with other authors that advanced age is a risk factor for prostate cancer and whose cause could be attributed to genetic alterations arising from the aging process [20,21]. Thus, several reasons are evoked namely, the variation of hormonal levels and the alteration of the testosterone ratio E2 [22,23]. Age is likely to decrease plasma levels of androgens and increase or increase estrogen, which in turn decreases the androgen ratio in plasma estrogens [24,25]. Therefore, other authors mention the probable role of the enzyme UGT2B28 (Uridine diphospho-glucuronosyltransferases) in the prostate tumor process, knowing that the prostate can glucuronide both testosterone and estradiol and therefore play on the balance of this ratio [26].
In our study, hypertensive patients have a high-risk factor, they are 7 times more likely to develop prostate cancer. (OR: 7.43, CI: 5.49-10.06, p< 0.0001). These results could be comparable to those obtained by Liang Z et al. who also found that hypertensive patients had 2 times odds of developing prostate cancer. (OR: 2.01, CI: 1.02-1.15, p=0.014) [27]. Navin et al who found the frequency of hypertension to be 73% in African American and they concluded that Prostate Cancer has a common androgen mediated mechanism [28]. Clinically, Miller and colleagues have documented higher aldosterone and higher blood pressure, respectively, in men compared with women [29]. Schunkert and associates found a positive correlation between dehydroepi-androsterone sulfate (a metabolite of testosterone), aldosterone levels, and blood pressure in a population of hypertensive men. It is also well known that decreasing androgen levels by orchiectomy or by chemical means slows the progression of prostate cancer. These findings suggest that androgens may have a causative role in prostate cancer and hypertension [30].
In our study, diabetes increased the risk of cervical cancer 95 times (OR: 4.1, CI: 3.22-9.14, p < 0.0001). These results are comparable with those obtained by Zaorsky et al who found out that diabetic patients are higher at risk of developing Prostate Cancer and subsequently dieing from it [31]. Chin-Hsiao Tseng et al also carried out a study on Diabetes and Prostate Cancer and found out that there was appositive link with diabetes and Prostate Cancer, with diabetic patients having 6 times odds of developing prostate cancer. (OR: 5.83, CI: 5.10-6.66, p< 0.0001) [32]. This could be explained by the fact that, Heterogeneity may exist in the association between diabetes and prostate cancer. Some suggested that prostate cancer risk differs by time since diabetes diagnosis or occurrence of metabolic aberrations associated with impaired glucose tolerance [33]. In a study carried out by Weinstein SJ et al. it was studied that serum creatinine is shown to be significantly predictive for prostate cancer risk [34]. Some other study by Muller et al. suggested that patients with more severe diabetes might have lower level of PSA and lower risk of prostate cancer [35]. With increasing duration and severity of diabetes, chronic complications may set in and interfere with the association between diabetes and prostate cancer. Some suggested that diabetes might only convey a higher risk of more advanced prostate cancer [36,37]. However, we did not have sufficient information for analysis.
According to ours results, consuming tobacco was a risk factor of prostate Cancer. (OR: 5.34, CI: 3.93-7.26, p=0.001). Study done by Plaskon et al had similar results where they found out that tobacco is associated with moderately increased risk of prostate Cancer (OR;1.4, CI;1.0-2.0, p= 0.03) [38]. A similar study was done by Micahel Huncharek et al where they sowed an association of smoking and prostate cancer incidence [39]. There are several potential mechanisms whereby cigarette smoking may increase risk of prostate cancer. One is the ability of cigarette smoking to increase bioavailable testosterone and decrease bioavailable estradiol, which may alter the hormonal milieu favoring higher androgenic exposure to the prostate [40,41]. Several lines of evidence suggest that hormones are involved in the etiology of benign and malignant prostate disease smoking-induced changes in steroid hormone levels which is a possible mechanism for the association between cigarette smoking and prostate cancer risk [42].
We found in the study that, carbohydrate consumers are 2.7 times more likely to develop prostate cancer (OR: 2.79, CI: 1.86-4.19,< 0.0001). These results are like those obtained by Drake et al who found out that an increase in the intake of carbohydrates may be associated with increased risk of Prostate Cancer, (OR: 1038, C: .04-1.84, p<0.05). However, they observed no significant associations with high-risk prostate cancer, and not all foods that are typically high in refined carbohydrates were associated with prostate cancer [43]. Landberg et al showed that whole grain and bran from rye resulted in significantly lower plasma PSA than did a cellulose-supplemented refined wheat diet in patients with prostate cancer [44].
Sexual activity has been hypothesized to play a role in the development of prostate cancer. we had as results for those who had sexual intercourse less than 5 times a month as (OR: 3.6; CI: 2.67-4.85, p = 0.013). A study confirmed that most frequent ejaculators had a lower risk of developing prostate cancer than those who ejaculated less frequently. In 2004, a large prospective study on 29.342 men by Leitzmann et al. concluded that higher ejaculation frequency was related to decreased risk of total and organ confined prostate cancer [45]. Jian Z et al, demonstrated that, moderate ejaculation (2-4 times per week) was significantly associated with a lower risk of PCa (OR: 0.91, 95% CI 0.87-0.96) [46].
Conclusion
Several factors increasing the occurrence of prostate cancer have been determined. An age superior to 50years, a history of diabetes and hypertension, the consumption of tobacco and foods rich in Carbohydrates. Having sex less than 5 times in a month had a significant association with Prostate cancer.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008 GLOBOCAN 2008. Int J Cancer. 2010; 127: 2893-2917.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide Sources methods and major patterns in GLOBOCAN Int J Cancer. 2015; 136: E359-E386.
- Quinn M, Babb Patterns and trends in prostate cancer incidence survival prevalence and mortality. Part I international comparisons. BJU Int. 2002; 90: 162-173.
- Gronberg Prostate cancer epidemiology. Lancet. 2003; 361: 859-864.
- Sankaranarayanan R, Swaminathan R, Jayant K, et al. An overview of cancer survival in Africa Asia the Caribbean and Central America the case for investment in cancer health IARC Sci Publi. 2011; 162: 257-291.
- Lloyd T, Hounsome L, Mehay A, et al. Lifetime risk of being diagnosed with or dying from prostate cancer by major ethnic group in England 2008-2010. BMC Med. 2015; 13: 171.
- Hemminki K, Czene K. Age specific and attributable risks of familial prostate carcinoma from the family-cancer database link is external. 2002; 95: 1346-1353.
- Mbakop A, Essame Ozona JL, Ngbangalm C, et Epidémiologieactuelle des cancers au Cameroun. Bull Cancer. 1992; 79: 1101-1104.
- Lauby-Secretan B, Scoccianti C, Loomis D. Body Fatness and Cancer Viewpoint of the IARC Working Group link is N Engl J Med. 2016; 375: 794-798.
- Rowlands MA, Gunnell D, Harris R. Circulating insulin-like growth factor peptides and prostate cancer risk a systematic review and meta-analysis LinkIs external. Int J Cancer. 2009; 124: 2416-2429.
- Fezeu L, Fointama E, Ngufor G, et Diabetes awareness in general population in Cameroon. Diabetes Res Clin Pract. 2010; 90: 312-318.
- Engbang NJP, Sala B, Moby H, et al. Cancers urogénitaux dans la région du littoral-Cameroun Epidémiologie et Revue de Medicine et de de Pharmacies. 2014; 4: 440-446.
- Angwafo III F, Zaher A, Befidi-Mengue R, et al. High-grade intra-epithelial neoplasia and prostate cancer in Dibombari, Prostate Cancer Prostatic Dis. 2003; 6: 34-38.
- Stangelberger A, Waldert M, Djavan B. Prostate Cancer in Elderly Rev Urol. 2008; 10: 111-119.
- Edwards SM, Eeles RA. Unravelling the genetics of prostate Am J Med Genet C Semin Med Genet. 2004; 129C: 65-73.
- SEER 9 areas and US Mortality Files National Center for Health Statistics CDC. Rates are age-adjusted to the 2000 US Std Population 19 age groups -Census P25-1103. Regression lines are alculated using the Joinpoint Regression Program Version 2.0. National Cancer Institute. 2015.
- Bunker CH, Patrick AL, Konety High prevalence of screening-detected prostate cancer among Afro-Caribbeans the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002; 11: 726-729.
- Zeigler-Johnson CM, Walker AH, Mancke B, et al. Ethnic differences in the frequency of prostate cancer susceptibility alleles at SRD5A2 and CYP3A4. Hum Hered. 2002; 54: 13-
- Park SY, Haiman CA, Cheng I, et Racial/ethnic differences in lifestyle related factors and prostate cancer risk the Multiethnic Cohort Study. Cancer Causes Control. 2015; 26: 1507-1515.
- Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly frequency of advanced disease at presentation and disease-specific Cancer. 2012; 118: 3062-3070.
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007; 8: 692-702.
- Yao S, Till C, Kristal AR, et al. Serum estrogen levels and prostate cancer risk in the prostate cancer prevention trial a nested case-control study. Cancer Causes Control. 2011; 22: 1121-1131.
- Black A, Pinsky PF, Grubb RL, et al. Sex steroid hormone metabolism in relation to risk of aggressive prostate cancer. Cancer Epidemiol Biomarkers 2014; 23: 2374-2382.
- Burton AJ, Tilling KM, Holly JM, et al. Metabolic imbalance and prostate cancer progression. Int J Mol Epidemiol Genet. 2010; 1: 248-271.
- Vermeulen AJM, Kaufman S, Goemaere I, et al. Estradiol in elderly Aging Male. 2002; 5: 98-102.
- Belledant A, Hovington H, Garcia L, et The UGT2B28 Sex- steroid Inactivation Pathway Is a Regulator of Steroidogenesis and Modifies the Risk of Prostate Cancer Progression. Eur Urol. 2016; 69: 601-609.
- Liang Z, Xie B, Li J, et al. Hypertension and risk of prostate cancer a systematic review and meta-analysis. Sci Rep. 2016; 6: 31358.
- Navin S, Ioffe The association between hypertension and prostate cancer. Rev Urol. 2017; 19: 113-118.
- Miller JA, Anacta LA, Cattran Impact of gender on the renal response to angiotensin II. Kidney Int. 1999; 55: 278-285.
- Schunkert H, Hense HW, Andus T, et al. Relation between dehydroepiandrosterone sulfate and blood pressure levels in a population-based Am J Hypertens. 1999; 12: 1140-1143.
- Zaorsky NG, Shaikh T, Ruth K, et Prostate Cancer Patients with Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment with Radiation Therapy. 2017; 15: 326-335.
- Tseng Diabetes and risk of prostate cancer a study using the National Health Insurance. Diabetes Care. 2011; 34: 616-621.
- Darbinian JA, Ferrara AM, Van Den Eeden SK, et Glycemic status and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2008; 17: 628-635.
- Weinstein SJ, Mackrain K, Stolzenberg-Solomon RZ, et al. Serum creatinine and prostate cancer risk in a prospective Cancer Epidemiol Biomarkers Prev. 2009; 18: 2643- 2649.
- Müller H, Raum E, Rothenbacher D, et al. Association of diabetes and body mass index with levels of prostate-specific antigen implications for correction of prostate-specific antigen cutoff values. Cancer Epidemiol Biomarkers Prev. 2009; 18: 1350-1356.
- Li Q, Kuriyama S, Kakizaki M. History of diabetes mellitus and the risk of prostate cancer the Ohsaki Cohort Cancer Causes Control. 2010; 21: 1025-1032.
- Leitzmann MF, Ahn J, Albanes D. Prostate Lung Colorectal and Ovarian Trial Project Team Diabetes mellitus and prostate cancer risk in the Prostate Lung Colorectal and Ovarian Cancer Screening Cancer Causes Control. 2008; 19: 1267-1276.
- Plaskon LA, Penson DF, Vaughan TL, et Cigarette Smoking and Risk of Prostate Cancer in Middle-Aged Men. Cancer Epidemiol Prev Biomark. 2003; 12: 604-609.
- Huncharek M, Haddock KS, Reid R, et al. Smoking as a Risk Factor for Prostate Cancer A Meta-Analysis of 24 Prospective Cohort Am J Public Health. 2010; 100: 693-701.
- Ferrini RL, Barrett-Connor Sex hormones and age a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998; 147: 750-754.
- Field AE, Colditz GA, Willett WC, et al. The relation of smoking age relative weight and dietary intake to serum adrenal steroids sex hormones and sex hormone-binding globulin in middle-aged J Clin Endocrinol Metab. 1994; 79: 1310-1316.
- Gann PH, Hennekens CH, Ma J, et Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst Bethesda. 1996; 88: 1118-1126.
- Drake I, Sonestedt E, Gullberg B, et al. Dietary intakes of carbohydrates in relation to prostate cancer risk a prospective study in the Malmö Diet and Cancer cohort. Am J Clin Nutr. 2012; 96: 1409-1418.
- Landberg R, Andersson SO, Zhang JX, et Rye whole grain and bran intake compared with refined wheat decreases urinary C-peptide plasma insulin and prostate specific antigen in men with prostate cancer. J Nutr. 2010; 140: 2180-2186.
- Leitzmann MF, Platz EA, Stampfer MJ, et Ejaculation frequency and subsequent risk of prostate cancer. JAMA. 2004; 291: 1578-1586.
- Jian Z, Ye D, Chen Y, et al. Sexual Activity and Risk of Prostate Cancer A Dose-Response Meta-Analysis. J Sex Med. 2018; 15: 1300-1309.