Sex Differences in HRV Under General Anesthesia in Rat Model
Author'(s): Pavol Švorc1,3, Darina PetráŠová2 and Pavol ŠvorcJr3
1Department of Physiology, Medical Faculty Safarik’s University,Kosice, Slovak Republic.
2Laboratory of Research Bio-models, Medical Faculty Safarik’s University, Kosice, Slovak Republic.
3Department of Physiology and Pathophysiology, Medical Faculty Ostrava University, Ostrava, Czech Republic.
*Correspondence:
Pavol Svorc, Assoc. Prof., Dr., PhD., Department of Physiology,Medical Faculty, Safarik’s University, Tr. SNP 1, 04001 Kosice,Slovak Republic, Tel: 00421907177340.
Received: 07 July 2020; Accepted: 04 August 2020
Citation: Pavol Švorc, Darina PetráŠová, Pavol ŠvorcJr. Sex Differences in HRV Under General Anesthesia in Rat Model. Anesth Pain Res. 2020; 4(2): 1-4.
Abstract
Background: It is known that general anesthesia weakens autonomic function and baroreflex control. Intravenous anesthetics may have different qualitative and quantitative effects on the peripheral autonomic nervous system (ANS) and, can thus, alter the activity of sympathetic or parasympathetic divisions of the ANS. Presently, there are relatively little data regarding sex differences in ANS activity or sex differences in ANS activities during anesthesia. The primary goal of the present study was to assess sex differences in ANS activity in dependence on the light-dark (LD) cycle in healthy, sexually mature, spontaneously breathing zoletil-anesthetized rats.
Methods: Experiments were performed using zoletil-anesthetized (30 mg/kg [intraperitoneal]) female and male Wistar rats after a four-week adaptation to an LD cycle (12h:12h). The animals were divided into four experimental groups according to sex and light period (n=20). Heart rate variability (HRV) was evaluated 20 min after administration of anesthesia.
Results: Sympathetic and baroreceptor activity were decreased, parasympathetic activity was increased in both sexes and in both lighted periods. LD differences were preserved mainly in the HF component; thus, circadian rhythm in parasympathetic activity probably persists in both sexes. Taking into account sex differences, HRV was significantly lower in females versus males in the light period. In the dark period, females exhibited higher HRV than males. Taking into account LD differences, in females, HRV was lower in the light versus the dark period, unlike males, in which HRV was higher in the dark versus light period of the rat regimen day.
Conclusions: It is concluded that the sex differences exist in rat ANS activity, persist in general anesthesia and depend on the LD cycle, what can be also related to its regulatory effect on the cardiovascular system. Future studies should address this issue and try to include females into the experiment if it is possible.
Keywords
Introduction
Small, periodic fluctuations in heart rate are well known to physicians and scientific investigators. Because these fluctuations are caused by varying activity of the ANS, an examination of heart rate variability (HRV) is needed to obtain information about the functional status of the ANS.
HRV analysis, which supports the evaluation of successive RR intervals using electrocardiographic methods, has been used as a powerful tool in the evaluation of autonomic cardiac control [1]. For example, in humans, reduced HRV is associated with an increased risk for ventricular arrhythmia and has been shown to be an independent prognostic mortality factor in patients with cardiac disease(s) [2,3]. On the other hand, some studies demonstrated that in rats, HRV spectral performance analysis is an ineffective method of detecting heart-related autonomic control disorders in some experimental models of myocardial infarction or diabetic neuropathy [4-6]. However, approaches that are based on electrocardiographic recordings in an anesthetic state are not ideal nor valid for HRV analysis due to significant heart rate fluctuations associated with impaired autonomic modulation of the heart [7,8]. In addition, anesthesia may contribute an important additional risk for animal mortality under some pathological conditions such as myocardial infarction and diabetes mellitus [9-11].
General anesthesia weakens autonomic function and baroreflex control. This side effect should be avoided as much as possible because it limits the ability of the subject to respond to physiological challenges during surgery (e.g., arterial pressure and decreases in chamber inotropy) [12]. Therefore, any research intervention that could affect aspects of the ANS and its impact on internal organs should take into account the anesthetic used. Intravenous anesthetics may have different qualitative and quantitative effects on the peripheral ANS and, can thus, alter the activity of sympathetic or parasympathetic divisions of the ANS.
Presently, however, there are relatively little data regarding sex differences in ANS activity or sex differences in ANS activities during anesthesia. During resting conditions, male rats exhibit a significantly higher heart rate and lower HRV parameters than female rats. This state is not only during the active, but also during the inactive, phase of the daily rat cycle [13].
These data support the concept that sex-based variations should also be taken into account given that females in human and animal studies use different mechanisms of cardiovascular regulation [14]. Although these data suggest that if there are sex differences in individual cardiovascular parameters, they are predominantly regulated by the ANS. Logically, therefore, if sex differences exist in cardiovascular activities, sex differences in the circadian oscillations of individual divisions of the ANS must also exist in parallel. However, supportive evidence of HRV changes in rats during a 24h period is lacking.
Zoletil anesthesia is used by veterinarians mainly for pets and wildlife animals but is not commonly used in experiments. It has been reported that this type of anesthesia is an appropriate anesthesia since it does not affect vital functions. The unanswered question is whether there are sex differences also in the dependence on the time of day at this type of anesthesia and whether the choice of this type of anesthesia is suitable for experiments with a rat model. Accordingly, the primary goal of the present study was to assess whether sex differences exist in ANS activity, measured according to HRV in dependence on the light-dark (LD) cycle (a parallel to circadian rhythm) in healthy, sexually mature, spontaneously breathing zoletil-anesthetized rats.
Animals
The present study conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (publication number 85-23, revised 1996). The study protocol was approved by the Ethics Committee of the Medical Faculty of Safarik University (Kosice, Slovak Republic) (permission number 2/05 and permission number ŠVPS SR: Ro- 4234/15-221).
The experiments were performed using albino Wistar rats (weight, 340 ± 40 g, three to four months of age) acquired from a breeding and vendor company (VELAZ, KoleÄ, Czech Republic, certificate number 70029/2013-MZE-17214), with the veterinary registration number CZ 21760118. The animals were quarantined for 2 weeks in the Laboratory of Research Biomodels of Medical Faculty Safarik’s University in KoŠice (official number SK UCH 08018) and adapted to an LD cycle (12h light:12h dark [intensity of artificial illumination 80 Lux]; 40% to 60% humidity; cage temperature, 24°C; two animals/plastic cage for four weeks. The rats were fed a standard, pelleted diet, with ad libitum access to food and water. Animal handling was performed by professional staff of the menagerie.
Anesthesia (Zoletil, 30 mg/kg, Virbac, France) in prescribed doses based on the weight of the animal was administered by intraperitoneal injection in the adaptation room. After testing the effect of anesthesia (loss of uprighting reflexes, reaction to painful stimulus), the animals were transferred to the operating room where they were fixed to an experimental table on which the subcutaneous electrodes used record ECG and HRV were applied. Again, the depth of anesthesia was assessed, depending on whether the painful stimulus caused noticeable motor movements (minimal limb movement, muscle tension change), or cardiovascular responses, such as change in heart rate or onset of heart rhythm disorders.
Materials and Methods
The effect of the light period on the monitored parameters was examined after adaptation to an LD cycle, with the light period from 06:00 h to 18:00 h. The effect of the dark period was monitored after adaptation to the inverse setting of the LD cycle (i.e., with the light period from 18:00 h to 06:00 h). Animals were divided randomly into four experimental groups (n = 20 each) according to sex and light conditions: group 1: female (light period); group 2: female (dark period); group 3: male (light period); and group 4: male (dark period). In in vivo experiments, at least 20 animals are valid sample for statistical processing.
HRV was analyzed using the ID Instruments computer system for biopotential recording from an average of 220 heart cycles, 20 min after administration of anesthesia at 09:00 and 12:00h using separate animals. In analyzing HRV parameters, the focus was on the evaluation of RR interval duration, spectral power at very-low frequency (VLF 0.003 Hz - 0.04 Hz), low-frequency (LF 0.04 Hz - 0.15 Hz) and high-frequency (HF 0.15 Hz - 0.4 Hz), total spectral power of HRV and the LF/HF ratio. Upon completion of the measurement, the animals were transferred to the menagerie.
Data are expressed as mean ± standard deviation (SD). Data were analyzed using GraphPad InStat (GraphPad, San Diego, CA, USA). The Tukey-Kramer test was used to identify significant differences between the groups, and p < 0.05 was considered to be statistically significant. The experiments were conducted throughout the year and the results were averaged independently of the season and, in females, independently of estral cycle. Animals included in statistical analysis was 20/20 and no animal was excluded from the statistical analysis. Before and after administration of the anesthetic as well as during the measurement, there were no adverse events or unexpected changes in the HRV or ECG parameters, although considerable variability has been observed.
Results
RR interval
The duration of the RR interval corresponds to increased heart rate in both sexes in both lighted periods of the regimen day. In females, LD differences were not observed in the duration of the RR interval (light 142.30 ± 25.19 ms vs. dark 134.97 ± 9.09 ms), in contrast to males, in which a significantly (p < 0.001) longer RR interval was recorded during the light part of the day (light 145.05± 8.51 ms vs. dark 124.68 ± 5.14 ms). Sex differences were found only in the dark (i.e., active) part of the day (Figure 1).
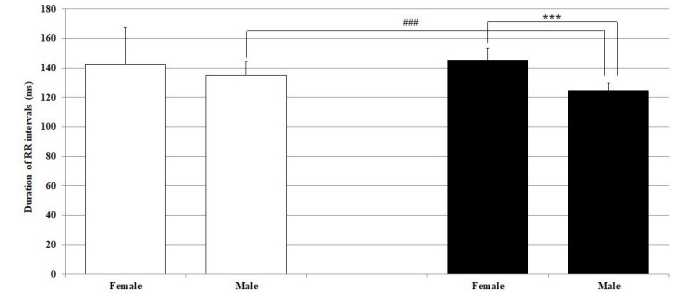
Figure 1: Duration of RR intervals under general zoletil anesthesia 20 min after zoletil administration in male and female Wistar rats in the light and dark parts of the regimen day. Hollow bars represent the light and the solid bars represent the dark part of the rat regimen day. ***p<0.001 statistically significant sex differences; ###p <0.001 statistically significant light-dark differences in the same sex.
HRV analysis
Despite the large variation in HRV spectral powers under zoletil anesthesia, in terms of sex differences, parasympathetic activity dominated in both sexes and in both light periods with nonsignificant higher sympathetic (VLF), baroreceptor (LF) activities and significant (p < 0.001) parasympathetic (HF) activity in females (Tables 1 and 2).
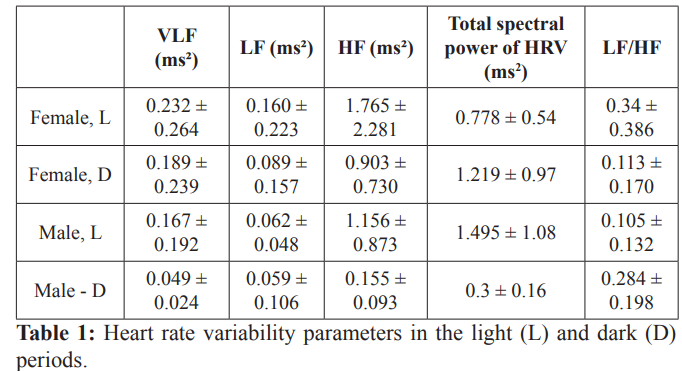
Data presented as mean ± standard deviation. VLF, very-low frequency; LF, low frequency; HF, high frequency.
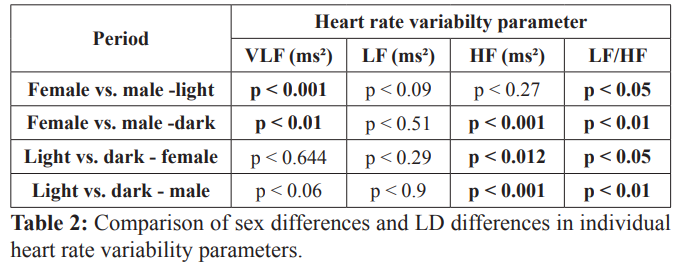
Data presented as p value. Bolded values indicate statistically significant differences. VLF, very-low frequency; LF, low frequency; HF, high frequency.
Significant sex differences in dependence on the LD cycle were recorded in total spectral power of HRV. In the light period in females, it was significantly lower than in males, and vice versa in males in the dark period of the regimen day (Figure 2). The opposite tendency was found in the LF/HF ratio, with dominance of parasympathetic activity in both light periods of the day (Figure 3).
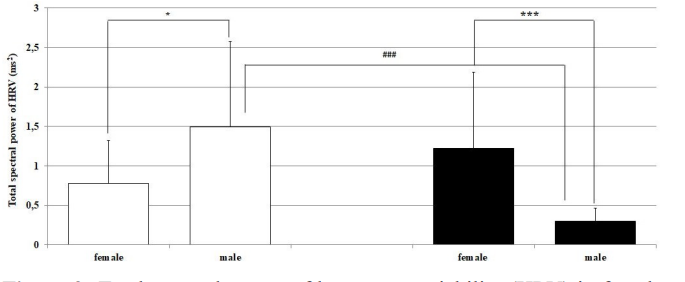
Figure 2: Total spectral power of heart rate variability (HRV) in female and male Wistar rats in the light and dark period of the regimen day. Hollow bars represent the light and solid bars represent the dark period of the regimen day. *p < 0.05; ***p < 0.001 statistically significant differences between sexes in both light periods of the rat regimen day; ###p < 0.001 statistically significant light-dark differences in the same sex.
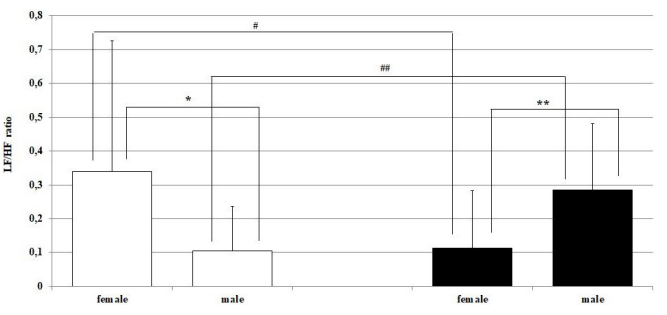
Figure 3: Low frequency (LF)/high frequency (HF) ratio in female and in male Wistar rats in the light and in the dark periods of the regimen day. Hollow bars represent the light and the solid bars represent the dark period of the rat regimen day. *p < 0.05; **p < 0.01 statistically significant differences between the sexes in both light periods of the rat regimen day, ##p < 0.01 statistically significant light-dark differences in the same sex.
Discussion
The limitations of the study were not whereas it was evaluation of the current status in ANS activity in healthy animals under anesthesia. The imprecisions in presentation of the results are primarily associated with relatively large variability of the measured parameters of HRV.
In rats, sympathetic activity dominates their normal life cycle [6,15]. Zoletil anesthesia increases parasympathetic activity in both sexes. Similar results have been reported in previous studies. Administration of the anesthetic agent tribromoethanol in male Wistar rats [16], ketamine hydrochloride and diazepam in albino Wistar rats [17], and ketamine/xylazine and pentobarbital in females [18] resulted in predominant parasympathetic activity. However, our results indicate that precisely defining changes in HRV is difficult due to significant variability which, therefore, makes it difficult to attribute sex differences. Thus, we agree with the opinion mentioned in the introduction that approaches based on ECG recording under general anesthesia are not fully valid for HRV analysis.
Although there is significant variation of HRV in Wistar rats, some parameters point to sex differences also in dependence on the LD cycle. If we consider the duration of RR intervals (adequate to heart rate) 20 min after zoletil administration, significant sex differences were found only in the dark (i.e., active) part of the day, with significantly shorter RR intervals (higher heart rate) in males. During the light period of the rat regimen day, sex differences in the duration of RR intervals were not detected. On the other hand, significant LD differences were maintained in males; however, they were eliminated in females. Our results, therefore, indicate that in zoletil-anesthetized rats, LD differences were maintained only in males but not in females. Considering the results of telemetric studies by Molcan et al. [19,20], heart rate exhibits a significant circadian rhythm in non-anesthetized rats, in which the heart rate in the dark period fluctuates from 347 beats/min to 363 beats/min, and from 309 beats/min to 321 beats/min in the light period. Thus, it appears that although zoletil exerts a tachycardic effect, it can eliminate-or, at least modify-circadian rhythm of heart rate, but only in females.
Such elimination or modification of LD differences in heart rate in females may also be partly explained by perhaps greater sensitivity of females to acidosis, hypoxia, and hypercapnia under general anesthesia [21]. Previous studies have described the effect of hypoxia on the modulation of daily rhythmicity [22-26]. The fact that hypoxia modifies circadian oscillations of important variables, such as body temperature and metabolism, can lead to the expectation that the rhythms of many functions are interrupted by hypoxia on the basis of their relationship with the primary variables. Such a hypoxic relationship probably contributes to a greater parasympathetic effect(s) on the heart [27]. Additionally, the effect of anesthetics can contribute to the loss or modification of rhythmicity. For example, in female rats under pentobarbital anesthesia, parasympathetic activity increases and sympathetic and baroreflex activity decreases; however, LD differences in heart rate are eliminated. In ketamine/xylazine anesthesia, a preference toward parasympathetic activity was increased and sympathetic and baroreflex activity was depressed, resulting in significant bradycardia but with maintenance of LD differences [18].
The question remains that if on the one hand, there is clearly proven increased parasympathetic activity and, on the other, increased heart rate. This paradox has been described by several authors [28-32], who assumed that stimulation of the vagal nerve releases catecholamines, which in turn can affect heart activity. This is also probably the case with zoletil anesthesia, which may have a similar effect on the release of catecholamines through higher parasympathetic activity, and is particularly evident in males in both light periods of the regimen day.
The LF to HF ratio (i.e., LF/HF) can be used to quantify the changing relationship between sympathetic and parasympathetic nerve activities (i.e., sympathetic-vagal balance) [33-35]. The exact interpretation of the LF/HF ratio also depends on the assumption that physiological interventions always cause mutual changes in parasympathetic and sympathetic activity.
Our results demonstrate that the LF/HF ratio depends on the light periods of the regimen day. In females in the light period, the LF/HF ratio was significantly higher and, in the dark period, significantly lower than in males. These conclusions, however, should be interpreted with caution. In the article by Billman [36], addressing the meaning of HRV examination, evaluation of the LF/HF ratio was questioned. The LF component of HRV does not provide a cardiac sympathetic response index, but rather reflects a complex and not readily recognizable mixture of sympathetic, parasympathetic and other unidentified factors with parasympathetic factors, which represent the largest part of variability in this frequency range. As a result, it is difficult to recognize the physiological basis for LF/ HF. In addition, a relatively large amount of data suggests that the spectral power of the HF component cannot be attributed solely to changes in cardiac vagal efferentation, further compromising the accurate interpretation of the LF/HF ratio [36].
In in vivo experiments, homeostatic regulatory mechanisms are not eliminated. It means that the obtained results are a reflection a direct but significantly intravariable response of the animals to the administration of the anesthetic. At evaluation of HRV in in vivo conditions, replacement and reduction of animals is not possible, but knowledge about the sex differences during anesthesia in the dependence on LD cycle in ANS activity may improve the quality of the experiment design. We do not have any data about sex differences and we do not have any data about changes of ANS activity depending on the LD cycle under general anesthesia. Further research is needed to assess the responses of other species, because the effect of zoletil is practically not described in experimental practice.
Conclusion
Based on our results, we conclude that under zoletil anesthesia, sympathetic (VLF) and baroreceptor (LF) activity were decreased, and parasympathetic (HF) activity was increased in both sexes and in both light periods. LD differences were preserved mainly in the HF component; thus, circadian rhythm in parasympathetic activity probably also persists in both sexes.
Taking into account sex differences based on total spectral power of HRV, our results suggest that HRV, in the light period of the rat regimen day, was significantly lower in females versus males. In the dark period, females exhibited higher HRV than males. Taking into account LD differences, in females, HRV was lower in the light versus the dark period, unlike males, in which HRV was higher in the dark versus light period of the rat regimen day.
Acknowledgment
This work was supported by VEGA [grant number 1/0423/11].
References
1.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat- to-beat cardiovascular control. Science. 1981; 213:220-222.
2.Kleiger RE, Miller JP, Bigger JT Jr, et al. Decreased heart- rate-variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987; 59: 256–262.
3.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure– Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-Heart). Circulation. 1998; 98: 1510–1516.
4.Krüger C, Landerer V, Zugck C, et al. The bradycardic agent zatebradine enhances baroreflex sensitivity and heart rate variability in rats early after myocardial infarction. Cardiovasc Res. 2000; 45: 900–912.
5.Sanyal SN, Arita M, Ono K. Inhomogeneous derangement of cardiac autonomic nerve control in diabetic rats. Circ J. 2002; 66: 283–288.
6.Pereira-Junior PP, Marocolo M, Rodrigues FP, et al. Noninvasive method for electrocardiogram recording in conscious rats: feasibility for heart rate variability analysis. An Acad Bras Cienc. 2010; 82: 431-437.
7.Uechi M, Asai K, Osaka M, et al. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac G(s alpha). Circ Res. 1998; 82: 416–423.
8.Mäenpää M, Penttilä J, Laitio T, et al. The effects of surgical levels of sevoflurane and propofol anaesthesia on heart rate variability. Eur J Anaesthesiol. 2007; 24: 626–633.
9.Tivesten A, Bollano E, Caidahl K, et al. The growth hormone secretagogue hexarelin improves cardiac function in rats after experimental myocardial infarction. Endocrinology. 2000; 141: 60–66.
10.Flumignan RL, Kanashiro RM, Saraiva RM, et al. Incidence of heart failure in infarcted rats that die spontaneously. Braz J Med Biol Res. 2006; 39: 1323–1328.
11.Cohen-Boulakia F, Valensi PE, Boulahdour H, et al. In vivo sequential study of skeletal muscle capillary permeability in diabetic rats: Effect of anthocyanosides. Metabolism. 2000; 49: 880–885.
12.Guzzetti S, Marchi A, Bassani T, et al. Univariate and bivariate symbolic analyses of cardiovascular variability differentiate general anesthesia procedures. Physiol Meas. 2015; 36: 715-726.
13.Koresh O, Kaplan Z, Zohar J, et al. Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behav Brain Res. 2016; 308: 128-142.
14.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension 2006; 47: 1019–1026.
15.Shi SB, Liu T, Wang DD, et al. Activation of N-methyl-D- aspartate receptors reduces heart rate variability and facilitates atrial fibrillation in rats. Europace. 2017; 19: 1237–1243.
16.Damasceno DD, Lima MP, Motta DF, et al. Cardiovascular and eletrocardiographic parameters after tonin administration in Wistar rats. Regulatory Peptides. 2013; 181: 30-36.
17.Yadav RK, Rawat JK, Gautam S, et al. Antidiabetic activity of mefloquine via GLP-1 receptor modulation against STZ-NA- induced diabetes in albino wistar rats. J Biotech. 2018; 8: 240.
18.Svorc P Jr, BaÄová I, Švorc P, et al. Autonomic nervous system under ketamine/xylazine and pentobarbital anaesthesia in a Wistar rat model: A chronobiological view. Prague Med Rep. 2013; 114: 72-80.
19.Molcan L, Teplan M, Vesela A, et al. The long-term effects of phase advance shifts of photoperiod on cardiovascular parameters as measured by radiotelemetry in rats. Physiol Meas. 2013; 34: 1623-1632.
20.Molcan L, Vesela A, Zeman M. Repeated phase shifts in the lighting regimen change the blood pressure response to norepinephrine stimulation in rats. Physiol Res. 2014; 63: 567-575.
21.Svorc P, Petrášová D, SvorcJr P. Arterial pH and blood gas values in rats under three types of general anesthesia: a Chronobiological study. Physiol Res. 2018; 67: 721-728.
22.Mortola JP, Seifert EL. Hypoxic depression of circadian rhythms in adult rats. J Appl Physiol. 2000; 88: 365–368.
23.Bishop B, Silva G, Krasney J, et al. Ambient temperature modulates hypoxic-induced changes in rat body temperature and activity differentially. Amer J Physiol. 2001; 280: 1190– 1196.
24.Bosco G, Ionadi A, Panico S, et al. Effects of hypoxia on the circadian patterns in men. High Alt Med Bio. 2003; 4: 305– 318.
25.Kaplan JL, Gao E, Garavilla L, et al. Adenosine A1 antagonism attenuates atropine-resistant hypoxic bradycardia in rats. Acad Emerg Med. 2003; 10: 923–930.
26.Mortola JP. Hypoxia and circadian patterns. Respir Physiol Neurobiol. 2007; 158: 274–279.
27.Hayashida Y, Hirakawa H, Nakamura T, et al. Chemoreceptors in autonomic responses to hypoxia in conscious rats. In: Zapata P, Eyzaguirre C, Torrance RW (eds) Frontiers in arterial chemoreception. New York: Adv Exp Med Biol. 1996; 410: 439–442.
28.Picker O, Scheeren TWL, Arndt JO. Inhalation anaesthetics increase heart rate by decreasing cardiac vagal activity in dogs. Br J Anaest. 2001; 87: 748-754.
29.Hoffmann TJ, Simon BJ, Zhang Y, et al. Low voltage vagal nerve stimulation reduces bronchoconstriction in guinea pigs through catecholamine release. Neuromodulation. 2012; 15:527-536.
30.Miner JR, Lewis LM, Mosnaim GS, et al. Feasibility of percutaneous vagus nerve stimulation for the treatment of acute asthma exacerbations. Acad Emerg Med. 2012; 19: 421- 429.
31.Steyn E, Mohamed Z, Husselman C. Non-invasive vagus nerve stimulation for the treatment of acute asthma exacerbations- results from an initial case series. Int J Emerg Med. 2013; 6: 7.
32.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part III. Headache. 2016; 56: 479-490.
33.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interactions in man and conscious dog. Circ Res. 1986; 59: 178–193.
34.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral density of heart rate variability as an index of symptho-vagal interactions in normal and hypertensive subjects. J Hypertens. 1984; 2: 383–385.
35.Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991; 84: 482–492.
36.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013; 4: 26.