Sexual Dysfunction Among Female Patients with Breast Cancer Attending Out Patient Clinic in Khartoum Center of Radiation and Isotope (2018)
Author'(s): Dr. Azza Mustafa Mohamed Eljaali1,2, Dr. Abdalla Abdelrahman3 and Dr Mohammed Ibrahim4,5*
1Taha Basher Teaching Psychiatric Hospital, Khartoum North,Sudan.
2Hayat Center for Treatment and Psychological Rehabilitation,Khartoum, Sudan.
3University of Khartoum Faculty of Medicine, Department of Psychiatry.
4Erada Complex for Mental Health, Tabuk, Saudi Arabia
5Sudanese Medical Research Association, Sudan.
*Correspondence:
Dr. Mohammed Ibrahim, Erada Complex for Mental Health, Tabuk, Saudi Arabia, Sudanese Medical Research Association, Sudan.
Received: 24 April 2021; Accepted: 20 May 2021
Citation: Eljaali AMM, Abdelrahman A, Ibrahim M. Sexual Dysfunction Among Female Patients with Breast Cancer Attending Out Patient Clinic in Khartoum Center of Radiation and Isotope (2018). Cancer Sci Res. 2021; 4(2): 1-9.
Abstract
Background: Breast cancer can bring about multiple physical and psychological challenges. Among the greatest challenges, are those associated with female sexual function?
Objectives: This study was designed to study sexual dysfunction among female patients with breast cancer attending outpatient clinic in Khartoum center of radiation and isotope Sudan in2018.
Method: A cross sectional study was performed on 180 female patients with breast cancer attending outpatient clinic in Khartoum center of radiation and isotope at Khartoum state during 2018. The Data were collected using questionnaire forms then data were analyzed using statistical package for social science (SPSS) v 24.0.
Result: In this study 57.2% were in the age group (31-45) years, 75.6% were homemakers, 35.6% had secondary education level. The majority of women had 11-15 years of marriage and 1-5 years of disease, 92.2% were used chemical treatment, 60.6% used surgery, 42.4% used radiation and only 18.3% used hormones. This study revealed that, 41.2% of women had lost sexual interest and desire, 34.5% lost sexual pleasure 22.8% had sexual aversion, 29.7% lost excitement, 25% vaginismus and dyspareunia, 13.4% problem in Sexual performance, 12.5% orgasm dysfunction , 28.8%reduce Sexual satisfaction, 18.9% can't reach Resolution.
Conclusion: The study concluded that sexual dysfunctions in women with breast cancer is high, there was correlation between sexual dysfunction with age, duration of disease and treatment methods
Keywords
Introduction
In the world, breast cancer is the most common cancer in women, regardless of race or ethnicity. Fortunately, more women are surviving their diagnosis, likely as the combined result of earlier detection and better therapies. As a result, over three million women have a history of breast cancer, making them the largest proportion of cancer survivors in the United States alone (41% of the entire population of cancer survivors) [1]. Female sexual function encompasses more than just arousal and orgasm; it is informed by multiple domains and other factors. These include desire, arousal, lubrication, orgasm, satisfaction and pain, body image, psychological health, and sensuality.
Several models of female sexual function have been characterized. Basson et al. attempted to incorporate the importance of psychological and mental health into the concept of female sexuality by proposing that intimacy, desire, arousal, orgasm, and satisfaction were all part of a propagating circle-separate, inter- related, and all equally important [2]. Manne and Badr discussed sexuality as a multi-compartmental model that governed intimacy and sexuality, particularly as it related to women in relationships [3]. Finally, Perelman described sexual response in terms of a “tipping point”, from which a self-designed threshold is set and once reached, elicits a sexual response [4]. Even in this model, the tipping point is subject to influences by both psychosocial and physical factors. All of these models highlight the importance of thinking of sexual health as a global concept, not one that is synonymous solely with sexual activity. While there is no consensus as to which one is universally applicable to women treated for breast cancer, patients at our center are educated about the interplay between intimacy, stimuli, and satisfaction using the Basson model.
The fifth edition of the Diagnostic and Statistical Manual now characterizes sexual health as three entities: disorders of sexual interest and arousal; difficulty with orgasm; and disorders associated with genito-pelvic pain/penetration [5]. However, it is important to note that more than one area of concern may be present given the interconnectedness of the domains that encompass female sexual health. More importantly, the diagnosis of any of these must meet criteria that mark them as not just a “nuisance” but also a disorder. This includes presence of symptoms for at least six months; self-reported and clinically significant distress; and not otherwise explained by a non-sexual condition (including other medical condition or secondary to substance or medication use); severe relationship distress; or other stressors.
It is important to note that changes in sexual health can be related to changes in either the patient or her partner (or both), whether it be related to changes in cosmesis, sensuality, or function. The end result may be a lack of engagement, partly as a result of a heightened sense of the risk of rejection. Fortunately, the available data suggest that of patients who are married, the majority remain stable after a diagnosis of breast cancer [6].
Impact of treatment on sexual function
Surgery
The treatment of breast cancer necessitates surgery of the breast and axilla, which can result in long-term issues both physically and mentally. In a recent prospective study of women with early- stage breast cancer, postoperative sexual function was notably worse when compared to their baseline pre-operative scores [7]. This was predominantly due to issues related to arousal following breast conserving surgery while desire, arousal, and difficulty with orgasm were reported by those who underwent mastectomy. However, when scores were compared to age-matched healthy controls, only those women who had undergone mastectomy had significantly more problems related to sexual dysfunction.
Radiation therapy (RT)
For most women treated with breast conserving surgery and those with high-risk features that warranted mastectomy (including those in whom neoadjuvant chemotherapy was chosen in lieu of primary surgery), RT is an essential part of therapy. However, RT can result in locoregional issues, including persistent breast pain, arm and shoulder discomfort and loss of flexibility, and lymphedema, and any of them are associated with reduction in sexual function [8-11]. Despite these findings, teasing out the exact contribution of RT to overall sexual health in breast cancer survivors is difficult, given that it is almost always a part of an interdisciplinary cancer treatment program involving both surgery and medical therapies, which have been both more closely tied to issues related to sexual health and function in women.
Chemotherapy
Although there is a major push towards personalized medicine, a large proportion of patients with breast cancer will undergo adjuvant chemotherapy, which for women who were not yet menopausal includes a risk of chemotherapy-induced ovarian failure and onset of an early menopause, which may include issues related to sexual dysfunction. In addition, agents such as the anthracyclines and taxanes can negatively affect global physical function, reducing interest, arousal, and desire. This includes common physical toxicities resulting from chemotherapy include fatigue, alopecia, gastrointestinaldistress, and myelosuppression.
While most women will experience improvement once chemotherapy ends, for others, the symptoms persist beyond end of treatment. Sexual functioning, desire, arousal, and quality of partnered relationships were shown to decline after chemotherapy when compared to baseline, and these changes persisted out to one year. Of note, these changes were not associated with worsening sense of body image. In a separate cross-sectional study that included 534 women, (69% of whom had received chemotherapy), chemotherapy was associated with both depression and unmet sexual needs [12]. These findings were predominantly reported acutely (<1 year from end of treatment) but appeared to recur later (>3 years out from treatment).
Endocrine therapy
The options for postmenopausal women with hormone receptor- positive breast cancers include an aromatase inhibitor (AI) for 5 years or tamoxifen for 5 years followed by 5 additional years of either tamoxifen or an AI [13]. Although premenopausal women were not considered candidates for an AI (which require non- functional ovaries), the option of ovarian suppression plus an AI is now an alternative option to tamoxifen (plus or minus ovarian suppression) [14].
While an important aspect of treatment in these patients, sexual health is often and negatively impacted. Estimates vary, but approximately 30% to 40% of women treated with tamoxifen report sexual complaints, while over 50% of those on an AI report issues related to sexual health. In addition to primary impacts on sexuality, these agents are tied to the onset or worsening of menopausal type symptoms.
Addressing sexual function:
The approach to sexual health in breast cancer survivors begins with a comprehensive history, including an understanding of the patient’s active medical problems and the medications currently being prescribed. This is important because sexual dysfunction can be a consequence of long-standing comorbidities (e.g., diabetes mellitus) and/or an iatrogenic result of medications (e.g.,beta- blockers) [15].
In addition, discussing sexuality necessitates that doctor-patient relationships build on three pillars: open communication; medical understanding; and education [16]. We encourage all clinicians to query patients as to their sexual history as part of the routine assessment of new patients. Doing so can provide the patient with comfort in knowing that sexual health is not “taboo” with their providers, and may open them up to communicating issues as they might arise.
Several methods are available to discuss sexual health, including the PLISSIT model of permission, limited information, specific suggestions, and intensive therapy. However, other ways to engage with patients have been published. Park and colleagues proposed a model of communication based on five A’s—Ask, Advise, Assess, Assist, and Arrange—to discuss and evaluate sexual health [17]. Notably, they propose that not all of these tasks require a sole provider to perform; rather, they propose that these issues be done as part of an interdisciplinary effort whenever possible.
For women who do bring up issues related to sexual function, validated questionnaires have proven useful in our practice to not only gage the severity of symptoms, but also to establish a baseline to help gage the impact of any subsequent interventions. While many are available, there is no one “gold standard” tool [18].
Of available tools, we screen for sexual issues using the FSFI at each visit. We also screen for depression and general quality of life with additional questionnaires. In practice, gaging for both global quality of life and the presence of depressive symptoms can help contextualize sexual health concerns and determine whether other experts may be of benefit to the individual patient. For example, a patient that high score for depression may benefit from referral to psychiatric or psychological support, especially since depression and sexual dysfunction are among the most common symptoms affecting breast cancer survivors and often overlap [19].
The approach to sexual health also requires specific evaluations of intimacy, as it is an inherent part of sexuality and often overlooked.
Intimacy can be described as the emotional connection or closeness, which can occur even in the absence of sexual activity (or the “coital imperative”). The importance of intimacy was underscored by work done by Perz et al. in which over 40 patients, their partners, and their providers were queried [20]. They confirmed that as clinicians, we often view sexual health concerns in the physical domain. However, patients and their partners emphasized that intimacy in its own right was important and that for some, even in the absence of a physical and sexual relationship, intimacy was not only important, it was highly valued.
For many women, sexual dysfunction is a symptom of menopause and its associated physical changes in the vulvar and vaginal areas. Atrophy and dryness are common and can make sexual encounters painful, resulting in both dyspareunia and loss of desire. Although the most effective treatment is estrogen replacement therapy (ERT), it is often not the first choice for treatment, particularly for the woman with an intact uterus and those who are on antiestrogen therapies. Alternatives to oral ERT include vaginal specific preparations, such as vaginal estrogen tablets or creams and the use of testosterone (which can be supplied as intradermal or an intravaginal preparation). However, the data on either of these are limited.
Beyond what has been reported in the contemporary literature, education and mind-body therapies can play a significant role in overcoming sexual dysfunction. Whether this is by providing much needed education on her anatomy, explanations on the complexity of the female sexual response cycle, or validation that sexual side effects are not only real but also common, such information (in our experience) can bring about a sense of relief and validation to patients.
In one study that included 83 breast cancer survivors (all of whom were 3 or more years after diagnosis) surveyed using the female sexual function index (FSFI) and the female sexual distress scale-revised (FSDS-R), 77% qualified for the diagnosis of sexual dysfunction on the FSFI alone [21] .
In another study by Ganz and colleagues, over 760 women who had participated in an earlier survey responded to a follow- up questionnaire, with an average time between survey points of 6.3 years. This study revealed that while physical and emotional functioning had normalized, sexual function remained compromised with a reduction in sexual activity with their partner compared to the initial survey (65% to 55%) and persistent vaginal dryness, and urinary incontinence [22].
The importance of sexual health in cancer survivorship was shown in the 2010 survey conducted by LiveStrong. In that survey that included over 3,000 people (24% with breast cancer, 41% one to five years post-treatment), sexual functioning and satisfaction were ranked the third most frequently reported concern. Despite this, less than half had received medical care. More importantly, sexual concerns resulted in significant emotional distress, including sadness/depression, issues related to personal appearance, stigma, and negative impacts on personal relationships [23].
In a recent survey of over 600 patients who underwent implant breast reconstruction, 219 of whom had prior RT, those who had RT reported lower satisfaction and health-related quality of life compared to those who did not undergo RT. These deficits included worse scores in sexual well-being and breast satisfaction [9].
In one study, 35 women completed a battery of questionnaires after surgery, after chemotherapy (or at least 6 months of endocrine therapy), and then at one year [24].
In a survey of over 180 women, Morales et al. showed that both agents significantly increased both the occurrence and the severity of hot flashes. While AIs significantly increased the risk of dyspareunia, tamoxifen significantly reduced sexual interest, showing that despite their different profiles, both agents can negatively impact female sexual health. More importantly, they reported that women at younger ages were significantly more likely to experience toxicities [25].
That AIs cause more sexual (and vaginal) side effects was shown in a study by Baumgart and colleagues. In this study, patients without a history of cancer who were either currently (n=54) or not currently taking estrogen (n=48) were compared to women taking tamoxifen (with or without estrogen, n=47) and those taking an AI (with or without estrogen, n=35). Although the proportion of women reporting they were sexually active was similar (60% to 65% across all groups), Women on an AI had significantly lower sexual interest than the two control groups taking estrogen (40% vs. 60%, P<0.05-0.001) or not taking estrogen (40% vs. 68%, P<0.05-0.01). Women on an AI also had significantly greater issues with other aspects of sexual function, such as insufficient lubrication (74%) and dyspareunia (57%), and reported general dissatisfaction with their sex lives (42%). For women on tamoxifen, the corresponding figures were 40%, 31%, and 18%, respectively. Of note, orgasmic dysfunction was not statistically different among women on an AI or tamoxifen (50% vs. 42%), though was higher when compared to controls taking or not taking estrogen (31% and 37%, respectively) [26].
Similar results were obtained in a survey of 129 women conducted by Schover et al. that queried the sexual health of women taking an AI during the first two years of therapy. Although the vast majority were adherent to medications, 93% met criteria for sexual dysfunction on the FSFI and 75% reported distress as a result. Of concern, almost a quarter of those women who were sexually active at the initiation of therapy ceased having sex with their partner as a result [27].
In a recent review, Halley et al. suggested there were at least three barriers to further discussion and treatment of sexual health after breast cancer. First, issues related to sexual function as brought up by patients were often restricted to the physical domain by their respective providers, rather than being acknowledged as a more complicated and global issue. Second, patients often were unsure where to turn to discuss these issues, which was particularly problematic for those receiving care in designated cancer-specific centers. Third, access to services during treatment was often not available due to a disconnection between cancer treatment and other medical care. As a result, sexual dysfunction is often an unmet medical complaint [28].
In a 2013 systematic review, Bartula and Sherman identified three tools that appeared to be most acceptable in terms of their psychometric properties: the FSFI, Arizona Sexual Experience Scale (ASES), and the sexual problem scale (SPS) [30].
A phase I/II study of topical testosterone (150 to 300-mcg cream applied intravaginal) was evaluated among 21 breast cancer survivors with reports that treatment improved both dyspareunia and vaginal dryness [29].
A randomized trial of transdermal testosterone preparation versus placebo in predominantly breast cancer survivors was conducted by the North Central Cancer Treatment Group and showed testosterone showed no improvement in sexual function compared to placebo [30].
Whether vaginal estrogen therapy is safe remains controversial. However, at least one study suggests there is no associated risk for recurrence. In this observational study that utilized the United Kingdom General Practice Research database, women with recurrent breast cancer on endocrine therapy were evaluated for the risk of recurrence based on whether or not they had been additionally prescribed vaginal estrogen therapy. Their population consisted of women treated with tamoxifen (n=10,806), AIs (n=2,673) and also those who had been treated with vaginal estrogen therapy (n=271). They did not find an association between vaginal estrogen therapy and recurrence risk (RR 0.78, 95% CI, 0.48-1.25) [31].
Another hormonal intervention evaluated in a randomized clinical trial is dehydroepiandrosterone (DHEA), which was compared to placebo in the Alliance Cooperative Group N10C1 clinical trial. In this trial, 441 women were randomly assigned to DHEA administered as a vaginal preparation (at 3.25 or 6.5 mg doses) compounded in a bio adhesive vaginal moisturizer or to the bio adhesive moisturizer alone. As presented, all women had improvements from their baseline regardless of treatment arm. However, compared to no DHEA, women receiving DHEA had statistically significant improvements in sexual function at 12 weeks based on their FSFI scores. The most common side effects associated with DHEA included headaches and voice changes [32].
Non-hormonal interventions have been proven to be effective in this population, especially the use of vaginal moisturizers. In one study of 86 patients, a 12-week course of a vaginal pH-balanced gel was compared to a placebo preparation [33].
Compared to placebo, the vaginal gel significantly reduced vaginal pH and enhanced vaginal maturation. In addition, treatment improved both vaginal dryness and dyspareunia scores. Similar results were obtained in a study of a polycarbophil-based vaginal moisturizer versus placebo among 45 women with breast cancer. There was trend towards improved dyspareunia scores with the vaginal moisturizer, although notably, both preparations were associated with improvements in vaginal dryness (62% and 64% in the placebo and moisturizer groups, respectively) [34].
For some patients who experience pain with penetrative intercourse, one potential therapy is aqueous lidocaine, particularly if the site of pain is located at the vulvar vestibule. This was shown in a trial conducted by Goetsch et al. that randomly assigned 49 postmenopausal patients with a history of breast cancer to an aqueous 4% lidocaine preparation or normal saline, applied for 3 minutes to tender areas before activity [35].
Aqueous lidocaine had a significant impact on worst pain score (from median of 5 to 0) whereas normal saline had only minimal relief (median 6 to 4). In this trial, side effects were minimal and of note, the partners of patients on this trial had no complaints, and none complained of numbness. Whether this treatment will be effective in patients with evidence of pelvic floor muscle dysfunction or vaginismus is unknown. Still, in a separate report,37 of 41 women who were treated with open-label lidocaine solution reported comfortable penetration and 17 of 20 women who had abstained from sex had resumed penetrative intercourse [36].
For women who experience pain with penetration not limited to the vestibule, treatment with lubricants and vaginal dilators is indicated. In contrast to moisturizers, lubricants do not change the vaginal pH and environment, but does address issues related to vaginal dryness that can make sexual contact uncomfortable. Although both water-based and silicone-based lubricants are available, there are no data to inform whether one is more useful in breast cancer survivors. Therefore, the choice is often individual and guided by the end results. Vaginal dilators are useful to help overcome pelvic floor muscle responses. While there are no data to inform the efficacy of treatment in a randomized trial setting in this population, one non-randomized study that included 15 women with vestibulodynia showed that treatment significantly improved dyspareunia scores using validated questionnaires, including the FSFI [37].
Beyond medications, some data suggest that vaginal exercise as part of a multimodal treatment plan can be effective. In the Oliver Oil, Vaginal Exercise, and Moisturizer (Overcome) trial, 25 women with dyspareunia were instructed to perform pelvic floor muscle exercises twice daily, use a polycarbophil vagina moisturizer three times per week, and use olive oil as a lubricant during sex. While not compared to a control arm, the treatment regimen resulted in significant improvements in dyspareunia scores, sexual function, and quality of life. Of those enrolled, 92%, 88%, and 73% of patients rated the pelvic floor exercises, vaginal moisturizer, and olive oil as beneficial, respectively. These data suggest that multimodal treatments play a role in sexual health recovery. Further studies should be performed to determine the benefits of these types of programs in women after cancer [38].
In addition, some data show that more formal programs in cognitive behavioral therapy (CBT) can provide relief. In one study, 422 patients were randomly assigned to CBT, CBT plus physical exercise, or were assigned to a waiting list (control group). CBT consisted of 6 weekly group sessions (focused on control of hot flashes and night sweats, as well as issues related to body image, sexuality, and mood) that also included relaxation exercises. The physical exercise component was 12-weeks long and individualized for patients to be active for 2.5 to 3 hours per week. Those patients who participated on the CBT plus physical exercise group had a significant improvement in sexual activity compared to the control group while the CBT only group had significant and sustained improvement in urinary complaints. These data suggest that CBT may help to address sexual health issues among breast cancer survivors; however, further studies are warranted [39].
Method
Study design
Descriptive, cross sectional, hospital-based study.
Study area
The study was carried out in Khartoum state - the radio isotope center. Khartoum Radiation and isotope center is the only governmental hospital in Khartoum state, established in 1966, funded by ministry of health. The hospital contains 300 beds. It received daily about 300-400 patients in refer clinic.Staffs consist of 9 consultants and 7 specialist's oncologists and 170 nurses.
Study population
Study population included all married female from 18 years old diagnosed with breast cancer attending outpatient clinic in Khartoum radiation and isotope center during study period.
Sample size
The sample size was calculated based on the following equation: n=N/1+N (d) 2 (n: sample size. N: population. d: precision = 0.05). Applying the equation gives n = 165.
Data collection method and instrument
A semi-structured questionnaire was used to collect data on study variables. Questionnaire was set by Dr.Sanna Abashar which is likert type scale. It is modified with local Sudanese accent from official Arabic, questions reduced from 17 questions to 11.
The questionnaire was designed with three sections; the first section demonstrated demographic information. The second section was measuring sexual dysfunction in patient with breast cancer. The third section measuring the effect of post-surgery in patient sexual attraction.
Data analysis
Data analysis was performed using Chi-square test to test significant differences between variables. The data was analyzed using Statistical Package for Social Sciences (SPSS version 24) and Microsoft office excel program. P-value ≤ 0.05 was considered significant. All data was expressed as text, illustrated tables and figures.
Ethical considerations
Ethical clearance was sought from S.M.S.B ethical review committee, Khartoum state ministry of health and hospital administration at Khartoum radiation and isotope center. A written consent was obtained from each participant after explaining the study and its aim. Confidentiality of data as well as anonymity of individual identity was adhered to as far as possible.
Results
Demographic Data
The study included 180 women; 57.2% within the age group (31-45) years. Most of women (75.6%) were house wives, and (35.6%) had secondary education level.
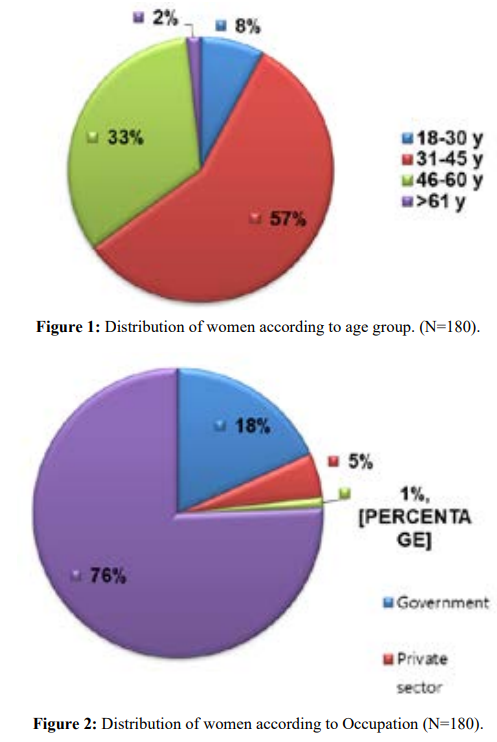
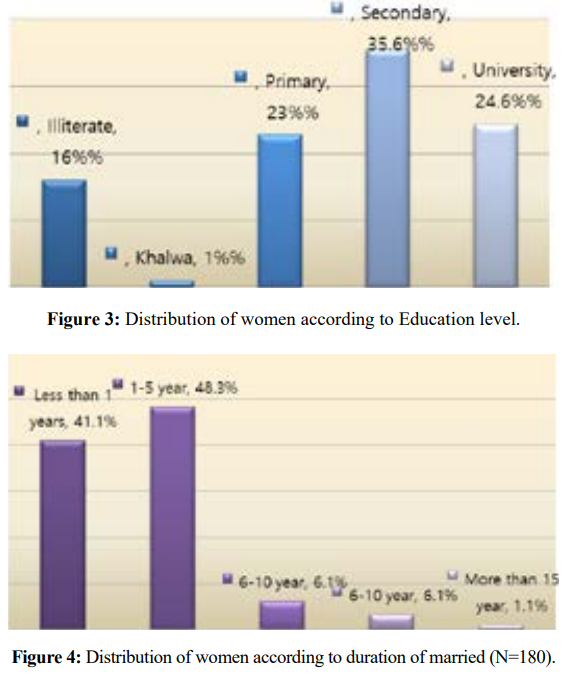
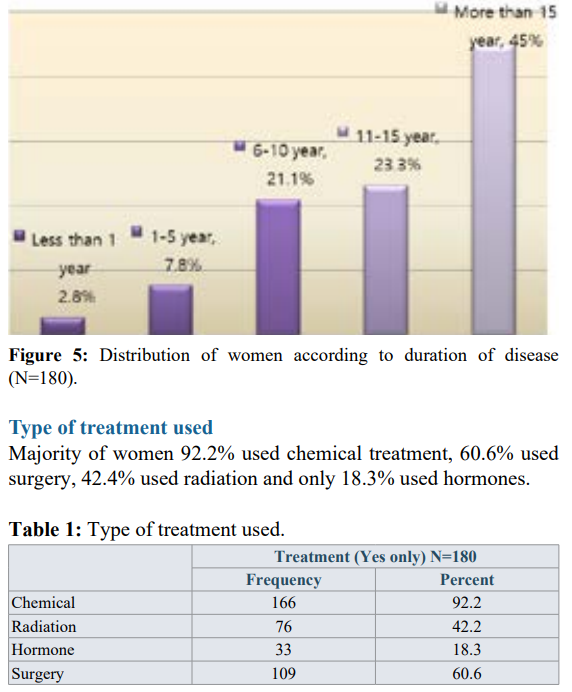
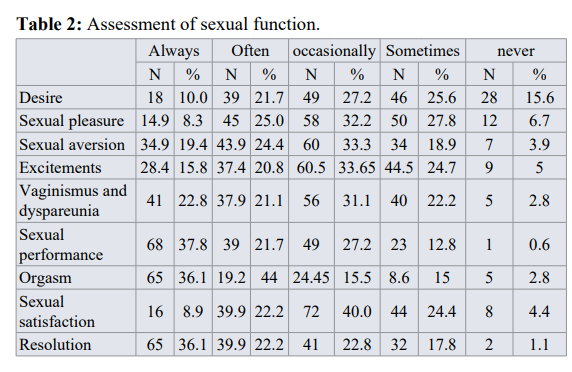
Assessment of sexual dysfunction
This study revealed that 41.2% of women had desire problem, 32.2%occasionally lost or decrease sexual pleasure, 34.5% had sexual aversion, 29.7% decrease or lost excitement, 25% had vaginismus and dyspareunia, 13.4% reduce sexual performance, 12.5% had orgasm problem, 28.8% decrease or lost Sexual satisfaction, 18.9 % can't reach Resolution.
Associations
Associations between sexual function and other variables
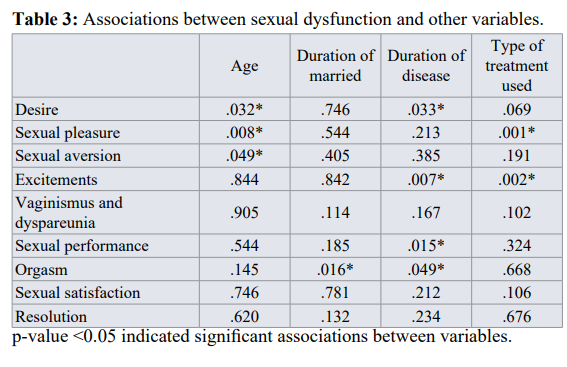
Chi -square correlation test was made between sexual dyfunctionand other variables. The result showed that depending on p-value (P- value <0.05) there were significant associations between them (Table 3).
Discussion
At this cross sectional study on assessing sexual dysfunction among female patients with breast cancer attending outpatient clinic in Khartoum center of radiation and isotope. The study enrolled 180 women.
The findings of this study indicated that sexual dysfunction among patients with breast cancer was high. The present study findings demonstrated that the sexual dysfunction is related to 41.2% lost or decrease desire, 34.5% lost or decrease sexual pleasure, 22.8% had sexual aversion, 29.7% decrease excitement [1], 25% experience sexuality was observed in 64% of patients, while 48% experienced lower motivation. Vaginismus, frigidity, vaginal lubrication and dyspareunia were among disorders reported by these patients. Yang et al. [42] in Korea showed that chemotherapy-based- treated patients with breast cancer have lower sexual activity and motivation while no relationship was found between patients’ sexual function and surgical-based treatments. Bakewell and Volker [43] discovered that all performed therapies on the breast cancer suffering women result in decreased sexual activity. In the current study, 92.2%chemical treatment, 60.6% used surgery, 42.4% used radiation and only 18.3% used hormones. Andersen[44] said that surgeries like hysterectomy and mastectomy have significant effects on cancerous women, resulting in dropped sexuality, sexual arousal, increased dyspareunia and disorder in orgasm. Surgery following breast cancer is a factor making the patients pessimistic about their body when compared to other women [45]. Study in Turkey, by Bektas and Ozkan [46] achieved the results indicating that post-operation body image has been more problematic for women undergoing mastectomy than those having surgery with preserving breast. It seems that the psychological complications have influenced women’s sexual function more that the physiological factors because in the majority of the reported studies, the most common sexual problem has been sexuality and arousal .This dimension mainly reflects their psychological status and conditions while the dimensions like sexual stimulation,vaginal moisture and dyspareunia are caused due to physiological disorders [47]. In this research, the most common sexual problem has been found in desire and sexual pleasure.vaginismus and dyspareunia, 13.4% not satisfied with sexual performance, 12.5% had never reach orgasm, 28.8% not sexualty satisfy, 18.9% never reach resolution and 26.7% of females who had mastectomy got the feeling that they are not sexually attractive after surgery. This finding is in line with those obtained by Mohammadi et al. [40] who investigated the relationship between sexual function disorder and life quality among female patients suffering from uterine, ovarian and breast cancer in Iran .In their research, the sexual dysfunction level has been 61% in desire and 55% in arousal.
In our research, there was a positive, significant relationship between age, type of treatment, duration of disease with sexual dysfunctions , that is, the higher the subjects’ education and age are, the less their sexual dysfunction is, or in other words, the more they suffer from sexual disorders.
This study also showed that there is significant relation between duration of marriage and sexual dysfunction, and this will be confounder with breast cancer.
In the study conducted by Barni and Mondin [41], the breast cancer relevant treatment affected individuals’ sexual activity, 90 % of the patients followed up their sexual activity after the treatment but many of them have faced with disorder. Lack of Chi –square correlation test was made between sexual dyfunctionand other variables. The result showed that depending on p-value (P- value <0.05) there were significant associations between them (Table 3).
This study revealed that age affects on desire, sexual pleasure and sexual aversion.
Also duration of marriage, reduce orgasm in female. The duration of disease also plays a role on desire, sexual excitement, performance and orgasm. Treatments can affect most on sexual pleasure and excitement.
A wide variety of sexual problems outbreak rates exist in breast cancer sufferers that may relate to their treatment type. The results derived from this research show that various treatment methods have had no influence on the patients’ sexual dysfunction, differing from the other researchers’ findings. The study result obtained by Sbitti et al. [48] indicated that 90% cases of post-chemotherapy, 9% of post-surgery, and 3% of post-radiotherapy showed no disorder getting started with hormone therapy. In the findings obtained by Thors et al. [49], the subjects with chemotherapy have displayed more intense function disorder while those with hormone therapy had been less affected by function disorders. The reason behind the difference between the frequencies obtained in this study and those of other studies may relate to the applied methods, demographic characteristics, and selecting different medical approaches towards the patients. One of the reasons for the incompatibility of the results of the present research results with other ones may be originated from the sample size. In addition, individual, cultural, and racial differences can be some of the probable reasons in this context. This is because, based on different reports, since cultural, social, economic, and ethical discrepancies can have a great effect on sexual behaviors [41].
Limitations
This research faced some limitations. For instance, the patient’s psychological and mental conditions have been effective in answering some questions. Lack of easy access to the patients was also limitation. It seems that the sample size was too small to allow the generalizability of the results to a larger population. The questionnaire provides the subjective interpretation of the problems while it does not pave the ground for real clinical diagnosis. There is a need to assess evidence that there is no sexual dysfunction before the illness. Also there is social issues like circumcision that can affect the results, so further studies should be conducted.
Conclusion
This study concluded that the prevalence of sexual dysfunctions in women with breast cancer is high, and there was a correlation between sexual dysfunction with age, duration of disease and treatment methods.
References
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics 2014. CA Cancer J Clin. 2014; 64: 252-271.
- Basson R, Wierman ME, van Lankveld J, et al. Summary of the recommendations on sexual dysfunctions in J Sex Med. 2010; 7: 314-326.
- Manne S, Badr H. Intimacy and relationship processes in couples psychosocial adaptation to Cancer. 2008; 112: 2541-2555.
- Perelman MA. The sexual tipping point a mind/body model for sexual J Sex Med. 2009; 6: 629-632.
- American Psychiatric Eds. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington VA American Psychiatric Association. 2013.
- Taylor-Brown J, Kilpatrick M, Maunsell E, et al. Partner abandonment of women with breast cancer. Myth or reality. Cancer 2000; 8: 160-164.
- Aerts L, Christiaens MR, Enzlin P, et al. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer a prospective controlled study. 2014; 23: 629-636.
- Hidding JT, Beurskens CH, van der Wees PJ, et Treatment related impairments in arm and shoulder in patients with breast cancer a systematic review. PLoS One. 2014; 9: e96748.
- Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation a multicenter analysis of long- term health-related quality of life and satisfaction. Ann Surg 2014; 21: 2159-2164.
- Ewertz M, Jensen Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011; 50: 187-193.
- Safarinejad MR, Shafiei N, Safarinejad S. Quality of life and sexual functioning in young women with early-stage breast cancer 1 year after lumpectomy. Psycho oncology. 2013; 22: 1242-1248.
- Hwang SY, Chang SJ, Park BW. Does chemotherapy really affect the quality of life of women with breast cancer? J Breast 2013; 16: 229-235.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer American society of clinical oncology clinical practice guideline focused J Clin Oncol. 2014; 32: 2255-2269.
- Pagani O, Regan MM, Walley BA, et Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014; 371: 107-118.
- Perez K, Gadgil M, Dizon Sexual ramifications of medical illness. Clin Obstet Gynecol. 2009; 52: 691-701.
- Me Corkle R, Grant M, Frank-Stromborg M, et al. Cancer Nursing A Comprehensive Philadelphia WB Saunders Co. 1996.
- Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J. 2009; 15: 74-77.
- Bartula I, Sherman Screening for sexual dysfunction in women diagnosed with breast cancer systematic review and recommendations. Breast Cancer Res Treat. 2013; 141: 173-185.
- Pinto AC, de Azambuja E. Improving quality of life after breast cancer dealing with symptoms. Maturitas. 2011; 70: 343-348.
- Perz J, Ussher JM, Gilbert Constructions of sex and intimacy after cancer Q methodology study of people with cancer their partners and health professionals. BMC Cancer. 2013; 13: 270.
- Raggio GA, Butryn ML, Arigo D, et Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol Health. 2014; 29: 632-650.
- Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term disease-free survivors of breast cancer a follow-up J Natl Cancer Inst. 2002; 94: 39-49.
- http://www.livestrong.org/what-we-do/our-approach/reports-findings/survivor-survey-report/
- Biglia N, Moggio G, Peano E, et al. Effects of surgical and adjuvant therapies for breast cancer on sexuality cognitive functions and body J Sex Med. 2010; 7: 1891-1900.
- Morales L, Neven P, Timmerman D, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer 2004; 15: 753-760.
- Baumgart J, Nilsson K, Evers AS, et Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013; 20: 162-168.
- Schover LR, Baum GP, Fuson LA, et al. Sexual Problems during the First 2 Years of Adjuvant Treatment with Aromatase J Sex Med. 2014; 11: 3102-3111.
- Halley MC, May SG, Rendle KA, et Beyond barriers fundamental disconnects underlying the treatment of breast cancer patients' sexual health. Cult Health Sex. 2014; 16: 1169-1180.
- Witherby S, Johnson J, Demers L, et Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors a phase I/II study. Oncologist. 2011; 16: 424-431.
- Barton DL, Wender DB, Sloan JA, et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido North Central Cancer Treatment Group protocol J Natl Cancer Inst. 2007; 99: 672-679.
- Le Ray I, Dell'Aniello S, Bonnetain F, et al. Local estrogen therapy and risk of breast cancer recurrence among hormone- treated patients a nested case-control Breast Cancer Res Treat. 2012; 135: 603-609.
- Barton DL, Sloan JA, Shuster LT, et al. Impact of vaginal dehydroepiandosterone DHEA on vaginal symptoms in female breast cancer survivors Trial N10C1 Alliance. J Clin 2014; 32: 9507.
- Lee YK, Chung HH, Kim JW, et Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors a randomized controlled trial. Obstet Gynecol. 2011; 117: 922-927.
- Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997; 15: 969-973.
- Goetsch MF, Lim JY, Caughey AB. Locating pain in breast cancer survivors experiencing dyspareunia a randomized controlled Obstet Gynecol. 2014; 123: 1231-1236.
- Goetsch MF, Lim JY, Caughey A solution for dyspareunia in breast cancer survivors a randomized controlled study. Obstet Gynecol. 2014; 123: 1S.
- Murina F, Bernorio R, Palmiotto The use of amielle vaginal trainers as adjuvant in the treatment of vestibulodynia an observational multicentric study. Medscape J Med. 2008; 10: 23.
- Juraskova I, Jarvis S, Mok K, et The acceptability feasibility and efficacy phase I/II study of the Overcome Olive Oil Vaginal Exercise and Moisturizer intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J Sex Med. 2013; 10: 2549-2558.
- Duijts SF, van Beurden M, Oldenburg HS, et Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer results of a randomized controlled multicenter trial. J Clin Oncol. 2012; 30: 4124-4133.
- Mohammadi SZ, Ghaffari Sexual dysfunction and its correlation with quality of life among women affected with cancer. Iran J Obstetrics Gynecol Infertil. 2009; 12: 39-46.
- Barni S, Mondin Sexual dysfunction in treated breast cancer patients. Ann Oncol. 1997; 8: 149-153.
- Yang EJ, Kim SW, Heo CY, et al. Longitudinal changes in sexual problems related to cancer treatment in Korean breast cancer survivors a prospective cohort study. Support Care 2011; 19: 909-918.
- Bakewell RT, Volker DL. Sexual dysfunction related to the treatment of young women with breast cancer. Clin J Oncol 2005; 9: 697-702.
- Andersen BL. Yes, there are sexual problems. Now what can we do about Gynecol Oncol. 1994; 52: 10-13.
- Ferrari Psychosexual sequence of gynecological condition. J Sex Marit Ther. 1994; 9: 239-249.
- Bektas H, Ozkan I. P66 Sexual dysfunction in breast cancer. Eur J Oncol 2010; 14.
- Mohammadi K, Rahnama P, Moayed S, et Sexual dysfunction and predisposing factors in women with multiple sclerosis. Payesh. 2013; 12: 71-77.
- Sbitti Y, Kadiri H, Essaidi I, et Breast cancer treatment and sexual dysfunction Moroccan women's perception. BMC Womens Health. 2011; 11: 29.Thors CL, Broeckel J, Jacobsen PB. Sexual functioning in breast cancer survivors. Cancer Control. 2001; 8: 442.