Shocked into Shock: Cardioversion-Induced Shock with Multi-Organ Injury
Author(s): Matthew Freedman MD1 , Kunal Kapoor MD2* and Sumon Roy MD2
1Department of Medicine, Virginia Commonwealth University, Richmond, Virginia, USA.
2Pauley Heart Center, Virginia Commonwealth University, Richmond, Virginia, USA.
*Correspondence:
Kunal Kapoor, Pauley Heart Center, Virginia Commonwealth University, Richmond, VA, USA, Tel: (804) 828-9000, Fax: (804) 828-0395.
Received: 14 Mar 2022; Accepted: 24 Mar 2022; Published: 30 Mar 2022
Citation: : Freedman M, Kapoor K, Roy S. Shocked into Shock: Cardioversion-Induced Shock with Multi-Organ Injury. Cardiol Vasc Res. 2022; 6(2): 1-5.
Abstract
Direct current cardioversion (DCCV) is an increasingly prevalent procedure to disrupt abnormal rhythms and restore normal sinus rhythm. While considered a relatively safe procedure, complications can be severe. We add to the emerging research surrounding delayed presentations of cardiogenic shock post-DCCV. Together with a review of the current literature, we extrapolate that a combination of post-conversion cardiac shifts, myocardial stunning, and a unique cardioversion stress-induced cardiomyopathy contributed to this occurrence. To our knowledge, this represents one of only a few examples of delayed, non-arrhythmic, cardioversion-induced cardiogenic shock and the first to cause the combination of non-ST elevation myocardial infarction, acute liver injury, and acute kidney injury. Our case adds to the growing body of research highlighting this rare, but serious consequence of direct current cardioversion.
Keywords
Introduction
Direct current cardioversion (DCCV) is an increasingly prevalent cardiac procedure [1]. Patients with arrhythmia are administered between 50 and 360 joules (J) of electricity to depolarize the myocardium in unison, aiming to disrupt an abnormal rhythm long enough for the heart to restore normal sinus rhythm [2]. With proper precaution and trained personnel, DCCV is a relatively low risk procedure with numerous potential short- and long-term benefits [2,3].
Complications of DCCV such as arrhythmia, transient hypotension, thromboembolic phenomena due to insufficient anticoagulation and dermatological burns are possible, albeit rare [2]. In this report, we present an unusual case of delayed DCCV-induced cardiogenic shock and multi-organ injury, which we propose was caused by transient hemodynamic instability, cardiac stunning, and an unusual biventricular stress-induced myocardial dysfunction.
Case Presentation
A 72-year-old female with persistent atrial fibrillation, coronary artery disease status post left anterior descending (LAD) artery percutaneous coronary intervention, and heart failure with reduced left ventricular ejection fraction (LVEF) of 45% presented for elective DCCV for symptomatic atrial fibrillation with rapid ventricular response (RVR). The patient was sedated with 70mg intravenous (IV) propofol (0.78 mg/kg) and underwent successful DCCV to normal sinus rhythm with one 200J shock. Following cardioversion, she was hypotensive but asymptomatic, and she was moved to the observation unit for routine post-procedural monitoring. Four hours after DCCV, the patient developed acute dyspnea, new hypoxia, and worsening hypotension, prompting inpatient admission. Workup was notable for acute kidney injury (AKI), new transaminitis, lactic acidosis, and troponin elevation. EKG showed sinus bradycardia at 56 beats per minute without acute ischemic changes. Bedside transthoracic echocardiogram (TTE) suggested moderate-severe biventricular dysfunction. Due to concerns of acute cardiogenic shock, dobutamine infusion was initiated at 5mcg/kg/min. Monitoring of serial bloodwork showed eventual peak levels of creatinine 1.54 mg/dL (creatinine clearance 29.7 mL/min), aspartate aminotransferase (AST) 4854 units/L, alanine aminotransferase (ALT) 1859 units/L, INR 3.4, lactate 10.3 mmol/L, and troponin 0.76 ng/mL (Figure 1).
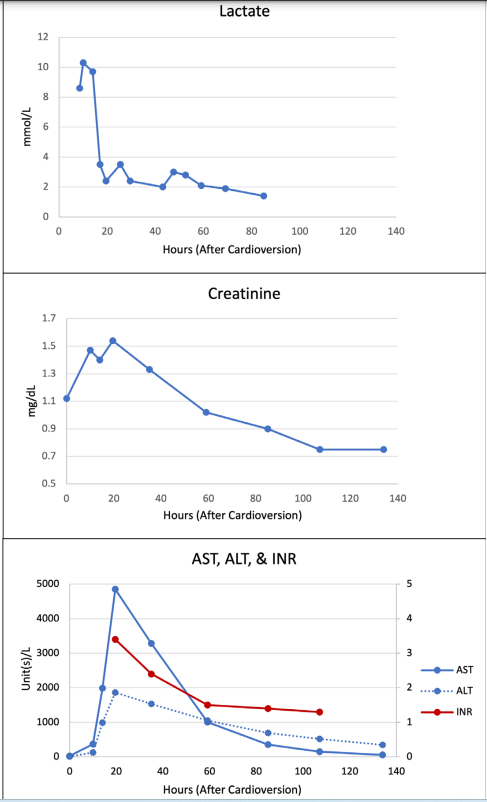
Figure 1: Laboratory data showing trends of lactate, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and international normalized ratio (INR) following cardioversion.
Electrolytes and thyroid function tests were within normal limits. Repeat TTE eight hours later while on dobutamine showed her LVEF had recovered to the previous baseline of 40-45%. Soon after, the patient’s clinical picture improved, and her symptoms and oxygen requirement resolved. Inotrope support was discontinued on hospital day 3. By day 5, her vitals were stable and bloodwork had normalized. Given the unusual hospital course, extensive infectious workup was pursued but resulted unremarkable, aligning with the absence of fever and leukocytosis consistent with no obvious component of sepsis. Cardiac catheterization showed non-obstructive coronary disease and a patent stent in the LAD artery. The patient’s remaining hospital course was unremarkable, and she was discharged home on hospital day 7.
Discussion
This is the first known report of non-arrhythmic post-DCCV cardiogenic shock with acute global biventricular dysfunction, significant lactic acidosis, and multi-organ failure manifesting as non-ST elevation myocardial infarction (NSTEMI), AKI, and acute liver injury (ALI), within hours of successful DCCV for atrial fibrillation with RVR. Clinical parameters rapidly improved with inotrope support and workup for a noncardiac etiology was unremarkable. Taken together, this report likely demonstrates a rare case of non-arrhythmic, DCCV-related cardiogenic shock. Unique to this case is the multi-hour delay in hemodynamic instability and multi-organ injury.
Although the precise mechanism underlying her cardiopulmonary collapse is unclear, we discuss multiple components, which likely contributed to the clinical picture, including post-conversion shifts in cardiac output, atrial and myocardial stunning, and stress- induced global myocardial dysfunction. To our knowledge, prior reports of DCCV-induced hemodynamic instability have not exhibited the combination of delayed cardiogenic shock, global biventricular dysfunction, NSTEMI, AKI, and ALI as was seen in this case.
Rare instances of DCCV resulting in non-arrhythmia-induced cardiogenic shock with various acute organ injuries have been previously reported [4-6]. In these cases, cardiovascular collapse generally occurred immediately following cardioversion [4,6]. One case described successful DCCV with a 100J shock for atrial flutter complicated by profound post-conversion hypotension thought to be due to myocardial dysfunction [4]. The patient rapidly improved with inotrope support and had no known long- term sequelae. Another case described successful DCCV with a 100J shock for atrial fibrillation complicated by cardiovascular collapse within minutes associated with a newly reduced LVEF of 25% [6]. Workup revealed concurrent thyrotoxicosis with the Burch-Wartofsky Point Scale score suggesting impending thyroid storm. Despite aggressive measures, the patient died on hospital day 3. Likely, the previously undiagnosed thyrotoxicosis contributed to the atrial fibrillation as well as ultimate DCCV- induced cardiac failure.
Delayed DCCV-induced cardiogenic shock has also been reported. In one case of atrial fibrillation, DCCV with a 100J shock was successful in restoring normal sinus rhythm, with the
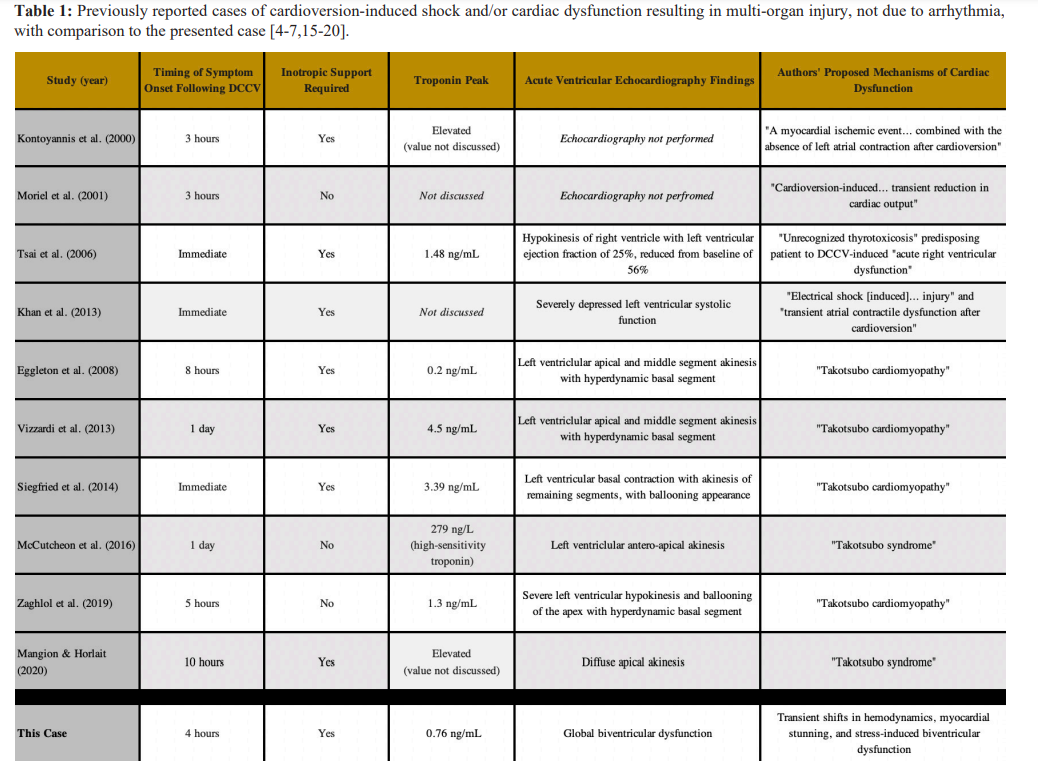
Course complicated three hours later by sudden dyspnea, a newly elevated troponin level, and acute hypotension that eventually led to electromechanical dissociation [5]. Following cardiopulmonary resuscitation, the patient remained in refractory cardiogenic shock and required placement of an intra-aortic balloon pump (IABP). The patient improved with hemodynamic support and the IABP was removed after 16 hours, with repeat TTE showing recovery of LVEF. The underlying mechanism was thought to be temporary myocardial ischemia, though no further testing was reported.
In another instance of delayed symptomatology following DCCV, a patient with atrial fibrillation was successfully cardioverted, but three hours post-procedure noted sudden, severe abdominal pain [7]. Laboratory testing revealed new transaminitis and AKI, with AST 6774 units/L, ALT 5189 units/L, elevated INR, and creatinine 5.3 mg/dL. Liver biopsy findings were most suggestive of ischemic hepatitis, despite no apparent hemodynamic instability throughout the hospital course. It was theorized that transient shifts in hemodynamics following DCCV led to this multi-organ injury.
Multiple factors likely contributed to the clinical course seen in this report, including acute post-DCCV changes in cardiac output. Hemodynamic shifts are expected following cardioversion, particularly transient hypotension in patients with longstanding uncontrolled atrial fibrillation [8]. The modest rise in stroke volume following cardioversion may not always compensate for the sudden drop in heart rate. In patients with chronic atrial fibrillation who undergo DCCV, more than one-third of patients had a cardiac output decrease of up to 14% within 30 minutes of cardioversion, which could persist for up to a week [7,8].
The effect of propofol used in our patient’s cardioversions should also be considered. Propofol is a regularly utilized sedative in DCCV and induces arterial and venous vasodilation, which may cause hypotension [9]. Typical dosing of propofol for DCCV ranges from 0.5-2.5 mg/kg depending on the patient’s stability and the desired level of sedation [9,10]. Propofol given in bolus doses of 2.5 mg/kg decreases systolic blood pressure, mean arterial pressure, and diastolic blood pressure by 25-40%, an effect that would likely be most pronounced minutes after cardioversion [9,11]. However, evaluation of the hypotensive effects of propofol used in DCCV for patients with similar comorbidities to ours concluded that DCCV in these settings generally does not cause hypotension significant enough to cause shock [9]. In procedures requiring continuous infusions of propofol, propofol has been associated with significant bradycardia and hypotension, a manifestation called propofol infusion syndrome (PRIS). PRIS has been associated with cardiac instability, AKI, ALI, and lactic acidosis, but at a minimum typically requires propofol dosing of 4 mg/kg/hr given over 48 hours [12]. Our patient received a single 70 mg propofol bolus prior to DCCV, a dose that represents 0.5% of the cumulative amount shown to cause PRIS. Thus, together with the delayed evolution of cardiovascular collapse, propofol likely contributed minimally to our patient’s hospital course.
Atrial stunning, characterized by transient dysfunction of the atrium and atrial appendage, may have contributed to the cardiovascular collapse witnessed in this case report [13]. Though the exact mechanism is not entirely understood, evidence suggests this phenomenon is due to a combination of tachycardia-mediated atrial cardiomyopathy, cytosolic calcium overload, and atrial hibernation that collectively develop in the interval of atrial fibrillation preceding DCCV and are subsequently unmasked following cardioversion. The most significant effect of atrial stunning is seen immediately following DCCV and progressively resolves from minutes to weeks later depending on the size of the atrium, duration of preceding atrial fibrillation, and the presence of prior structural heart disease.
While this transient atrial dysfunction has been shown to reduce cardiac function temporarily, atrial stunning alone has not been linked to hemodynamic instability or shock [8]. In our case, atrial stunning could have contributed to the hospital course, though likely together with ventricular dysfunction given the significant cardiogenic shock.
Following DCCV and with the onset of cardiovascular collapse in our patient, global biventricular dysfunction was observed on TTE, raising concern for myocardial stunning. Myocardial stunning is a process in which myocardial dysfunction persists for hours to days following resolution of a reversible ischemic cardiac event [14]. While not entirely understood, the current hypothesis is that following temporary ischemia, generated oxygen radicals, calcium overload, and excitation-contraction coupling changes combine to cause temporary myocardial dysfunction. This phenomenon generally only occurs following resolution of temporary ischemia, such as after reperfusion of an occluded coronary artery or resuscitation after cardiac arrest. Whether DCCV imparted a transient ischemic effect causing myocardial stunning is debatable and cannot be excluded. Given the otherwise unremarkable workup, a stunning mechanism is thought to have contributed at least in some capacity to our patient’s post-DCCV course.
Ventricular myocardial dysfunction has rarely been associated with DCCV through stress-induced Takotsubo cardiomyopathy, and has been reported in six previous cases [15-20]. These cases generally describe hemodynamic collapse following DCCV with elevated troponin and acutely reduced LVEF, with delayed symptom presentation ranging between 3 and 24 hours. The described course is similar to this case report. However, TTE findings represent a key differentiator. The previously reported DCCV-associated Takotsubo cases describe apical akinesis, with most cases also showing a hyperdynamic basal segment, demonstrating the classic echocardiographic findings seen in Takotsubo cardiomyopathy [21]. Apical Takotsubo cardiomyopathy, together with the mid- ventricular, basal or “Reverse Takotsubo”, and focal variants are the more commonly recognized presentations of Takotsubo cardiomyopathy [22]. In our case, TTE findings were consistent with global biventricular dysfunction, which would be highly unusual for stress cardiomyopathy. The ventricular dysfunction seen in stress cardiomyopathy is thought to occur due to the relative variation in localization and density of catecholamine receptors, which would be difficult to explain in the clinical context of biventricular dysfunction, though not unprecedented [23]. It makes sense that since DCCV has been associated with classical stress-induced cardiomyopathy, it is possible that DCCV could cause this unusual global variant, and this phenomenon may have contributed to our patient’s temporary cardiac dysfunction and hemodynamic instability.
Conclusion
Profound hemodynamic instability manifesting as cardiogenic shock is a rare but dangerous complication of DCCV. This report describes, to our knowledge, the first case of delayed DCCV- induced cardiogenic shock with global biventricular dysfunction, NSTEMI, AKI, and ALI. These manifestations were likely multifactorial in etiology, resulting from the combined impact of rapid changes in cardiac output, myocardial stunning, and stress- induced global cardiomyopathy, leading to severe mechanical impairment of cardiac function. This case highlights both an uncommon complication of cardioversion and the importance of maintaining a broad differential while implementing aggressive support in these unexpected cases.
References
- Kato E, Ngo-Metzger Q, Fingar KR, et al. Inpatient Stays Involving Atrial Fibrillation, 1998–2014: Statistical Brief #236. 2018 Feb. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. Available from: https://www.ncbi.nlm.nih.gov/books/ NBK499571/
- Sucu M, Davutoglu V, Ozer O. Electrical cardioversion. Ann Saudi Med. 2009; 29: 201-206.
- Sandhu RK, Smigorowsky M, Lockwood E, et al. Impact of Electrical Cardioversion on Quality of Life for the Treatment of Atrial Fibrillation. Can J Cardiol. 2017; 33: 450-455.
- Khan MU, Khouzam RN, Khalid H, et al. Cardiogenic shock following electro-cardioversion of new onset atrial flutter. Hear Lung J Acute Crit Care. 2013; 42: 462-464.
- Kontoyannis DA, Nanas JN, Toumanidis ST, et al. Severe cardiogenic shock, after cardioversion, reversed by the intraaortic balloon pump. Intensive Care Med. 2000; 26: 649.
- Tsai SH, Chu SJ, Wu CP, et al. Acute right ventricular dysfunction after cardioversion in an unrecognized thyrotoxicosis patient. Am J Emerg Med. 2006; 24: 631-633.
- Moriel M, Morali G, Rosenmann E, et al. Cardioversion- induced fulminant ischaemic hepatitis. Eur J Gastroenterol Hepatol. 2001; 13: 1481-1483.
- Upshaw CB. Hemodynamic Changes After Cardioversion of Chronic Atrial Fibrillation. Arch Intern Med. 1997; 157: 1070.
- Wafae B, Da Silva R, Veloso H. Propofol for sedation for direct current cardioversion. Ann Card Anaesth. 2019; 22: 113-121.
- Kaye P, Govier M. Procedural sedation with propofol for emergency DC cardioversion. Emerg Med J. 2014; 31: 904- 908.
- Marik P. Propofol: Therapeutic Indications and Side-Effects. Curr Pharm Des. 2005; 10: 3639-3649.
- Kam PCA, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007; 62: 690-701.
- Khan IA. Atrial stunning: Basics and clinical considerations. Int J Cardiol. 2003; 92: 113-128.
- Bolli R. Mechanism of Myocardial “Stunning.” Circulation.1990; 82: 723-738.
- Eggleton S, Mathur G, Lambros J. An Unusual Precipitant of Tako-Tsubo Cardiomyopathy. Hear Lung Circ. 2008; 17: 512-514.
- McCutcheon K, Butler I, Vachiat A, et al. Takotsubo syndrome in an elderly woman due to electrical cardioversion. Int J Cardiol. 2016; 224: 69-71.
- Siegfried JS, Bhusri S, Guttenplan N, et al. Takotsubo cardiomyopathy as a sequela of elective direct-current cardioversion for atrial fibrillation. Texas Hear Inst J. 2014; 41: 184-187.
- Vizzardi E, Rovetta R, Bonadei I, et al. A case of Tako-Tsubo cardiomyopathy after electrical cardioversion. Minerva Med. 2013; 104: 115-117.
- Zaghlol R, Hritani R, O’Donoghue S. Shock begets shock: A case of direct current cardioversion-induced takotsubo cardiomyopathy. Hear Case Reports. 2019; 5: 310-313.
- Mangion JP, Horlait G. Recurrent Post-Cardioversion Takotsubo Syndrome. Int J Case Reports. 2020; 160: 1-4.
- RadiÄ? K, VrbaniÄ? M, ŠvadumoviÄ? L. Stress cardiomyopathy: diagnosis and treatment. Cardiol Croat. 2018; 13: 495-495.
- Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018; 39: 2032-2046.
- Win CM, Pathak A, Guglin M. Not Takotsubo: A Different Form of Stress-Induced Cardiomyopathy-A Case Series. Congest Hear Fail. 2011; 17: 38-41.