Tenofovir Induced Hepatitis B Surface Antigen Loss and Seroconversion in Two Patients with E-Antigen Negative Chronic Hepatitis B Infection: Case Series
Author'(s): Ijarotimi O1*, Betiku O.A2, Osasona E.O1, Umenze I1, Adekanle O1, and Ndububa D.A1
1Department of Medicine, Obafemi Awolowo University/Teaching Hospital, Ile-Ife, Osun State, Nigeria.
2Department of Morbid Anatomy, Obafemi Awolowo University/Teaching Hospital, Ile-Ife, Osun State, Nigeria.
*Correspondence:
Oluwasegun Ijarotimi, Department of Medicine, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, Tel: +2348038214351.
Received: 06 Jun 2022 Accepted: 11 Jul 2022 Published: 15 Jul 2022
Citation: Ijarotimi O, Betiku OA, Osasona EO, et al. Tenofovir Induced Hepatitis B Surface Antigen Loss and Seroconversion in Two Patients with E-Antigen Negative Chronic Hepatitis B Infection: Case Series. Gastroint Hepatol Dig Dis. 2022; 5(2): 1-3.
Abstract
Two cases of HBsAg seroconversion, following treatment with Tenofovir disoproxil fumarate, a nucleotide reverse transcriptase inhibitor, were reported amongst the patients with chronic hepatitis B infection attending gastrointestinal clinic in a tertiary hospital. The patients had liver biopsy done during their evaluation, following which they were commenced on the medication. Tenofovir was taken for four years and two years respectively before they lost the hepatitis B surface antigen and developed quantifiable antibodies to the surface antigen (Anti-HBs).
Loss of HBsAg is a rare event, and seroconversion in patients with CHB is even less common. Studies have shown that HBsAg loss and seroconversion is less likely with patients on the oral antiviral agents compared with pegylated interferon. Although, both patients had very low pretreatment hepatitis B viral load which might indicate strong immune response to the virus, a factor that could have been partly responsible for the seroconversion. They however, had significant liver fibrosis on histology prior to their seroconversion and will require hepatocellular cancer surveillance until normalization of the liver histology can be ascertained
Keywords
Introduction
Chronic hepatitis B infection (CHB) remains a public health problem with over 240 million people chronically infected worldwide [1]. It is the leading cause of chronic liver disease globally and it accounts for over one million deaths annually through complications of liver cirrhosis, liver cancer and decompensated liver disease [2]. Nigeria is an endemic region for hepatitis B virus with a CHB prevalence of 10% [3]. Furthermore, about 75% of Nigerians have been likely exposed to the virus at one time or the other in their lifetime [4].
There are several drugs used in the management of CHB infection depending on the treatment requirement under the different local and international guidelines. These drugs are mainly the immune- modulators and the oral antiviral drugs. The three first line medications are Tenofovir disoproxil fumarate (TDF) or Tenofovir alafenamide (TAF), Entecavir and Pegylated Interferon [1]. The ideal treatment goal is total eradication and cure of the virus. However, the presence of covalently closed deoxyribonucleic acid (CCC DNA) in the hepatocytes and genomic integration of hepatitis B viral Deoxyribonucleic Acid (HBV DNA) makes this almost impossible to achieve [5]. Therefore, there are different surrogate treatment goals both immediate and long term to guide treatment plans and durations. The main achievable long-term goal of treatment is to prevent liver related morbidity and mortality associated with the virus by preventing disease progression and development of liver cancer [1]. The most desirable and optimal treatment endpoint to achieving this goal is the loss of Hepatitis B surface antigen (HBsAg) and seroconversion to antibody to Hepatitis B surface antigen (anti-HBs) [6]. This HBsAg seroconversion is regarded as a ‘functional cure’, and it signifies a favorable outcome in the natural history of the disease particularly if it occurs before the accrual of significant liver disease [7]. This is because HBsAg loss with or without anti-HBs seroconversion is said to lead to a profound suppression of HBV replication [8]. This, however, is rarely achieved using the currently available medications. At present, treatment with Pegylated Interferon offers the highest chance of a functional cure in both Hepatitis B envelope antigen (HBeAg) positive and negative type of CHB infection in highly selective patients [1]. The oral antiviral drugs have been shown, to a lesser extent, capable of HBsAg seroconversion in HBeAg positive CHB infection and even rarer in HBeAg negative CHB. Hence, the need to report these two cases of HBsAg seroconversion in HBeAg negative CHB infection following treatment with Tenofovir disoproxil fumarate.
Case Report 1
A 33-year-old man presented to the Gastroenterology clinic in our hospital because of an incidental finding of HBsAg positivity detected during pre-employment screening. Patient had no complaints at presentation and there was no previous history of yellowish discoloration of the sclera, pruritus, passage of pale colored bulky stool or right upper quadrant pain. There was history of sharing of clippers for haircut, occasional use of alcohol- based herbal concoction for intercurrent illness, but no history of significant alcohol consumption or blood transfusion in the past. He had no chronic medical conditions.
General physical examination was normal. There were no peripheral stigmata of chronic liver disease. Abdominal examination was also normal with a liver span of 9cm and no ascites. Rectal examination was normal. Examination of other systems were essentially normal.
Laboratory investigations revealed the following results: hepatitis B virus (HBV) panel test as follows: HBsAg was positive, Anti- HBS was negative, HBeAg was negative but antibody to the e antigen (anti-HBe) was positive, antibody to the core antigen (anti- HBc), the total antiHBc was positive while the IgM anti-HBc was negative. He tested negative to Human immunodeficiency virus (HIV) and antibody to hepatitis C virus (anti-HCV). Packed cell volume (PCV) was 46% and white blood cell (WBC) count was 6000 cells/mm3. Coagulation profile done revealed Prothrombin time (PT) of 15.3 secs, control of 14.0 secs and an international normalized ratio (INR) of 1.1. Alpha-feto protein (AFP) was 5.7ng/ ml (normal values < 10.9 ng/ml). Ultrasound scan of the abdomen (Abd USS) was essentially normal. Liver function test (LFT) revealed the following: total bilirubin was 16 µmol/L, conjugated Bilirubin was <5 µmol/L while alkaline phosphatase (ALP) was 153 iu/L (normal 60 iu/L – 170 iu/L). Alanine aminotransaminase (ALT) was 30 iu/L (normal <12 iu/L). Total protein was 73g/L, Albumin 42g/l. The serum HBV DNA count was 22 iu/ml. Liver biopsy was done due to the disparity in the hepatitis B viral count and serum ALT level, and it showed moderate hepatic necroinflammation and moderate fibrosis with no evidence of malignancy (METAVIR score A2/F2).
Patient was commenced on Tabs TDF 300mg daily after a normal baseline renal function test. A repeat serum Hepatitis B viral count done a year after commencing treatment showed no viral DNA detected and this was sustained on therapy through to the fourth year. It was discontinued when patient eventually tested negative to HBsAg. A follow up anti-HBs done about the same time was 84.91miu/ml (levels greater than 10miu/ml are seroprotective). He declined a repeat liver biopsy and fibroscan, a non-invasive procedure, due to cost. However, patient is still on surveillance for hepatocellular carcinoma (HCC) until a fibrosis free liver can be ascertained.
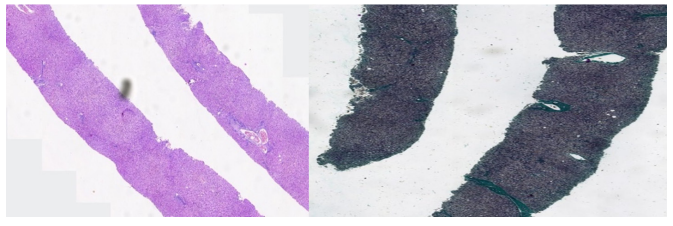
Figure 1: Showing liver histology with features of chronic hepatitis B with moderate necroinflammation and fibrosis (METAVIR A2/F2). (Left); Hematoxylin and Eosin staining, (Right); Masson trichrome staining.
Case Report 2
A 32-year-old woman presented to the Gastroenterology clinic in our hospital on referral, after testing positive to HBsAg during pre-marital counselling and medical screening. She presented with no symptoms and there was no previous history of yellowish discoloration of the sclera, pruritus, passage of pale colored bulky stool or right upper quadrant pain. Risk factors for viral hepatitis noted in her included: history of sharing of sharps and unprotected sexual intercourse with multiple partners.
Her general physical examination was normal. She did not have any peripheral stigmata of chronic liver disease. Abdominal examination did not reveal any abnormality, liver span was 10cm and there was no clinically detectable ascites. Rectal examination was also normal. Other systemic examinations were normal.
Her laboratory investigations revealed the following results: HBV panel test as follows: HBsAg was positive, anti-HBs was negative, HBeAg was negative, anti-HBe was positive, the total anti-HBc was positive while the IgM anti-HBc was negative. She tested negative to HIV and anti-HCV. Her Abd USS was essentially normal. AFP was less than 5.0ng/ml. Her liver function tests were as follows: total Bilirubin was 18 umol/L, conjugated bilirubin was <5umol/L, ALP was 149 iu/l (normal 60 iu/L – 170 iu/L) and ALT- 19 iu/L (normal < 12 iu/L). PCV was 30%, WBC was 4600 cells/ mm3 and platelet count was 230,000 cells/mm3. Clotting profile was as follows: PT-14 sec, control-13 sec, INR-1.2. Serum HBV DNA count level was less than 20 iu/ml. Liver biopsy was advised on account of disparity in the Hepatitis B viral count and serum ALT level and it showed moderate hepatic necroinflammation and moderate fibrosis with no evidence of malignancy (METAVIR score A2/F2).
She was commenced on tabs TDF 300mg daily after a normal baseline renal function test. Patient tested negative to HBsAg two years after commencement of TDF. Anti-HBs quantification done was 10 miu/ml. She was counselled for a repeat liver biopsy of which she declined. She, however, was placed on six monthly checks with LFT, Abd USS and AFP due to the moderate fibrosis seen on the liver histology from the biopsy done before seroconversion. This is for surveillance for HCC until a fibrosis free liver can be ascertained.
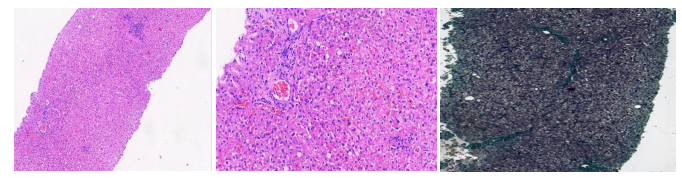
Figure 2: Showing liver histology with features of chronic hepatitis B with moderate necroinflammation and fibrosis (METAVIR A2/F2). (Left/Middle); Hematoxylin and Eosin staining, (Right); Masson trichrome staining.
Discussion
The loss of HBsAg and subsequent seroconversion seen in these patients while on oral antiviral drugs is a very rare occurrence, especially because they had the HBeAg negative type of CHB. Literature search through Hepatitis B related studies in Nigeria showed this has never been reported before now in Nigeria. The rate of HBsAg loss and seroconversion with pegylated interferon treatment has been reported to be as high as 50% in HBeAg positive CHB patients and 12% in HBeAg negative CHB patients after 5-8 years post-treatment [1]. The highest rate reported for oral antiviral treatment was 12% for HBeAg positive CHB patients and less than 1%-2% for HBeAg negative CHB patients after 5-8 years of treatment [1]. The inactive carrier phase of infection is said to be the one with the highest rate of spontaneous HBsAg loss and seroconversion, about 1%-3%, of all the phases of CHB infection [1]. This is probably because of the e antigen seroconversion leading to low viral replication and mild-to-no hepatic inflammation. The immune reactivation phase has the lowest seroconversion rate of all the phases . The two patients in this case series were not in the inactive carrier phase, they were in the immune reactivation phase. However, the factor that was similar in them, which may have contributed to the HBsAg loss and seroconversion might be the strong immune response elicited by both. This was shown by the very low pretreatment viral count and the significant hepatic inflammation on histology in both patients. This strong immune response might have been partly responsible for the HBsAg loss and seroconversion aided by the administration of the oral antiviral medication. A low HBsAg quantification below the level of 1000i.u/l, which has been attributed to higher likelihood of HBsAg loss and seroconversion especially in patients with concomitant low HBV DNA, below 2000i.u/l [9], could have also helped to explain the reason for this extremely rare treatment response in both patients, but HBsAg quantification was not done in both patients. The durability of this HBsAg loss in both patients cannot be guaranteed, but the strength of the seroconversion viz- a-viz the high serum level of anti-HBs produced by the patients is reassuring. HBsAg loss and seroconversion has been said to allow for safe discontinuation of oral antiviral drugs [1] as it signifies sustained immune control of HBV infection leading to improved clinical outcomes and prognosis, but because both patients had significant hepatic fibrosis on liver histology pretreatment, HCC surveillance was continued until a fibrosis free liver could be ascertained.
In conclusion, HBsAg loss and seroconversion are rare events in patients with CHB, regardless of whether they occur spontaneously or following treatment. Patients on oral antiviral therapy, especially those with HBeAg-negative CHB, have been shown to rarely achieve seroconversion compared to those on immune modulators. However, seroconversion seems to be possible in such patients, especially those that exhibit a strong immune response to the virus even in the presence of significant fibrosis on liver histology.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate informed patient consent. In the form, the patient gave her consent for the clinical information and the included image to be reported in the journal. The patient understand that her name and initials will not be published, and due effort will be made to conceal her identity. However, anonymity cannot be guaranteed.
References
- Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus Journal of Hepatology. 2017; 67: 370-398.
- Organization WH. World health organization fact sheet No. 204 Hepatitis Geneva: The Organization. 2002.
- Ijarotimi O, Ijarotimi AO, Ndububa DA, Comparing Serological Markers of Hepatitis B Virus Infection among People Living with HIV/AIDS and HIV Seronegative Individuals. In J Hepat Res. 2015; 2: 1022.
- Sirisena N, Njoku M, Idoko J, et Carriage rate of hepatitis-B surface antigen (HBsAg) in an urban community in Jos, Plateau State, Nigeria. The Nigerian Postgraduate Medical Journal. 2002; 9: 7-10.
- Liver EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of Hepatology. 2012; 57: 167-185.
- Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: Antigens, antibodies and viral Best Practice and Research:Clinical Gastroenterology. 2008; 22: 1031-1048.
- Dusheiko G, Wang Hepatitis B Surface Antigen Loss: Too Little, Too Late and the Challenge for the Future. In Gastroenterology. 2019; 156: 548-551.
- Liaw YF, Chu Hepatitis B virus infection. In The Lancet. 2009; 373: 582-592.
- Terrault NA, Lok ASF, McMahon BJ, et Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018; 67: 1560-1599.