The Benefit of Added Ranolazine to the Association of Angiotensin Receptor Blockers and Mineralocorticoid Receptor Antagonists Therapy for Ventricular Extrasystoles Treatment without the Use of Anti-Arrhythmic Drugs
Author(s): Carlos Alberto Paterno Marchioli*
Fellow European Society of Cardiology, Member of European Society of Hypertension, Laboratory of Cardiovascular Research, Castiglion Fiorentino (Tuscany), Italy.
*Correspondence:
Carlos Alberto Paterno Marchioli, Fellow European Society of Cardiology, Member of European Society of Hypertension, Laboratory of Cardiovascular Research, Castiglion Fiorentino (Tuscany), Italy, E-mail: carlos_paterno@tiscali.it.
Received: 11 Sep 2023; Accepted: 29 Oct 2023; Published: 06 Nov 2023
Citation: Paterno Marchioli CA. The Benefit of Added Ranolazine to the Association of Angiotensin Receptor Blockers and Mineralocorticoid Receptor Antagonists Therapy for Ventricular Extrasystoles Treatment without the Use of Anti-Arrhythmic Drugs. Cardiol Vasc Res. 2023; 7(5): 1-9.
Abstract
Hypertension is a complex, multifactorial disease that has a significant positive association with adverse cardiovascular outcomes. The development of hypertension is usually accompanied by wall arterial structural remodelling, such as large artery stiffness, and increased central systolic blood pressure and pulse pressure. Also, is well established that hypertension is an important risk factor for ventricular extrasystoles. The goal of antihypertensive therapy is to prevent cardiovascular complications. Therefore, treatment with a combination of antihypertensive agents acting on multiple targets is necessary for success in the majority of patients. New therapeutics models are necessary to reduce both arterial stiffness and cardiovascular volume to ameliorate the stress of the arterial-ventricular-atrial dynamic coupling and the abnormal function of the pulsatile system. The objective of the study was to assess if the levels reached on both systolic and diastolic blood pressure, and the central haemodynamic parameters during the angiotensin II receptor blockers, mineralocorticoid receptor antagonists, and ranolazine therapy could avoid or diminish the presence of ventricular extrasystoles during 24- hour Holter monitoring. The results suggest that the association of simultaneous and sequential double blockade on the renin-angiotensin aldosterone system plus ranolazine therapy achieves the best haemodynamic conditions to avoid or reduce the presence of ventricular extrasystoles in hypertensive patients with normal kidney function.
Keywords
Background
Arterial Hypertension
Normal blood pressure is the level that does not cause cardiovascular injuries. Arterial hypertension is a complex, multifactorial, and chronic disease, frequently beginning during childhood or adolescence, it has a significant positive association with adverse cardiovascular outcomes, such as myocardial infarction, stroke, kidney disease, and death [1,2].
Hypertensive Cardiovascular Disease has innumerable causes according to variables proportions and onset times, with an irreversible evolution, it develops from an early age with a paucisymptomatic characteristic and when symptoms appear with "normal" systolic-diastolic values, their identification is still difficult. The pathophysiology of hypertensive cardiovascular disease shows increased peripheral vascular resistance, and increased sodium and water retention, with elevated cardiac output. The development of the clinical evolution of hypertension is usually accompanied by arterial structural remodelling, increasing large artery stiffness, and central systolic blood pressure arising are well documented [3-5].
An increase in arterial stiffness is regarded as a direct measure of target organ damage, indicating the occurrence of pathological changes in large artery walls under the action of cardiovascular risk factors [6]. The roles of arterial stiffness and its wave reflections in hypertension have been elucidated by modern interpretations of the blood pressure curve in relation to its propagation mechanisms of systolic-blood-pressure amplification, and the pulse-pressure amplitude. The importance of large artery stiffening has been further highlighted by the observation that aortic pulse wave velocity, which is inversely related to distensibility [7], and central augmentation index, a composite measure that depends on the site and degree of wave reflection [8]. Noninvasive cardiovascular imaging has a crucial role in the appropriate evaluation of patients with cardiovascular diseases. Structural and/or functional cardiac and vascular damage represent the effect of exposure to different cardiovascular risk factors over time [9]. Perivascular fibrosis that occurs through aldosterone reduces vascular compliance [10].
Angiotensin II
Tissue RAAS is involved in numerous physiological and pathological processes, such as growth and remodelling [11], renal development [12], inflammation and cardiac hypertrophy [13], and vascular hypertrophy and thrombosis [14]. Undoubtedly, Angiotensin II is one of the most potent vasopressor substances known, it is able to regulate cardiac contractility [15], cell coupling, and impulse propagation [16-19], as well as being responsible for remodeling and the induction of apoptosis [20,21]. Lastly, Angiotensin II was demonstrated that may directly cause podocyte injury [22]. Also, a cross-talk between Angiotensin II and Aldosterone has been demonstrated, because some of the cellular effects of Angiotensin II occur through aldosterone-dependent pathways [23].
Aldosterone
Aldosterone, which acts by binding to mineralocorticoid receptors, is now considered a major cardiovascular-risk steroid hormone, it exerts its effects through genomic and non-genomic pathways. The harmful effects of aldosterone are innumerable known since the early work of Laragh et al. [24]
In experimental studies, administration of aldosterone produced extensive cardiac fibrosis and hypertrophy, independent of blood pressure, induced Na-pumps in blood vessels, and worsened arterial stiffness [25-27]. One of the main determinants of reentry arrhythmias is myocardial fibrosis, which in turn produces diastolic dysfunction. This action of aldosterone is independent of angiotensin II plasma levels. Cardiac hypertrophy is less likely than fibrosis to be an arrhythmic substrate. Myocardial fibrosis as a predictor of sudden cardiac death in patients with chronic ischaemic heart disease has been the topic of a recent retrospective investigation using cardiac magnetic resonance [28].
Aldosterone exerts its deleterious effects at several levels: Increase sympathetic nervous system activation [29], induced metabolic effects such as glucose intolerance, insulin resistance, cardiac [30,31] and renal [32-35] inflammation and fibrosis [36,37]. Increases in expression of COX-2 and modulating oxidative stress through induction of NADPH oxidase, contribute to the development of left ventricular interstitial fibrosis and myocardial apoptosis, which involves arterial and myocardium remodelling [11,38], impairs endothelial function by reducing the bioavailability of nitric oxide which alter the diastolic function [39,40]. Decrease sodium and water excretion with a concomitant increase in plasma volume increases the filling pressure, induces heart failure, increases sodium influx in vascular smooth muscle cells, leads to vascular wall inflammation [41] and cell hypertrophy, with a reduction in vascular compliance, and produce a prothrombotic state [42]. Increases vascular responsiveness to pressor agents such as norepinephrine and angiotensin [43]. Developed arrhythmias by hypokalaemia and myocardial calcium channel expression [44,45], increases atrial fibrosis with subsequent structural remodelling developing a substrate for atrial arrhythmias [46] by electrical instability.
The phenomenon of "aldosterone escape" or “aldosterone breakthrough” occurs even in the presence of combination therapy with Angiotensin-Converting Enzyme inhibitors, regardless of the doses used [47]. A proarrhythmic role of aldosterone has been suggested by Ramires et al. based on the finding of a 74% reduction in ventricular extrasystoles and an 80% reduction in episodes of non-sustained ventricular tachycardia in patients treated with spironolactone [48].
Arrhythmia
The electrophysiological characteristics of the arrhythmias depend on the underlying structural heart disease [49]. Late sodium current has been shown to play a key role in action potential prolongation and arrhythmogenesis in acquired and congenital cardiac diseases. Increasing ventricular volume can stimulate the development of abnormal ventricular rhythms, which in turn is a trigger factor for sudden death [50]. This mechanism is supported by the Survival and Ventricular Enlargement (SAVE) trial, where ectopic ventricular arrhythmias increased as left ventricular end- systolic and end-diastolic volumes increased [51].
Angiotensin Receptor Blockers
The protective effect of Angiotensin II Receptor Blockers on major cardiovascular events might be partly independent of the degree of blood pressure reduction and has been recommended in all the arterial hypertension treatment guidelines.
Mineralocorticoid Receptor Antagonists
The MRAs are not recommended as first-line drugs. The European Society of Hypertension/European Society of Cardiology guidelines actually placed them as fourth-line agents to be used in resistant hypertension [52]. Non-steroidal mineralocorticoid antagonists reduce adverse remodelling, inflammation, and fibrosis in the heart, kidneys, and vasculature. Aldosterone blockade reduces left ventricular remodeling and collagen deposition, improves endothelial function, reduces inflammation, and increases myocardial perfusion [53,54]. A positive effect has been documented in patients with chronic heart failure, in whom spironolactone, added to chronic angiotensin-converting enzyme inhibition, improved endothelial function secondary to increased nitric oxide availability [55].
Early use of MRAs as first-line antihypertensives would avoid the harmful action of aldosterone, thus preventing its serious consequences such as heart failure. At the last ISH 2022 congress, Prof Shibata Hirotaka said that it is conceivable that MRA should be included in the first-line therapy for hypertension. (Lecture ISH 2022: Mineralocorticoid Receptor Blockers as the possible first-line therapy in hypertension. Journal of Hypertension 41, Suppl 1, 2023)
Ranolazine
Ranolazine was developed in the late 1990s and has been approved as an anti-anginal medicine. It is an anti-ischaemic agent that can reduce diastolic myofilament activation leading to improved intraventricular relaxation and potentially improving microcirculation. It has additional electrophysiological antiarrhythmic properties by inhibiting the cardiac late Na+ current, reducing the Ca2+ overload, and has been found to inhibit the IKr-current is different from conventional agents in that it does not reduce heart rate or blood pressure [56,57]. Ranolazine is indicated for use in angina and its specific use for ventricular arrhythmias is still off-label.
Objective
This study aimed to know if Ranolazine when added to Angiotensin II Receptor Blockers (ARB) and Mineralocorticoid Receptor Antagonists (MRA) therapy by its sequential and simultaneous actions on the Renin Angiotensin Aldosterone System in hypertensive patients with normal kidney function could reduce or avoid the appearance of ventricular extrasystoles without the use of antiarrhythmics drugs such as flecainide or propafenone.
Design & Methods
To develop this observational and prospective study, two groups of patients were analyzed. In a retrospective group of 72 hypertensive patients, females 42 and males 30, during therapy with the triple association ARB + MRA + Ranolazine (375/500 mg, 2xd) without anti-arrhythmic drugs assessed with 24-Holter monitoring, was observed that the presence of ventricular extrasystoles was scarce. This study gave the idea to develop a second prospective study in a group of hypertensive patients without anti-arrhythmic drugs, in treatment with ARB + MRA who presented ventricular extrasystoles, whose Ranolazine was added considering its properties anti-ischemic/anti-arrhythmic, to know the potential benefit of this triple association on ventricular arrhythmias. The patients of the retrospective group had a follow-up of 18.8 months for the women and 14.9 months for the men. All patients had normal kidney function and did not use antiarrhythmic drugs.
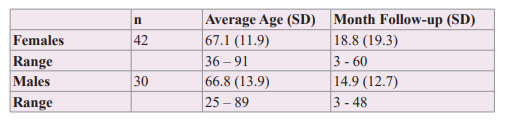
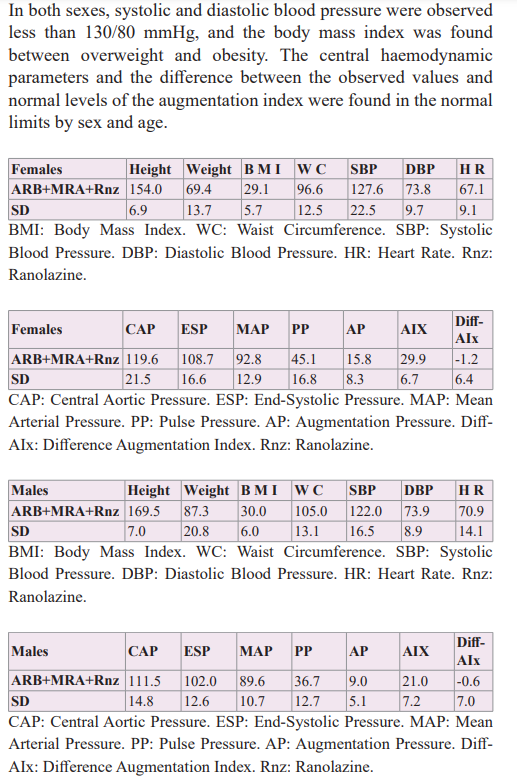
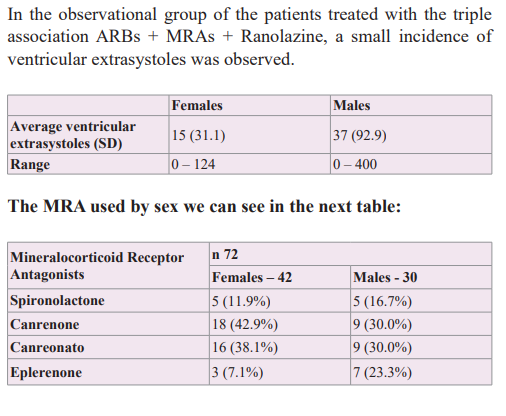
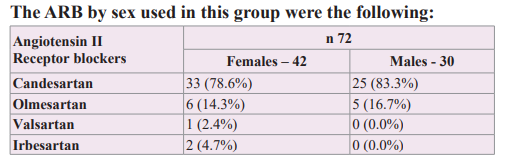
The synergistic association of therapy with ARB and MRA, which act sequentially and simultaneously on the Renin-Angiotensin Aldosterone system, was studied, managed to improve arterial elasticity and cardiovascular volume, decreasing the wall tension of the arterial-ventricular-atrial coupling and showing a net decrease in the brief phases of atrial fibrillation during 24-hour Holter monitoring, [58] therefore the possibility of adding a molecule such as Ranolazine, with anti-ischemic/anti-arrhythmic action without direct action on heart rate or blood pressure was considered, in order to know its potential benefit over mono or multifocal ventricular extrasystoles.
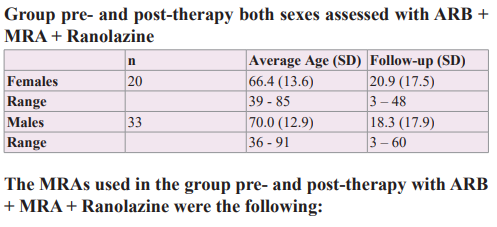
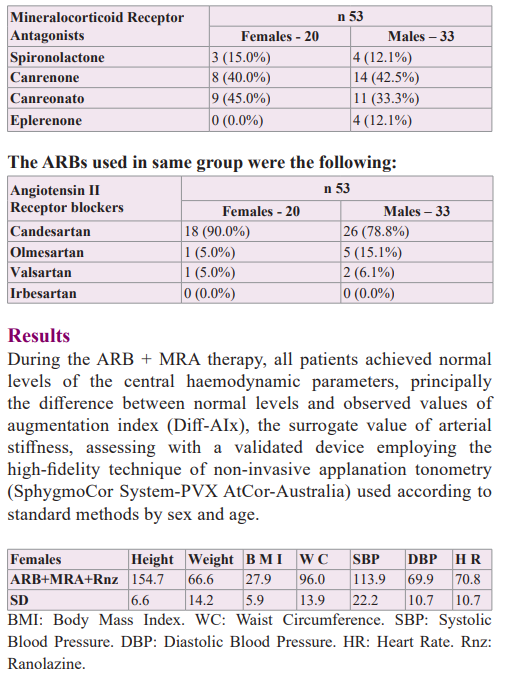
A group of 53 hypertensive patients who were analyzed during an association of ARB + MRA therapy was entered females 20 with an average age of 66.4 years (SD 13.6) and males 33 with an average age of 70.0 years (SD 12.9). All patients had normal kidney function and presented ventricular extrasystoles without the use of anti-arrhythmic drugs were assessed with a 24hs-monitoring Holter, pre- and post-Ranolazine therapy (375/500 mg, 2xd), with an average therapy time-length on females-males by 20.9 (SD 17.5) - 18.3 (SD 17.9) months, respectively. The patients were highly encouraged to suppress or diminished salt intake in the diet to the lowest tolerable quantity, a very important variable considering that aldosterone exerts its deleterious action principally in the presence of sodium chloride. Also, patients received information about moderating the consumption of alcohol and giving up the habit of smoking, absolutely, in addition, they were advised to maintain regular physical activity according to sex and age.
The patients were informed that they should have their renal function checked regularly. Thus, when the MRA was added to ARB therapy considering could be a rationale possibility by a double sequential blockade over the RAAS even knowing that the serum potassium level could increase in patients with a previous normal kidney function with an occult injury.

Discussion
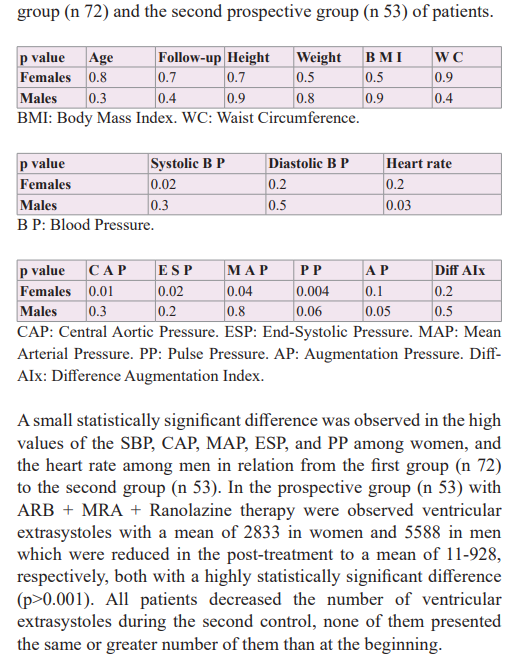
In a previous study, we observed that the simultaneous and sequential double blocking with ARB + MRA therapy, both the systolic and diastolic blood pressure levels, and central hemodynamic parameters were optimized because improved was observed that arterial stiffness, reducing the stress of the pressure/ volume system into the arterial-ventricular-atrial dynamic coupling, ameliorating the whole heart electrical structure without added of antiarrhythmic drugs avoiding the presence of brief phases of atrial fibrillation [58]. Therefore, we thought of adding Ranolazine, a drug with anti-ischaemic and anti-arrhythmic actions, to the aforementioned therapy with the aim of avoiding or diminishing the appearance of the ventricular extrasystoles. Using the triple therapeutic association ARB + MRA + Ranolazine in hypertensive patients with normal kidney function, it was observed that ventricular extrasystoles decreased in all patients and in a high percentage of them, they were completely abolished.
It is a relatively common occurrence for cardiovascular clinicians to see patients with frequent premature ventricular contractions. There is no doubt that sudden cardiac death by ventricular arrhythmias is among the most devastating events affecting cardiovascular patients. Premature ventricular contractions may be a marker of underlying heart disease, the presence of which would confer an unfavourable prognosis even in asymptomatic individuals and is thought to be the result of the triggered activity. There is no absolute threshold for the number of premature ventricular contractions that can be used as a cut-off underlying disease. Sudden death in the majority of cases (>90%) is caused by malignant ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation [59]. Given that sudden death is generally caused by ventricular arrhythmias, it seemed logical to think that antiarrhythmic drugs could be useful to prevent it, a fact disproved because the CAST study demonstrated that antiarrhythmic therapy (class IC drugs), when it is able to suppress arrhythmias not only does it not improve prognosis but paradoxically it makes it worse [60].
Ventricular tachycardias are usually related to structural heart disease. However, in 10% of patients with ventricular tachycardias, no structural heart disease, metabolic, electrolyte abnormalities, or long QT syndrome can be found. These arrhythmias have been called idiopathic ventricular tachycardias.
Professor Santiago Ramón y Cajal said: “It is not the problem that is blocked, it is the people that are blocked in the problem”. Frequently when we controlled these patients with “normal” blood pressure and a cardiomyopathy, using the applanation tonometry we found that they had stiff arteries (high augmentation index) with normal central aortic and end-systolic pressure, therefore we could think that the illness could be present for a long-time as the cause of the development of hypertensive cardiovascular disease and the ventricular extrasystoles as one of its complications. This phenomenon tells us that the numbers obtained with the sphygmomanometer could be "relative data" but not absolute data. It is possible to think that the measurement of blood pressure could not be sufficient to make a diagnosis of hypertensive cardiovascular disease.
To see Box 1 a case clinic with “normal” blood pressure and cardiomyopathy.
On other hand, thiazide diuretics are one of the most commonly prescribed antihypertensive drugs worldwide, but the major concern is that can activate the neurohormonal system through volume depletion and could release aldosterone. The addition of hydrochlorothiazide to Angiotensin II Receptor Blockers to treat hypertension might act on releasing aldosterone, which in turn sensitizes the endothelium to the action of Angiotensin II.
In addition, can produce hypokalaemia and hyponatraemia, which are potentially arrhythmogenic.
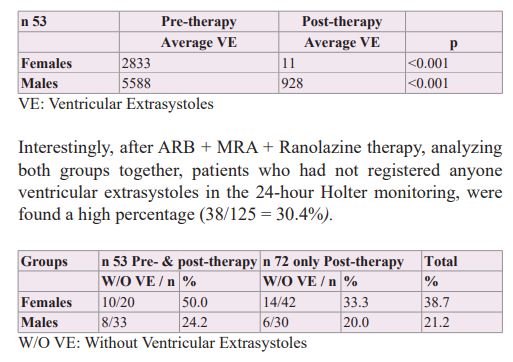
Hypokalaemia was deemed hazardous by many investigators; associations with ventricular arrhythmias were especially worrisome [61]. Some observational studies have suggested that diuretic-induced hypokalaemia may be associated with an increased incidence of arrhythmias [62-64]. Knowing that diuretics can stimulate the production of arrhythmias replaced them with MRAs associated with ARBs to treat hypertension producing a dual sequential block to the Renin-Angiotensin-Aldosterone System in a previous study which was observed a better result in the prevention of the appearance phases of atrial fibrillation when confronted with ARB alone and ARB + hydrochlorothiazide [58]. Also, when we observed in our registry the 24-hour Holter recordings of patients during the association ARB + MRS therapy, no evident decrease in ventricular extrasystoles was found. However, when we observed the 24-hour Holter recordings of the patients during the triple association ARB + MRS + Ranolazine therapy, which is the first retrospective group of this study, we observed that ventricular extrasystoles were rare, and which gave the idea to make a second prospective study group. Therefore, a second study was developed in hypertensive patients with ventricular extrasystoles and started the triple association ARB + MRA + Ranolazine, who were assessed with a 24-hour Holter monitor before and after therapy with the objective to find out if there was a statistically significant difference. The addition of Ranolazine was intended to use a drug with anti-ischaemic/anti- arrhythmic properties and so avoid the use of anti-arrhythmic drugs considering that the appearance of ventricular extrasystoles could be caused by a micro-vascular dysfunction produced by the action of hypertensive cardiovascular disease, which was not diagnosed or treated early. Ranolazine was the objective of various studies in order to evaluate its anti-ischemic and anti-arrhythmic potential, which helped us to support the hypothesis of its potential benefit in the treatment of ventricular extrasystoles, although not in isolation as a drug but in association with ARB + MRA therapy that had already demonstrated an effective action on wall stress and cardiovascular volume, reducing or avoiding atrial fibrillation phases.
Following are some studies about the anti-anginal/anti-arrhytmic effect of the ranolazine:
CARISA: Ranolazine increased exercise capacity and provided additional antianginal relief [65].
MARISA: In chronic angina patients, ranolazine monotherapy increased exercise performance [66].
ERICA: Ranolazine significantly reduced the frequency of angina [57].
MERLIN-TIMI 36: Ranolazine in the acute and chronic management of patients presenting with Non-ST-Elevation Acute Coronary Syndrome [67].
MERLIN-TIMI 36: Our findings provide support for the safety and efficacy of ranolazine as antianginal therapy [68].
MERLIN TIMI-36: Recurrent ischemia was significantly reduced by ranolazine [69].
MERLIN-TIMI 36: Effect of ranolazine on the incidence of arrhythmias in patients with non-ST segment elevation in acute coronary syndrome [70].
RESTYLE-HCM: Ranolazine was associated with reduced premature ventricular complex burden [71].
RAID: Ranolazine was associated with a significant reduction in recurrent ventricular tachycardia or ventricular fibrillation requiring implantable cardioverter-defibrillator therapy [72].
In the small study of Curnis A et al., in seventeen implantable cardioverter defibrillators, ranolazine proved to reduce ventricular arrhythmia episodes. In addition, none of the patients with chronic angina developed major ventricular arrhythmias [73]. According to the ESC-2019 guidelines, the available results support the use of ranolazine as a second-line drug in patients with chronic coronary syndromes and refractory angina [74].
At the moment, Ranolazine has no indication as an anti-arrhythmic drug alone or in combination, therefore would be possible to use it associated with ARB + MRA to treat ventricular extrasystoles with the aim of avoiding the use of anti-arrhythmic drugs or when are contraindicated. Why treat the consequences (arrhythmias) with anti-arrhythmic drugs when it is possible to treat their causes (arterial stiffness, end-systolic pressure, diastolic dysfunction), considering it is more specific, economical, safe, and effective?
Box 1 Clinical case
Patient:GA,male,68yearsold.Height160cm,Weight53Kg.
Former smoker, COPD. CCAG normal.
Two weeks before the medical visit, the patient was implanted with a cardioverter defibrillator to treat ventricular extrasystoles resistant to anti-arrhythmic drugs. Long-life TA 90/60 mmHg. Echocardiogram: diastolic dysfunction, FE 25%, Aortic ectasia. Aortic and mitral regurgitation mild/moderate, tricuspid regurgitation mild. 24-h Holter monitoring: complex ventricular extrasystoles. Creatinine 0.9, LDL-C 85, Glycemic 107. Non- Invasive Applanation tonometry: Augmentation Index 44%,very high arterial stiffness, End-Systolic pressure 79 mmHg.
Conclusions
In a previous study, we observed that with the association of ARB + MRA therapy, the patients reached normal levels of both systolic- diastolic blood pressure and central hemodynamic parameters, stabilized the volume/pressure curve, reducing the stress of the arterial-ventricular-atrial dynamic coupling and thus avoiding the appearance of atrial fibrillation.
In this study, Ranolazine was added to the aforementioned drug combination, with the aim of obtaining a beneficial antiarrhythmic action to avoid or reduce the occurrence of ventricular extrasystoles without the use of anti-arrhythmic drugs. The best therapy for ventricular extrasystoles could be Prevention, which is safer, effective, and economical.
References
- Psaty BM, Furberg CD, Kuller LH, et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001; 161: 1183-1192.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360: 1903-1913.
- Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC study Hypertension. 1999; 34: 201-206.
- O’Rourke MF, Adji A. An updated clinical primer on large artery mechanics: implications on pulse waveform analysis and arterial tonometry. Curr Opin Cardiol. 2005; 20: 275-281.
- Melenovsky V, Borlaug BA, Fetics B, et al. Estimation of central pressure augmentation using automated radial artery tonometry. J Hypertens. 2007; 25: 1403-1409.
- Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2007; 25: 1105-1187.
- Bramwell JC, Hill AV. Velocity of transmission of the pulse- wave in man. Proc R Soc London Biol Sci. 1922; 93: 298-306.
- Safar ME, London GM. Therapeutics studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertension. 2000; 18: 1527-1535.
- Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006; 114: 2850-2870.
- Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010: 52: 401-409.
- Tamura T, Said S, Harris J, et al. Reverse modeling of cardiac myocyte hypertrophy in hypertension and failure by targeting of the renin-angiotensin system. Circulation. 2000; 102: 253-259.
- Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for renal development. Journal of Hypertension. 2000; 18: 123-137.
- Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin6 in human coronary atherosclerotic plaques. Potential implications for inflammation and plaque instability. Circulation. 2000; 101: 1372-1378.
- Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Advances in Internal Medicine. 2000; 45: 419-429.
- Koch Weser J. Nature of inotropic action of angiotensin on ventricular myocardium. Circulation Research. 1995; 16: 230-237.
- De Mello WC. Is an intracellular renin angiotensin system involved in the control of cell communication in heart?. Journal of Cardiovascular Pharmacology. 1994; 23: 640-646.
- De Mello WC. Renin angiotensin system and cell communication in the failing heart. Hypertension. 1996; 27: 1267-1272.
- De Mello WC, Cherry R, Mannivanan S. Electrophysiologic and morphologic abnormalities in the failing heart; effect of enalapril on the electrical properties. Cardiac failure. 1997; 3: 53-62.
- De Mello WC. Cell coupling and impulse propagation in the failing heart. Journal of Cardiovascular Electrophysiology. 1999; 10: 1409-1430.
- Harada K, Sugaya T, Murakami K, et al. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999; 100: 2093-2999.
- Horiuchi M, Ashita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999; 33: 613-621.
- Che G, Gao H, Hu Q, et al. Angiotensin II promotes podocyte injury by activating Arf6-Erk1/2-Nox4 signaling pathway. PLos One. 2020; 15: e0229747.
- Virdis A, Neves MF, Amiri F, et al. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002; 40: 504-510.
- Laragh JH, Angers M, Kelly WG, et al. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA. 1960; 174: 234-240.
- Weber KT, Sun Y, Campbell SF. Structural remodelling of the heart by fibrous tissue: role of circulating hormones and locally produced peptides. Eur Heart J. 1005; 12-18.
- Alzamora R, Marusic ET, Gonzalez M, et al. Nongenomic effect of aldosterone on Na+, K+-adenosine triphosphatase in arterial vessels. Endocrinology. 2003; 144: 1266-1272.
- Oberleithner H. Aldosterone makes human endothelium stiff and vulnerable. Kidney Int. 2005; 67: 1680-1682.
- Zegard A, Okafor O, de Bono J, et al. Myocardial 1 fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol. 2021; 77: 29-41.
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res. 1997; 35: 30-34.
- Horisberger JD, Rossier BC. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992; 19: 221-227.
- Weber KT. Aldosterone in congestive heart failure. New England Journal of Medicine. 2001; 345:1689-1697.
- Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney disease: pathophysiological basis. Kidney Int. 2019; 96: 302-319.
- Epstein M. Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: from Hans Selye to the present. Am J Nephrol. 2021; 52: 209- 216.
- Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004; 66: 1-9.
- Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019; 42: 293-300.
- Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008; 51: 161-167.
- Robert V, Besse S, Sabri A, et al. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest. 1997; 76: 729-738.
- Brilla CG, Pick R, Tan LB, et al. Remodelling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990; 67: 1355-1364.
- Brilla CG, Janicki JS, Weber KT. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circulation Research. 1991; 69: 107-115.
- Funder J. Mineralocorticoids and cardiac fibrosis: the decade in review. Clin Exp Pharmacol Physiol. 2001; 28: 1002-1006.
- Rocha R, Funder JW. The pathophysiology the aldosterone in cardiovascular system. Annals of the New York Academy Science. 2002; 970: 89-100.
- Sechi LA, Novello M, Colussi GL, et al. Relationship of plasma renin with a prothrombotic state in hypertension: relevance for organ damage. Am J Hypertens. 2008; 21: 1347- 1353.
- Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res. 1999; 41: 55-64.
- Bénitah JP, Vassort G. Aldosterone upregulates Ca2+ current in adult rat cardiomyocytes. Circulation Research. 1999; 85: 1139-1145.
- Carmeliet E, Vereecke J. General description of electrical activity. In: Carmeliet E, Vereecke J, eds. Cardiac Cellular Electrophysiology. Boston, Dordrecht/London: Kluwer Academic Publishers Group. 2002; 1-7.
- Reil JC, Hohl M, Selejan S, et al. Aldosterone promotes atrial fibrillation. Eur Heart J. 2012; 33: 2098-2108.
- Pitt B. “Escape” of aldosterone production in patients with left ventricular dysfunction treated with an angiotensin converting enzyme inhibitor: implications for therapy. Cardiovascular Drugs Therapy. 1995; 9: 145-149.
- Ramires FJ, Mansur A, Coelho O, et al. Effect of spironolactone on ventricular arrhythmias in congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. American Journal of Cardiology. 2000; 85: 1207-1211.
- Katja Zeppenfeld, Jacob Tfelt-Hansen, Marta de Riva, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022; 43: 3997-4126.
- Babuty D, Lab MJ. Mechanoelectric contributions to sudden cardiac death. Cardiovasc Res. 2001; 50: 270-279.
- St John SM, Lee D, Rouleau JL, et al. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation. 2003; 107: 2577-2582.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and of the European Society of Cardiology. Eur Heart J. 2013; 34: 2159-2219.
- Modena MG, Aveta P, Menozzi A, et al. Aldosterone inhibition limits collagen synthesis and progressive left ventricular enlargement after anterior myocardial infarction. Am Heart J. 2000; 11: 41-46.
- Zannad F, Dousset B, Alla F. Treatment of congestive heart failure: interfering the aldosterone-cardiac extracellular matrix relationship. Hypertension. 2001; 38: 1227-1232.
- Abiose AK, Mansoor GA, Barry M, et al. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004; 15: 1564-1566.
- Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004; 110: 904-910.
- Stone PH, Gratsiansky NA, Blokhin A, et al. Antianginal Efficacy of Ranolazine When Added to Treatment With Amlodipine: The ERICA (Efficacy of Ranolazine in Chronic Angina) Trial. J Am Coll Cardiol. 2006; 48: 566-575.
- Paterno Marchioli CA. The double sequential actions of the Angiotensin II Receptor Blockers and Mineralocorticoid Receptor Antagonists Therapy on the Renin Angiotensin Aldosterone System produce a better reduction of both Blood Pressure and Central Haemodynamic Parameters and can prevent the appearance of the atrial fibrillation. Cardiology & Vascular Research. 2023; 7: 1-12.
- Uretsky BF, Sheahan RG. Primary prevention of sudden cardiac death in heart failure: will the solution be shocking?. J Am Coll Cardiol. 1997; 30: 1589-1597.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989; 321: 406-412.
- Kaplan NM. Our appropriate concern about hypokalemia. Am J Med. 1984; 77: 1-4.
- Myers MG. Diuretic therapy and ventricular arrhythmias in persons 65 years of age and older. Am J Cardiol. 1990; 65: 599-603.
- Siegel D, Hulley SB, Black DM, et al. Diuretics, serum and intracellular electrolyte levels, and ventricular arrhythmias in hypertensive men. JAMA. 1992; 267: 1083-1089.
- Siscovick DS, Raghunathan TE, Psaty BM, et al. Diuretic therapy for hypertension and the risk for primary cardiac arrest. N Engl J Med. 1994; 330: 1852-1857.
- Chaitman BR, Pepine CJ, Parker JO, et al. Combination Assessment of Ranolazine in Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004; 291: 309-316.
- Chaitman BR, Skettino SJ, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004; 43: 1375-1382.
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J. 2006; 1516: 1186.e1-9.
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007; 297:1775-1783.
- Melloni C, Newby LK. Metabolic efficiency with ranolazine for less ischemia in non-ST elevation acute coronary syndromes (MERLIN TIMI-36) study. Expert Rev Cardiovasc Ther. 2008; 6: 9-16.
- Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non-ST segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007; 116: 1647-1652.
- Olivotto I, Camici PG, Merlini PA, et al. Efficacy of ranolazine in patients with symptomatic hypertrophic cardiomyopathy: the RESTYLE-HCM randomized, double-blind, placebo- controlled study. Circ Heart Fail. 2018; 11: e004124.
- Zareba W, Daubert JP, Beck CA, et al. RAID trial Investigators. Ranolazine in high-risk patients with implanted cardioverter- defibrillators: the RAID trial. J Am Coll Cardiol. 2018; 72: 636-645.
- Curnis A, Salghetti F, Cerini M, et al. Ranolazine therapy in drug-refractory ventricular arrhythmias. J Cardiovasc Med. 2017; 18: 534-538.
- Knuuti J, Wijns W, Saraste A, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41: 407-477.