The Epidemiology of Colorectal Cancer in Erbil
Author'(s): Hawro Hamza1* and Kakil Rasul2
1Postgraduate Fellow (Medical Oncology), Medical Oncology Department, Oncology Center, Rizgary Teaching Hospital, Erbil,Kurdistan, Iraq.
2Senior Consultant Hematology/Oncology, National Center for Cancer Care and Research (NCCCR), Associate Professor in Clinical Medicine, Weil Cornell Medical College, Qatar.
*Correspondence:
Hawro Taha Hamza, Medical Oncology Department, Oncology Center, Rizgary Teaching Hospital, Erbil, Kurdistan, Iraq, Tel:00964 750 467 20 77; E-mail: hawro_hamza@yahoo.com.
Received: 16 March 2018; Accepted: 08 April 2018
Citation: Hawro Hamza, Kakil Rasul. The Epidemiology of Colorectal Cancer in Erbil. Cancer Sci Res. 2018; 1(2); 1-6.
Abstract
Background and Objectives: Colorectal cancer (CRC) is a major cause of morbidity and cancer related mortality throughout the world. It is the third most common cancer worldwide and the fourth most common cause of cancerrelated death. The objective of this study is to study the epidemiology of colorectal cancer in Erbil city.
Methods: Retrospective analysis of the data from registry units in Rizgary Oncology Center and Nanakaly Cancer Hospital in Erbil during the period of Jan – Dec 2016.
Results: Data of total 118 patients collected, 55.08% of patients were male and 44.92% were female with a male to female ratio of approximately "1.22:1". The highest number of patients was in the age range of 60-69 years. Left side tumors were more common, 77.12% of patients. The presence of 41.53% of the patients in stage III makes that stage majority. The most common histopathological type was adenocarcinoma NOS, 90.68%. Overall one-year
survival was 77.1%. The one-year survival of rectal tumors is 66%.
Conclusions: The study concluded that the male to female ratio is "1.22:1". The highest number of patients was in the age range of 60-69 years. The percentage of patients below the age of 40 years was 20.51%. The left side tumors were 77% while right side tumors were 23%. The majority of the patients were in stage III and IV, 67%. The most common histopathological type was adenocarcinoma NOS, 90.68%. Overall one-year survival was 77.1%. Rectal tumors had the worst one-year survival, 66% only.
Keywords
Introduction
Colorectal cancer (CRC) is a major cause of morbidity and cancer related mortality throughout the world [1]. It is the third most common cancer worldwide and the fourth most common cause of cancer-related death [2].
Colorectal cancer is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second in women (614,000 cases, 9.2% of the total) worldwide. Almost 55% of the cases occur in more developed regions [3].
Globally, it is believed that approximately 1,200,000 new CRC cases diagnosed annually which accounts for nearly 10% of all incident cancers and mortality from CRC is estimated at about 609,000 [4].
Age has an impact on CRC incidence being greater than all other demographic factors. Therefore, sporadic CRC increases significantly above the age of 45 years for all groups. Colorectal cancer is generally believed to be a disease of older people, as more than 90% of patients being diagnosed above the age 55 years [5]. In Western countries, about 2%-8% of all CRCs occur in young age (< 40 year-old) patients [6-10]. By contrast, many studies have revealed that 15%-35% of CRCs in Middle-Eastern countries occur in young age patients [11,12]. These observations led some authors to suggest a difference in genetic susceptibility to cancer to interpret this wide different proportion of CRC among Middle-eastern and Western countries.
A study that using data from the Surveillance Epidemiology and End Results (SEER) program found an increasing incidence of CRC over the last 20 years in patients aged 20 to 49. The most significant rise was in the age group 40 to 44 where colon and rectal cancers raised 56% and 94%, respectively. Depending on the findings and the fact that CRC leans to be more aggressive in younger patients, the authors recommended the colonoscopic age-based screening for average risk patients beginning at the age of 40 [13].
In nearly all countries, age-standardized incidence rates are less for women than for men. CRC is 25% more likely to occur in men than in women, and the rate is 20% higher in African Americans compared with whites [14].
Colonic carcinogenesis is thought to be a multifactorial process; nevertheless, the direct etiology of CRC remains uncertain [15].
Nearly 20% of cases of CRC are associated with familial clustering, and first-degree relatives of patients with colorectal adenomas or invasive colorectal cancer are at increased risk for colorectal cancer [16-20].
Genetic susceptibility to colorectal cancer includes well-defined inherited syndromes, such as Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer HNPCC) and familial adenomatous polyposis (FAP) [21-23].
Approximately 5%-10% of CRC cases arise because of well- recognized hereditary conditions; however, the vast majorities are sporadic forms in subjects without family history or any apparent predisposing conditions [24].
Epidemiological studies, in addition to familial tendency and the influence of genetic susceptibility, have recognized environmental risk factors that connected to an increased risk, including Western dietary practices, alcohol consumption, smoking tobacco, and physical activity [25-27].
Dietary factors may affect the oncogenesis by modifying intestinal transit time, altering the recycling and flow of bile, or changing the composition of intestinal bacterial flora. Increased body mass index (BMI), and central obesity are emergent risk factors for colorectal cancer. The Framingham Study found that a BMI > 30 increases the risk of colon cancer by 1.5 fold among middle-aged (30 - 54 year individuals) and by 2.4-fold for individuals aged 55- 79 years [28].
Objectives
The epidemiology of colorectal cancer is not well studied in many of the developing countries. The objective of this study is to know the epidemiology and the pathological pattern of colorectal cancer patients in Erbil city, to compare the local data with the regional and global data, to collect demographic and anatomic pathology data, and to find the effect of well-known risk factors on these patients.
Patients and Methods
Retrospective analysis of the data that is being collected from registry units in Rizgary Oncology Center and Nanakaly Cancer Hospital, which are the only public cancer centers in the Erbil city during the period Jan 2016 Dec 2016. All hospital medical records that contain history and physical examination, histopathology reports, imaging reports, endoscopy reports, and treatments received by histologically proven colorectal cancer patients have been taken. The needed information was taken from the records, and the patients or their relatives were contacted by phone if any data was missing or incomplete.
Results
Data of total 118 patients collected, 55.08% (65) of patients were male and 44.92% (53) were female with a male to female ratio of approximately 1.22.
The patients' age ranged from 17 - 86 years, with the median age at diagnosis of 54 years (mean age of approximately 53 years). The highest number of patients was in the age range of 60-69 years (29 patients 24.79%) (Figure 1).
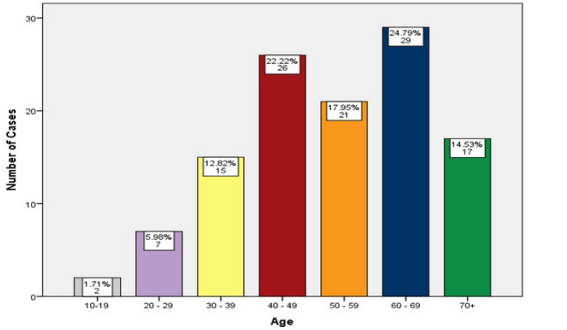
Figure 1: Age Distribution.
The number of patients below the age of 40 years was 24 (20.51%). The left side tumors were the most common, 91 (77.12%) of the patients.
The majority of the patients were in stage III, 49 (41.53%) of the patients (Figure 2).
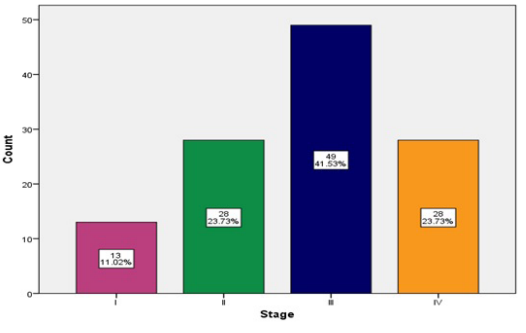
Figure 2: Stage Distribution.
The most common histopathological type was adenocarcinoma NOS, 107 (90.68%) of the patients.
The most common histologic grade was grade II, 88 (74.58%) of the patients (Figure 3).
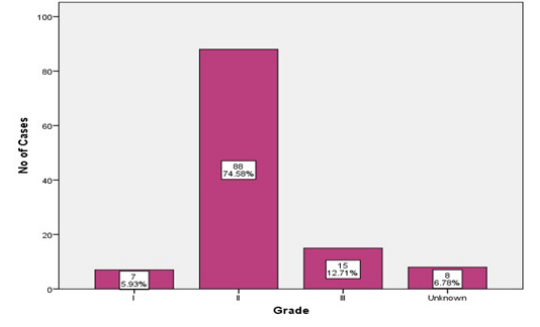
Figure 3: Grade Distribution.
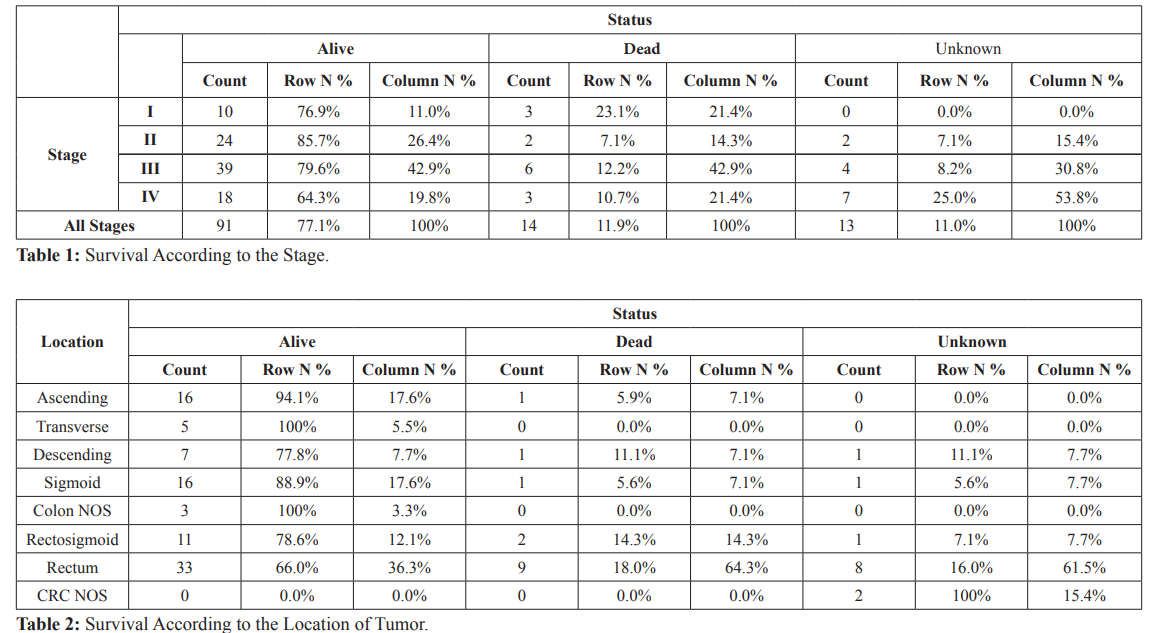
Overall one-year survival was 77.1% and one-year survival for stages I, II, III and IV were 76.9%, 85.7%, 79.6% and 64.3% respectively (Table 1).
Rectal tumors had the worst one-year survival (66% only) accounting for about 64.3% of CRC deaths in one-year duration (Table 2).
Discussion
During the period of Jan Dec 2016, data of 118 patients collected, there is a slight male predominance, 55.08% (65) of patients were male and 44.92% (53) were female with a male to female ratio of approximately "1.22:1". This is nearly consistent with the previous studies and cancer data registries in Qatar (58% vs 42% and M:F 1.4:1) [29], Jordan (57.5% vs 42.5% and M:F 1.35:1) [30], Iran (54.9% vs 44% and M:F 1.24:1) [31], Croatia (57.3% vs 42.7% and M:F 1.34:1) [32], UK (M:F 1.27:1) [33], (M:F 1.2:1) [34], (55% vs 45% and M:F 1.2:1) [35], Canada (51.9% vs 48.1% and M:F 1.08:1) [36] and US (46 vs 35.1/100.000 and M:F 1.31:1) [37].
The patients' age ranged from 17 - 86 years, with mean age of 53 years. This is lower than the other studies and cancer data registries in Qatar (age range 33 83 years, mean age 57.1 years) [29], (age range 31 80 years, median age 62 years) [38], Iran (median age 57 years) [31], Croatia (mean age 65.3 years) [32], UK (mean age 70 years) [33], US (median age 67 years) [37], Taiwan (mean age 62 years) [39], Austria (mean age 67 years and median age 69 years) [40], but slightly higher than a Jordanian study (age range 14 81 years, mean age 49) [30].
The highest number of patients was in the age range of 60-69 years (29 patients 24.79%) (Figure 1) which is consistent with the other studies in Qatar [29], Croatia [32], Taiwan [39], lower by about a decade in comparison with other studies and cancer data registries in UK [34,35], Canada [36] and France [41], but higher by a decade in comparison with the other studies and cancer data registries in Qatar [38] and Egypt [42].
The number of patients under the age of 40 was 24 (20.51%) (Figure 3). This is a bit comparable with the other studies in the neighboring countries (Qatar 20% [29], Iran 17% [31], Jordan 20.2% [43] and Saudi Arabia 23% [44]). However, it is more than twice or thrice in comparison to the other studies in some other Asian countries (Taiwan 6.2% [39], Singapore 5.1% [45] and Japan 10% [46]), and three to five times higher than studies done in Europe (France 3% [41], Georgia 8.6% [47], Italy 4.2% [48] and Sweden 2.5% [49]), New Zealand 5.5% [44] and USA 6% [50]. The number is much less than other studies in Egypt 38% [42] and India 38% [51]. The increasing proportion of young age CRC cases recorded in Iraq, and many regional and neighboring countries, may be due to the young age-structure of these countries.
The left side tumors were the most common, 91 (77.12%) of the patients which is consistent with the other study in Qatar (79.5%) [29], but less than other studies and cancer data registries in UK (69%) [35], Austria (64.7%) [40], and US (50%) [52]. The majority of the patients were in stage III, 49 (41.53%) of the patients (Figure 2) which is higher than the other studies and cancer data registries in Qatar (35.5%) [29] and (41%) [38], UK (26%) [34].
The histopathological types were adenocarcinoma NOS, signet ring carcinoma, and mucinous carcinoma, 107 (90.68%), 6 (5.08%) and 5 (4.24%) of the patients, respectively. The adenocarcinoma NOS and signet ring carcinoma are more in comparison to other studies and cancer data registries in Qatar, (78.62%) and (2.07%) respectively, but the mucinous carcinoma is comparable (4.83%) [38]. The signet ring carcinoma and mucinous carcinoma are much less in comparison to another study done in Jordan (14%) and (31%) respectively [30].
Overall one-year survival was 77.1% and one-year survival for stages I, II, III and IV were 76.9%, 85.7%, 79.6% and 64.3% respectively, but this is not statistically significant (P value > 0.05) (Table 1). Although survival of stages II and III appears to be better than stage I numerically, but this might be explained by the post-operative staging of rectal cancers that received neoadjuvant chemoradiotherapy. The one-year survival is lower than other studies and cancer data registries in Qatar (84%) [38], Croatia (84.4%) [32], US (83.6%) [53], (83%) [54].
Rectal tumors had the worst one-year survival in comparison with other parts of colorectum (66% only), accounting for about 64.3% of CRC deaths in one-year duration (Table 2). The one-year survival for rectal cancers is much lower than the other studies and cancer data registries in Europe (UK 74.9%), (Denmark 79.6%), (Norway 81.8%), (Sweden 84.1%), Canada (84.8%) and Australia (84.6%) [55].
Four patients had previous history of cancer (2nd malignancy), one patient had familial adenomatous polyposis (FAP), and one patient had previous history of ulcerative colitis (UC).
Limitations
Despite all efforts, there are some limitations in this study. The information provided by the medical records may not be accurate especially for the patients that are clinically staged or received neoadjuvant treatment. Some variables that included in the questionnaire could not be analyzed because of incomplete histopathological reports that did not mention all recommended information especially molecular studies like MMR, RAS and BRAF studies due to lack of advanced laboratory services. Finally, not all patients might be registered in these two public centers during that period, since there are private sectors that manage cancer patients.
Conclusions
The study concluded that the male to female ratio is "1.22:1". The highest number of patients was in the age range of 60-69 years. The percentage of patients below the age of 40 years was 20.51%. The left side tumors were 77% while right side tumors were 23%. The majority of the patients were in stage III and IV, 67%. The most common histopathological type was adenocarcinoma NOS, 90.68%. Overall one-year survival was 77.1%. Rectal tumors had the worst one-year survival, 66% only.
Acknowledgements
Dr. Bestoon S. Hasan, MBChB, HD CO, for his support with some of the data…
References
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk Clinics in colon and rectal surgery. 2009; 22: 191-197.
- World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global Amer Inst for Cancer Research. 2007.
- http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and Cancer Epidemiology and Prevention Biomarkers. 2010; 19: 1893- 1907.
- Atkin WS, Northover JM, Cuzick J, et Prevention of colorectal cancer by once-only sigmoidoscopy. The Lancet. 1993; 20: 341: 736-740.
- Bülow S. Colorectal cancer in patients less than 40 years of age in Denmark, 1943-1967. Diseases of the Colon & 1980; 23: 327-336.
- Griffin PM, Liff JM, Greenberg RS, et al. Adenocarcinomas of the colon and rectum in persons under 40 years Gastroenterology. 1991; 100: 1033-1040.
- MacGillivray DC, Swartz SE, Robinson AM, et Adenocarcinoma of the colon and rectum in patients less than 40 years of age. Surgery. Gynecology & Obstetrics. 1991; 72: 1-7.
- Guillem JG, Puig-La Calle J, Cellini C, et Varying features of early age-of-onset “sporadic” and hereditary nonpolyposis colorectal cancer patients. Diseases of the colon & rectum. 1999; 42: 36-42.
- Mitry E, Benhamiche AM, Jouve JL, et Colorectal adenocarcinoma in patients under 45 years of age: comparison with older patients in a well-defined French population. Diseases of the colon & rectum. 2001; 44: 380-387.
- Al-Jaberi TM, Ammari F, Gharieybeh K, et al. Colorectal adenocarcinoma in a defined Jordanian population from 1990 to Diseases of the colon & rectum. 1997; 40: 1089-1094.
- Soliman AS, Bondy ML, Levin B, et al. Colorectal cancer in Egyptian patients under 40 years of age. International journal of 1997; 71: 26-30.
- Davis DM, Marcet JE, Frattini JC, et Is it time to lower the recommended screening age for colorectal cancer? Journal of the American College of Surgeons. 2011; 213: 352-361.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010; 60: 277-300.
- Wilmink AB. Overview of the epidemiology of colorectal Diseases of the colon & rectum. 1997; 40: 483-493.
- Ahsan H, Neugut AI, Garbowski GC, et Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Annals of internal medicine. 1998; 128: 900-905.
- Bonbllj L, Martines H, Conio M, et al. Family history of colorectal cancer as a risk factor for benign and malignant tumours of the large A caseâ?control study. International journal of cancer. 1988; 41: 513-517.
- Hemminki K, Eng Clinical genetic counselling for familial cancers requires reliable data on familial cancer risks and general action plans. Journal of medical genetics. 2004; 41: 801-807.
- Hemminki K, Chen Familial risk for colorectal cancers are mainly due to heritable causes. Cancer Epidemiology and Prevention Biomarkers. 2004; 13: 1253-1256.
- Quintero E, Carrillo M, Leoz ML, et Alonso-Abreu I. Risk of Advanced Neoplasia in First-Degree Relatives with Colorectal Cancer: A Large Multicenter Cross-Sectional Study. PLoS medicine. 2016; 13: e1002008.
- Hampel H, Frankel WL, Martin E, et Feasibility of screening for Lynch syndrome among patients with colorectal cancer. Journal of Clinical Oncology. 2008; 26: 5783-5788.
- Lynch HT, De la Chapelle A. Hereditary colorectal cancer. New England Journal of 2003; 348: 919-932.
- Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. The American journal of gastroenterology. 2006; 101: 385-
- Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer Oncogene. 2004; 23: 6445-6470.
- Le Marchand L, Wilkens LR, Kolonel LN, et al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer research. 1997; 57: 4787-4794.
- Lin Acquired risk factors for colorectal cancer. Cancer Epidemiology: Modifiable Factors. 2009; 472: 361-372.
- Lieberman DA, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic Jama. 2003; 290: 2959-2967.
- Moore LL, Bradlee ML, Singer MR, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. International journal of obesity. 2004; 28: 559-567.
- Rasul KI, Awidi AS, Mubarak AA, et al. Study of colorectal cancer in Saudi medical journal. 2001; 22: 705-707.
- Dajani YF, Zayid I, Malatjalian DA, et al. Colorectal cancer in Jordan and Nova Scotia a comparative epidemiologic and histopathologic Cancer. 1980; 46: 420-426.
- Ansari R, Mahdavinia M, Sadjadi A, et Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer letters. 2006; 240: 143-147.
- Tomas D, Belicza M, BaliceviÄ? D, et al. Colorectal Cancer Trends by Age and Sex Distribution, Anatomic Subsite and Survival (1989-2002). Acta Clinica Croatica. 2003; 42: 175-
- Gomez D, Dalal Z, Raw E, et al. Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: is there a true “rightward shift”?. Postgraduate medical journal. 2004; 80: 667-669.
- National Collaborating Centre for Cancer (UK. Colorectal cancer: the diagnosis and management of colorectal cancer).
- https://www.cancerresearchuk.org/home
- Gao RN, Neutel CI, Wai E. Gender differences in colorectal cancer incidence, mortality, hospitalizations and surgical procedures in Canada. Journal of public health. 2008; 30: 194-201.
- http://seer.cancer.gov/statfacts/html/colorect.html
- Qatar National Cancer Registry QNCR - Annual Report
- Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. British journal of surgery. 2003; 90: 205-214.
- Brozek W, Kriwanek S, Bonner E, et al. Mutual associations between malignancy, age, gender, and subsite incidence of colorectal Anticancer research. 2009; 29: 3721-3726.
- Adloff M, Arnaud JP, Schloegel M, et Colorectal cancer in patients under 40 years of age. Diseases of the colon & rectum. 1986; 29: 322-325.
- Abou-Zeid AA, Khafagy W, Marzouk DM, et al. Colorectal cancer in Egypt. Diseases of the colon & rectum. 2002; 45: 1255-1260.
- Al-Jaberi TM, Yaghan RJ, El-Heis HA. Colorectal cancer in young patients under 40 years of age. Comparison with old patients in a well-defined Jordanian Saudi medical journal. 2003; 24: 871-874.
- Isbister WH. Colorectal cancer below age 40 in the Kingdom of Saudi ANZ Journal of Surgery. 1992; 62: 468-472.
- Chung YF, Eu KW, Machin D, et al. Young age is not a poor prognostic marker in colorectal British journal of surgery. 1998; 85: 1255-1259.
- Okuno M, Ikehara T, Nagayama M, et Colorectal carcinoma in young adults. The American journal of surgery. 1987; 154: 264-268.
- Parramore JB, Wei JP, Yeh KA, et al. Colorectal cancer in patients under forty: Presentation and outcome/Discussion.The American surgeon. 1998; 64: 563.
- Torsello A, Garufi C, Cosimelli M, et al. P53 and bcl-2 in colorectal cancer arising in patients under 40 years of age: distribution and prognostic relevance. European Journal of 2008; 44: 1217-1222.
- Öhman Colorectal carcinoma in patients less than 40 years of age. Diseases of the Colon & Rectum. 1982; 25: 209-214.
- O'Connell JB, Maggard MA, Livingston EH, et Colorectal cancer in the young. The American journal of surgery. 2004; 187: 343-348.
- Pal Proportionate increase in incidence of colorectal cancer at an age below 40 years: an observation. Journal of cancer research and therapeutics. 2006; 2: 97.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, CA: a cancer journal for clinicians. 2017; 67:177-193.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000-2014) , National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016
- Acs AC. Cancer facts and figures 2010. American Cancer Society, National Home Office, 2010: 1-44.
- Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: A population-based study of patients diagnosed during 2000- Acta Oncologica. 2013; 52: 919-932.