The HIV Patient and their Antibody Response to SARS-CoV-2 Infection
Author'(s): Robert L. Stout, PhD, Steven J. Rigatti, MD
Clinical Reference Laboratory, Lenexa, Kansas Lenexa, KS United States of America.
*Correspondence:
Robert L. Stout, PhD, Clinical Reference Laboratory, Lenexa, Kansas Lenexa, KS United States of America.
Received: 10 August 2021; Accepted: 06 September 2021
Citation: Stout RL, Rigatti SJ. The HIV Patient and their Antibody Response to SARS-CoV-2 Infection. Clin Immunol Res. 2021; 5(2): 1-2.
Abstract
SARS-CoV-2 infected HIV patients have a similar antibody response to infection as age and sex-matched controls.
Well, HIV patients previously infected with COVID-19 have a similar rate of infection and antibody response as age and sex-matched controls.
Keywords
Introduction
We investigate the antibody response to COVID-19 infection in otherwise healthy HIV-positive life insurance applicants.
Methods
From January 2020 to March 2021, we evaluated a national adult convenience sample of 1,191 HIV positive self-reported healthy life insurance applicants for the frequency and level of antibody to nucleocapsid protein with the Roche SARS CoV-2 total antibody test [1]. We did all testing at Clinical Reference Laboratory, Lenexa, KS.
To investigate if HIV was associated with a detectable difference in immunological response, we examined COVID-19 antibody levels in age and sex-matched HIV- negative applicants.
We recorded the applicant's age, sex and deleted all personal data. Statistics were Chi square, T-test, and Mann Whitney U test to examine differences between positive and negative groups as appropriate with a selected significance level of 99%. All statistical analyses were with R (version 3.6.1) [2] and R-studio (version 1.2.1335) [3]. The study conforms to the recommendations of STROBE for Cross-sectional epidemiology studies, www.equator- network.org.
All participants signed disclosures allowing the use of their de- identified data for research purposes. Western IRB (Puyallup, WA) reviewed the study under the Common Rule and applicable guidance and determined it is exempt under 45 CFR § 46.104(d)(4) using de-identified study samples for epidemiologic investigation. WIRB Work Order #1-1324846-1.
Results
We tested 1,191 HIV-positive applicants for antibodies to COVID-19 with the Roche Diagnostics SARS-CoV-2 (total antibody to nucleocapsid protein). The age and sex distribution were 893 (74.5%) were male, and 296 (24.5%) were female with median ages of 40 years (IQR: 33, 40) and 45 years (IQR: 38, 53), respectively. Likewise, we tested the age and sex-matched control group of HIV-negative applicants.
The overall SARS-CoV-2 seroprevalence in HIV-positive females was 12.8% (38 out of 297) and was 12.9% (115 out of 894) in HIV-positive men.
Between the HIV positive and HIV negative groups the COVID-19 seronegative and seropositive, the difference in age and sex were not statistically significant Table 1 (page = 0.23, psex = 0.54). COVID-19 antibody levels in the age and sex-matched control plus HIV positive groups varied from 1.1 to > 500 cut-off intensity units (COI), a measure of the amount of antibody present. For the age and sex-matched COVID positives, the HIV-positive population had a higher proportion of individuals with lower COVID antibody levels than the HIV- negative individuals. Still, the distributions of antibody levels are not statistically different (Figure 1).
Discussion
HIV-positive applicants must be negative for the virus, have been on successful anti-retrovirus therapy for at least three years, have acceptable CD4 levels, and be under annual health care. Therefore, HIV-positive insurance applicants may represent a select group of HIV patients.
Jérémy Dufloo et al. [4] have reported that antibody to COVID-19 in symptomatic and asymptomatic patients is protective. The authors also note that while the asymptomatic patient may have lower antibody levels, the antibodies are still protective. They report that sera from asymptomatic individuals neutralize the virus, activate Antibody-Dependent Cellular Cytotoxicity (ADCC) and trigger complement deposition using replication-competent SARS CoV-2 or reporter cell systems. If Dufloo's study results can be extended, they suggest that asymptomatically infected COVID-19 antibody- positive HIV patients may have a similar level of protection as the HIV-negative patient.
We report that SARS-CoV-2 anti-nucleocapsid antibody levels are similar in HIV positive and age and sex-matched HIV negative individuals in a healthy well population, independent-samples Mann Whitney U Test (p* = 0.147), no statistical difference between groups. Thus, our results suggest that the healthy HIV patient may have a similar risk for adverse events or reinfection as the HIV negative patient.
Limitations
Limitations of the study include self-reported health status (well), the applicant may fail to report a prior symptomatic COVID-19 infection and possible misrepresentation of their health status. In addition, there was no attempt to determine if the antibody was protective.
Conclusion
This report is for a sample of a healthy HIV-positive population that appears to be compliant with current clinical treatment guidelines. Anti-SARS-CoV-2 antibody levels are similar in HIV positive and age and sex-matched HIV negative individuals in a healthy well population.
Acknowledgement
Funding was by Clinical Reference Laboratory, Lenexa, KS. Dr. Rigatti is a paid consultant for the evaluation of mortality data for Clinical Reference Laboratory. The funding agency had no input to the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, nor the decision to submit the manuscript for publication.
Both authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Table 1: SARS-CoV-2 Seropositivity by Age in the HIV-positive population.
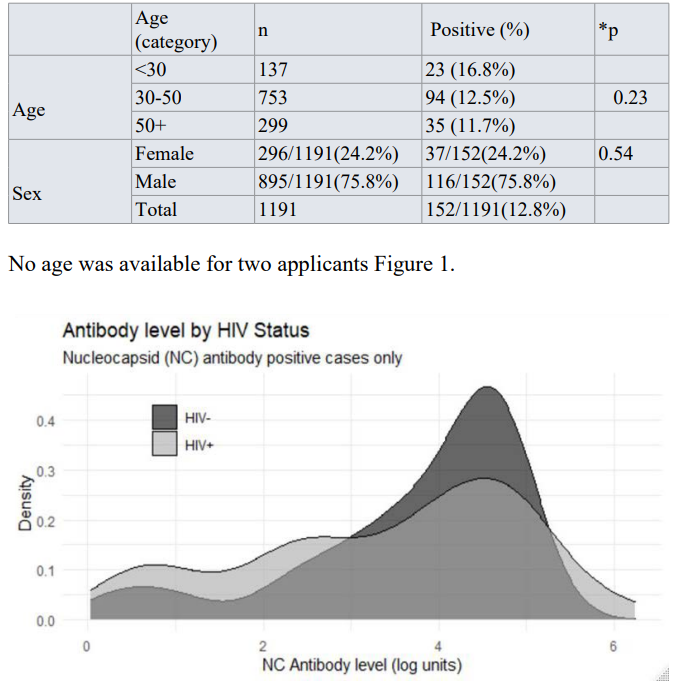
Comparison of COVID-19 antibody levels in HIV positive and negative serum samples.
References
- Roche Elecsys® Anti-SARS-CoV-2. Package Insert 2020-07, V4.0; Reference 09203095190 and 09203079190.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. https://www.R-project.org/
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. http://www.rstudio.com/
- Jérémy Dufloo, Ludivine Grzelak, Isabelle Staropoli, et al. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Reports Medicine. 2021; S2666- 3791(21)00103-8.