The Importance of Il8 Gene Expression and Il-8 A251t in Diagnosis and Prognosis of Ovarian Cancer in Iraqi Women
Author'(s): Khaled A. Habeb1* and Maysaa Ghazi. Jumaah2
1Department of Biology, College of Science for Women,University of Baghdad, Baghdad, Iraq.
2Department of Microbiology, College of medicine, University of Maisan, Maisan, Iraq.
*Correspondence:
Khaled A. Habeb, Department of Biology, College of Science for Women, University of Baghdad, Baghdad, Iraq.
Received: 02 Oct 2022; Accepted: 10 Nov 2022; Published: 15 Nov 2022
Citation: Habeb KA, Jumaah MG. The Importance of Il8 Gene Expression and Il-8 A251t in Diagnosis and Prognosis of Ovarian Cancer in Iraqi Women. Cancer Sci Res. 2022; 5(3): 1-7.
Abstract
The present study aimed to shed light on the association between the gene expression of IL8 and its genetic polymorphism IL-8 A251T in the incidence and pathogenesis of ovarian cancer. A total of 43 Paraffin-embedded tissue blocks from patients with different stages of newly diagnosed ovarian cancer were provided by certain Iraqi hospitals as well as 14 samples of patients with benign ovarian tumors tissues as a control group were used in this study. In the present study, the level of IL8 gene expression was investigated by comparing it with that of benign tumors, the results detected that IL-8 mRNA was expressed in 47(81.02%) of samples, 36 samples with ovarian cancer and 11 benign ovarian. IL-8 mRNA levels in ovarian cancer tissues were statically significant higher than those in benign ovarian tissue (P=0.0328<0.05). The samples were divided into high and low mRNA-expression depending on the mean value of IL8 gene expression in benign tumors which used as a cutoff, the results showed that samples with high mRNA-expressing 25(69.4%) which showed high significant differences compare with samples that showed low expressing 11(30.55%) (P value=0.0028<0.01). In correlation with histopathological type of ovarian tumors, mucinous tumors showed statistically significant difference in compare with other histopathologic tumor type (P=0.0516,<0.05). According to the tumor stages, statistically significant difference was found between 31(86.11%) of samples with stage I which showed the highest level of expression and 5(13.88%) of samples with stage III (P value=0.0410<0.05). For A251T polymorphism, the result showed that 21(58.33%) patients were heterozygous A/T, 14(38.88%) were homozygous T/T, and 1(2.77%) was homozygous A/A. For patients with benign ovarian tumors 11(91.66%) were heterozygous A/T, 1(8.33%) was homozygous T/T, and no one of the patients were homozygous for the A/A genotype. High significant prevalence of the IL-8 251T allele was detected in both ovarian cancer patients (P value 0.0047 <0.01) and patients with benign ovarian tumors (P value 0.014 <0.02) as compared with IL-8 251A allele. In the present study the association of the A251T polymorphism with ovarian cancer were investigated by attending to levels of IL-8 mRNA in benign and malignant ovarian tissues related with the respective genotypes. In benign ovarian tumors statically analysis showed that there were no significant differences between genotypes, while for cancer samples, the average of IL-8 gene expression in ovarian cancer patients carrying +251TT genotype was highly significant than that in ovarian cancer patients with the +251AT and +251AA genotypes (p value= 0.0037<0.01). In conclusion, the results reflected the possibility of detecting the IL8 gene transcript in benign as well as the malignant ovarian tissues but with wide differences in the sample percentages and level of gene expression which in turn reflect the value of IL8 gene as a useful tool for discriminating malignant breast tumors from non-malignant ones. On the other hand the high level of gene expression associated with stage I of tumor may be reveals the diagnostic value of this gene for early diagnosis of ovarian cancer. The results showed that the genetic variation of IL-8 gene influences susceptibility to ovarian cancer, we also suggested that the TT genotype of IL-8 -251A/T may associate with increase the risk of ovarian cancer in Iraqi women because of altered IL-8 gene expression.
Keywords
Introduction
IL-8 is a member of the CXC chemokine family and is classified and referred to as CXCL8 according to the new nomenclature sys- tems [1]. Transcription of the IL- 8 gene encodes for a protein of 99 amino acids that is subsequently processed to yield a signaling competent protein of either 77 amino acids in nonimmune cells or 72 amino acids in monocytes and macrophages. IL-8 belongs to a super family of chemokines that has chemotactic activity for neu- trophils, esosinophils, basophils, monocytes, mast cells, dendric cells, nature killer (NK) cells, and T and B lymphocytes [2] The chemokine are divided into four subgroups, CXC, CC, CX3C and C chemokines, in which C indicates NH2- terminal cysteines and X indicates intervening amino acid, respectively [1]. The gene en- coding cytokine IL-8/CXCL8 is found on human chromosome 4, q12-21, and consists of four exons and three introns [3]. IL-8 binds to two distinct receptors, CXCR1 and CXCR2, with a similar high affinity [4] CXCR1 and CXCR2 have been shown to be expressed in a variety of cells, such as leukocytes, endothelial cells and ma- lignant cells [5]. The biologic effect of IL-8 originally described includes chemotaxis for a variety of leukocytes, facilitating leuko- cyte transmigration into the tissue by inducing adhesion molecule expression and promoting neutrophil adhesion to extracellular ma- trix, activating various functions of neutrophil, including degran- ulation and the release of leukotrienes B4 and platelet-activating factors [6]. Evidence has revealed that IL-8 has several biologic functions not related to leukocyte chemotaxis and migration and may play an important role in cancer progression. Tumor associ- ated IL-8 expression was first found in malignant melanoma cell lines, and its expression was considered to play a role in regulat- ing the growth and metastasis of melanoma [7]. Tumor associated IL-8 is thought to play at least five roles in the biology of primary and metastatic cancers [8], including control leukocyte infiltrate into tumor tissue, modification of tumor immune response, regu- lation of angiogenesis, autocrine or paracrine, regulation of tumor growth and survivals, and promoting tumor cell migration. Evi- dence shows that IL-8 biologic activity in tumors and the microen- vironment may contribute to cancer progression, and in other cir- cumstances to host anti-tumor response, and this biologic response may be different in different types of human cancers [9]. Some studies have shown that IL-8 was constitutively expressed in sev- eral human cancer cell lines derived from astrocytoma, hepatoma, transitional cell carcinoma, and melanoma, and is associated with angiogenesis and metastatic potential in human melanoma cell lines in a nude mice model [10]. IL-8 is also a well-known chemo- attractant factor for leukocytes, and leukocytes infiltration is fre- quently seen in some types of human cancers, including NSCLC. These recruited inflammatory cells can enhance angiogenesis by secreting several cytokines, such as tumor necrotic factor (TNF)-α. Thus, in addition to its direct angiogenic effect, IL-8 secreted by tumor cells might also indirectly induce angiogenesis by recruiting inflammatory cells to the tumor tissues [11].
IL-8 expression can be induced by various stimuli, such as lipo- polysaccharides, cytokines (IL-1, TNF-α), and bacterial or viral products, while IL-8 is also constitutively expressed in many hu- man cancers [12]. Other stimuli can also regulate constitutive IL-8 expression in tumors. IL-8 expression has been shown to be up- regulated by inflammatory cytokines and ultraviolet light in mel- anoma, and downregulated by interferon (INF)-α and INF-β. Ac- cumulating evidence generated in clinical studies shows increased tumoral IL-8 expression correlates with adverse patient prognosis in a variety of human cancers. Another study has shown that IL-8 expression is associated with a poor prognosis in epithelialvovar- ian cancer patients [13]. Although a wide variety of cytokines can be measured in ovarian cancer ascites, interleukin-6 (IL-6) and interleukin- 8 (IL-8) are among the most abundant. The concentra- tion of these pro-inflammatory cytokines in ascites is 40- to 500- fold higher as compared to the levels found in serum [14]. The source of the IL-8 found in ascites has not been well defined. These pro-inflammatory cytokines are involved in different pathophysio- logical processes including carcinogenesis. IL-8 that was recently reported to promote ovarian tumor growth in vivo [15]. In the pres- ent study, we aimed to shed light on the role and importance of IL8 gene expression and its particular polymorphism IL-8 A251T as a tool for diagnosis and prognosis of ovarian cancer.
Materials and Methods
Subjects and samples collection: The tissue samples used in this study included 43 Paraffin-embedded tissue blocks from patients with different stages of newly diagnosed Invasive ovarian cancer were provided by certain Iraqi hospitals (including Al-Kadhemia, AL -Yarmouk Teaching Hospital, Baghdad Hospital, the Teaching Laboratories of Medical City, Nuclear Medical Hospital in Bagh- dad and Alsader Hospital in Missan) after patients underwent to total abdominal hysterectomy and bilateral salpangio-oopherecto- my (TAH-BSO), subtotal abdominal hysterectomy, vaginal hys- terectomy, and endometrial biopsy, 14 samples of patients with benign ovarian tumors tissues were used as a control. The required information about the patients and the histopathologic properties of the tumors were recorded from the patients’ files. The Paraf- fin-embedded tissue blocks were sectioned into 10µm in DNase- RNase tubes for molecular evaluation. Samples subjected to RNA extraction and molecular study by using Revers Transcription and Real Time PCR at Molecular Oncology Unit in Guy´s hospital – Kings college/London.
RNA extraction, reverse transcription
Total RNA was extracted from benign and malignant tumor tis- sues and normal tumors was extracted using the RNeasy FFPE Kit, which designed for purifying total RNA from FFPE tissue sections (Qiagen-USA) according to the protocol provided by the manufacturer. Total RNA was reversely transcribed using Ther- mo-Script™ Reverse Transcription kit (Invitrogen/USA). The procedure was carried out in a reaction volume of 50 μl composed of 15 µl Denaturized RNA,0.2 μl Random hexamere primers 3µg/ µl, 5μl of 10 mM dNTP Mix, 10μl of 5x cDNA synthesis buffer, 2.5µl RNase OUT (40U/µl), 2.5µl ThermoScript RT (15 units/µl),14.8µl DEPC-treated water. The samples were then placed in a 96 Well Thermal Cycler, and cycled at the following conditions: 25ºC for 10 min., 10 min. at 37ºC, and 60 min. at 42ºC followed by 75ºC for 5 minutes. The converted cDNA. Was stored at -80°C and used as a template for PCR amplification of VEGF. Primers and probes were designed using Primer Express software (ABI, USA). The primers and prop for IL8 were as follows:5' CTGG- CCGTGGCTCTCTTG3'(foreword),5'CCTTGGCAAAACTG- CACCTT3'(reverse),5'CAGCCTTCCTGATTTCTGCAGCTCT-GTG -3(prop). PGK1 gene used as an endogenous control gene. The amplification of IL8 cDNA for real time RCR analysis was performed in duplicate using the Applied Bio systems 7900. The 25 µl of reaction volume containing 10 µl of master mix ,3 µl of primer mixes, 3µl of RNase free water and 4µl of cDNA template. Real-Time PCR protocol was as follows; stage 1 50ºC for 1 min- utes, (stage 2: 95ºC for 45 sec., 55C for 45 Sec. and 72ºC for 1 min.) repeated for 32 cycles. The slope of a standard curves was used to estimate the PCR amplification efficiency of a real-time PCR reaction. A calculation for estimating the efficiency (E) of a real-time PCR assay was performed as following:
E = (10 –1/slope –1) × 100
E = (10 –1/3.35 –1) × 100
For each sample, the cycle threshold (Ct) is defined as the number of PCR cycles required to achieve the user defined level of fluo- rescence. This Ct value is used to compare across all samples. The Ct is inversely proportional to the amount of starting mRNA of the target gene (IL8) as well as the endogenous control gene (PGK1). The relative fold change ratio of the target gene in the sample was calculated as described below:
Log copy(enodogenus control gene) = (Ct -32.85) /-3.3592 Copy number(enodogenus control gene) = 10^Log copy Log copy(IL8) = (Ct -34.82) /-3.5126 Copy number(VEGF) = 10^Log copy
Fold change = Copy number (IL8)/Copy number (endogenous control gene)
DNA Extraction, Genotyping and Sequencing Analysis
DNA was extracted and purified from Formalin-Fixed Paraf- fin-Embedded (FFPE) tissue sections of 58 ovarian tumor sam- ples with QIAamp DNA FFPE Tissue Mini Kit (Qiagen,USA) which was designed for purifying of DNA from FFPE tissue sections. The primers for IL-8 A251T were as follows: 5'- TTGTTCTAACACCTGCCACTCT-3' (forward) and 5'- TGAC-
GAAAGTTTTCTTTGATCTTT-3' (reverse). The procedure was carried out in a reaction volume of 20 μl. The PCRs conditions were as follows: an initial step of 10 min at 95°C foe enzyme acti- vation, followed by 40 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 30 s for denaturation, annealing and extension respectively, followed by final extension for 5 min. at 72°C, and then incubation at 4°C to time end. The resulting DNA fragments were separat- ed by 2% agarose gel electrophoresis and visualized under UV light after ethidium staining. Before sequencing the PCR products purified by using Charge Switch PCR Clean-up kit according to the manufacturer's instructions, the sequencing performed using ABI BigDye terminator ready reactions Kit (Applied Biosystems,USA) and ABI Automated DNA Sequencer 3730. Data were an- alyzed using Mutation Surveyor Software of sequencing reading Version 3.24.
Statistical Analysis
The Statistical Analysis System-SAS (2012) program was used to effect of difference factors in study parameters. Chi-square test was used to significant compare between percentage and Least significant difference –LSD test was used to significant compare between means in this study.
Results
A total of 58 samples of 44 samples with ovarian cancer and 14 benign ovarian tumors tissues were examined for the expression of IL8 rt-PCR. The patients’ age range was 14-70 years and the median is 47 years. According to the family history, all samples were negative for family history to the ovarian cancer. Clinical features of ovarian cancer samples are listed in table 1. About the menopausal state of ovarian cancer patients, 20(46.5%) of samples were premenopausal, while 23(53.48%) of them were postmeno- pausal. According to the International Federation of Gynecology and Obstetrics (FIGO) surgical staging system, most of samples 35(81.4%) came with stage I, while the other 8 (18.6%) samples were with stage III. According to the tumor histological types, the samples were divided into three clinical groups; surface epithelial tumors 38(88.3%) samples, sex cord tumors 3(6.9%) samples, and germ cell tumors 2(4.65%) samples. qRT-PCR analysis revealed that IL8mRNA was expressed in the tissues of both malignant and benign tumors (figure 1). The present study showed that IL8 mRNA was expressed in 47(81.02%) of samples, 36 samples with ovarian cancer and 11 benign ovarian. The higher level IL8 mRNA in ovarian cancer samples was statistically significant (Mean ± SE: SE:8.68 ± 2.49, P=0.0328<0.05) compared to benign tumors (Mean ± SE: 1.99 ± 0.84). The mean value of IL8 gene expression in benign tumors was used as a cutoff to separate tumors into high and low mRNA-expressing samples which showed high statisti- cally significant differences (P value = 0.0028<0.01) between high mRNA-expressing 25(69.4%), and low mRNA-expressing sam- ples 11(30.55%). In correlation with the histopathological type of ovarian tumors mucinous tumors showed statistically significant difference in the level of IL-8 gene expression (Mean ± SE: 11.37± 9.90, P=0.0516,<0.05) compared with other histopathologic tu- mor type. According to the tumor stages, statistically significant difference was found between 31(86.11%) of samples with stage I which showed the highest level of expression (Mean ± SE: SE: 8.38 ± 3.48, P value=0.0410<0.05) and 5(13.88%) samples with stage III (Mean ± SE: : 2.58 ± 0.71). The results of genotyping in benign and ovarian cancer patients viewed in (table 2). The result of IL-8 A251T polymorphism showed that out of 36 ovarian cancer patients 21(58.33%) patients were heterozygous A/T,14(38.88%) patients were homozygous T/T, and 1(2.77%) were homozygous A/A (figure 2). For patients with benign ovarian tumors, 11 pa- tients(91.66%) were heterozygous A/T, one patient (8.33%) was homozygous T/T, and no one of the patients were homozygous for the A/A genotype. High significant prevalence of the IL-8 251T allele was detected in both ovarian cancer patients (P value 0.0047<0.01) and patients with benign ovarian tumors (P value 0.014<0.02) as compared with IL-8 251A allele. In compare with IL-8 gene expression, the study showed that the average of IL-8 gene expression in ovarian cancer patients carrying +251TT genotype (9.115415) was highly significant (p value= 0.0037<0.01) than that in ovarian cancer patients with the +251AT and +251AA gen- otypes (4.508481, 4.867132), while In benign ovarian tumors sta- tistically analysis showed that there were no significant differences between genotypes. The analysis of the distribution of the three IL-8 A251T genotypes in correlation with stages of cancer showed that for patients with stage I the percentages of these genotypes were 61%, 39%, and 0% for the genotypes AT,TT, and AA respec- tively. While for patients with stage III, the percentages of these genotypes were 43%, 43%, and 14% for these genotypes. The AT genotypes in patients with stage I showed highly statistically sig- nificant among other genotypes (P value 0.00043 < 0.001).
Discussion
In the present study, the level of IL8 gene expression was investigated by comparing it with that of benign tumors. IL-8 mRNA was expressed in 47(81.02%) of samples, 36 samples with ovarian cancer and 11 benign ovarian. IL-8 mRNA levels in ovarian cancer tissues were statistically significant higher. Kassim et al.,[13] showed in his study, which was performed on 24 tumors from patients with epithelial ovarian cancer and 20 tissue samples of benign ovarian lesions as a control group that IL-8 mRNA was expressed only in 18.2% of 44 samples he tested. There are difficulties in discussing the differences that had been showed between benign and malignant tumors because most of the studies for IL8 gene expression were performed either using techniques other than qRT-PCR like in situ hybridization or immunohistochemistery (IHC) or the experiments were applied on animal models, cell line and malignant tumors without comparing with benign tumors, ovarian cancer types, and epithelial ovarian tumor subtypes [16,17]. Using the mean value IL-8 gene expression in benign tumors as the cutoff value to showed high significant differences (P value: 0.0028<0.01) as the samples with high mRNA- expressing 25(69.4%) with samples that showed low expressing 11(30.55%). The present study results are different from those reported in other studies including Merritt et al.,[17] who showed that 43 (42%) had high IL-8 expression and 59 (58%) had low or no IL-8 expression, and Kassim et al.,[13] who showed that only seven (29.1%) of 24 samples with epithelial ovarian cancer samples were positive for IL-8 Mrna. In correlation with the histopathological type and stages of ovarian tumors mucinous tumors as well as stage I both showed statistically significant difference in the level of IL-8 gene expression (, P=0.0516,<0.05) and (P value=0.0410<0.05) respectively. The present study results were differed from those reported by Kassim et al.,[13] showed that eight samples were positive for IL-8 mRNA, seven of them were in malignant group, with the highest frequency in stages III and IV of the disease and Merritt et al.,[17] who showed that high IL-8 expression was associated with advanced tumor stage. Davidson et al.,[16] showed that there was no correlation between angiogenic gene expression
(IL-8,VEGF,and bFGF) with patient age, previous treatment, residual tumor size, FIGO stage or disease outcome in survival analysis. He also showed that IL-8 and VEGF are down regulated in carcinoma cells, so he reported that mRNA expression of VEGF, bFGF, and IL-8 does not appear to be a predictor of disease outcome in advanced-stage ovarian carcinoma. The -251T>A polymorphism results showed that 21(58.33%) were heterozygous A/T, 14(38.88%) were homozygous T/T, and 1 (2.77%) were homozygous A/A. These results were similar to those obtained by Savage et al. [18] who showed that the frequencies of these polymorphisms were 44.3%, 29.6%, and 26.1% in patients with gastric cardia adenocarcinoma (GCC) and 42.6%, 37.2% and 20.2% in patients with esophageal squamous cell carcinoma (ESCC). Other study performed by Dumitrescu et al.,[19] showed that the frequencies of these polymorphisms in control samples were 45.33 %,34.67 %, and 20% in patients with colorectal cancer, the frequencies were 46.30 %, 35.19 % and 18.51 %. Aledson et al.,[20] showed that the frequencies of these polymorphisms in control samples were 43.4%,30.1% and 26.5%, while the frequencies were 55.8%,29.8% and 14.4% in patients with gastric cancer. Schultheis et al.,[21] showed that 24.6% of patients were homozygous A/A, 49% of patients were heterozygous A/ T, and 26.4% of patients were homozygous T/T. High significant prevalence of the IL-8 251T allele was detected in both ovarian cancer patients (P value 0.0047<0.01) and patients with benign ovarian tumors (P value 0.014 <0.02) as compared with IL-8 251A. Aledson et al.,[20] showed that the T Carriers 85.6% and 73.5% while those not carriers for T allele were 14.4% and 26.5% in both gastric cancer patients and controls. Andia et al.,[22] showed that A allele frequency was 36% and 48% while the frequency of T allele was 64% and 52% in controls and patients with Chronic Periodontitis respectively. In the present study the association of the SNP IL-8 251A with ovarian cancer were investigated by attending to levels of IL-8 mRNA in benign and malignant ovarian tissues related with the respective genotypes. This study showed that the average of IL-8 gene expression in ovarian cancer patients carrying +251TT genotype (9.115415) was highly significant (p value= 0.0037<0.01) than that in ovarian cancer patients with the +251AT and +251AA genotypes (4.508481, 4.867132). Most of patients were carrying the genotype +251AT in which the level of gene expression was (2.092204). Other studies including Hull et al.,[23] showed that the A variant of this polymorphism was associated with increased IL-8 production, Kamali-Sarvestani et al.,[24] observed an increased frequency in the production of IL-8 in breast-cancer patients carrying the AA genotype. These findings correlate with data reported by Schultheis et al.,[21] who showed that patients carrying at least one A allele, hypothesized having increased IL-8 production, had a statistically significant lower response rate than those being homozygous for the wild type (T-allele). Wei et al.,[25] showed that higher promoter activity of IL-8 -251AA polymorphism might increase production and expression of IL-8. Wacharasint et al.,[26] reported that IL-8 mRNA expression in stimulated lymphoblastoid cells with the AA genotype of -251A/T was significantly greater than stimulated lymphoblastoid cells with the AT or TT. Andia et al.,[22] showed that IL-8 mRNA levels were higher in patients with generalized chronic periodontitis, mainly in those who presented the TA genotype. However the results of the current study showed completely different results, they revealed that IL-8 mRNA expression in samples with the TT genotype of 251A/T was significantly greater than samples with the AT or AA. These results were similar to that reported by Selvaraj et al.,[27] who showed that normal healthy subjects and pulmonary tuberculosis positive for TT genotype showed significantly higher IL-8 production compared to the AA genotype. The study suggests that the TT genotype may be associated with higher IL-8 production and increased leucocyte accumulation and inflammation at the site of Mycobacterium tuberculosis infection. Ahn et al.,[28] also showed that IL-8 protein level in bronchoalveolar lavage fluid was significantly increased in the subjects with idiopathic pulmonary fibrosis having the common allele (T) of IL8251A/T compared to those with the minor allele (A). This result indicates that the 251T allele within the promoter may result in increased IL-8 production when compared with the minor allele. These differences in our results may be due to the conclusion that there are two major haplotypes containing the–251A allele, only one of which is associated with disease and increased IL-8 production, as a result the –251A allele may not be the functional one and it may be in linkage disequilibrium with a functional variant elsewhere in the IL-8 gene [29]. Moreover, the reporter plasmid constructs containing 1409 base pairs of the 5` flanking region of IL-8 gene differing only at the –251 position transfected into A549 cells, showed a higher expression for (–251T) than (–251A) constructs when stimulated with TNF in vitro [30]. These findings suggest that the –251T allele, in association with other functional variants in the IL-8 gene, may be involved in higher IL-8 production. According to the knowledge, the present study is the first study on the association of the IL-8 gene -251 T>A polymor- phism and ovarian cancer risk in association with level of IL-8 mRNA. The results of this study showed that the genetic variation of IL-8 gene influences susceptibility to ovarian cancer. the results of the present study suggest that the TT genotype of IL-8 -251A/T may associate with increase the risk of ovarian cancer because of altered IL-8 gene expression.

Figure 1: Amplification target and endogenous gene product run with duplicate samples Cycle number is plotted on the x-axis with level of fluores- cence on the y-axis. The threshold fluorescence level is depicted by the green line.

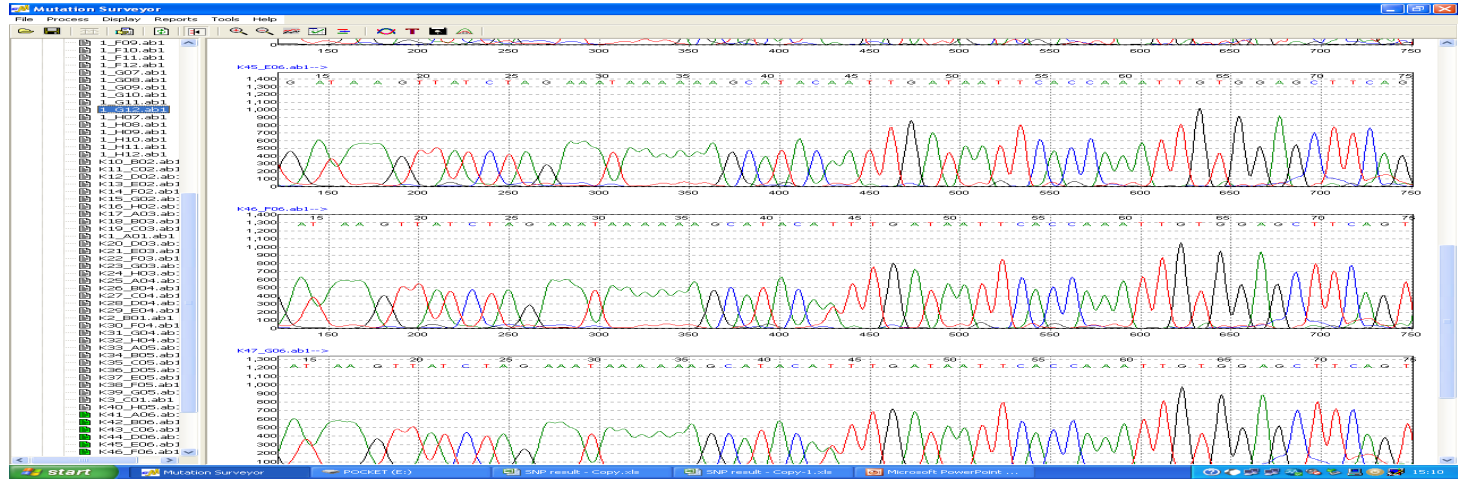
Figure 2: Example of DNA sequencing results showing the three genotypes of IL8 A251T polymorphisms: polymorphisms and the transision of the wild type allel T in figure a to A in figure b. The arrow refer to the change in single nucleotide in the sequence: 5-TAAAGTTATCTAGAAATA- AAAAAGCATACAT/A TTGATAATTCA-3

Acknowledgments
The authors thank all patients for their permission to get samples and clinical information, we also thank the head and staff members of the molecular oncology department/ Guys hospital / London, for their cooperation in part of the study related with molecular analysis.
References
- Zlotnik A, Yoshie Chemokines a new classification system and their role in immunity. Immunity. 2000; 12: 121-127.
- Strieter Interleukin-8 a very important chemokine of the human airway epithelium. Am J Physiol. Lung Cell Mol Physiol. 2002; 283: L688-L689.
- Luster Chemokines chemotactic cytokines that mediate inflam- mation. N Engl J Med. 1998; 338: 436-445.
- Holmes WE, Lee J, Kuang WJ, et al. Structure and functional expression of a human interleukin-8 receptor. Science. 1991; 253: 1278-1280.
- Murphy PM, Baggiolini M, Charo IF, et International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000; 52: 145-176.
- Mukaida Interleukin-8 an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000; 72: 391-398.
- Zachariae, Ferrara N, Kerbel Angiogenesis as a therapeutic target. Nature. 2005; 438: 967-974.
- Balkwill F. Chemokine biology in cancer. Sem in Immunol. 2003; 15: 49-55.
- David JJ, Wilson W. The Interleukin-8 Pathway in Cancer. Clin Cancer Res. 2008; 14: 6735-6741.
- Singh RK, Gutman M, Radinsky R, et Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994; 54: 3242- 2347.
- Yuan A, Yang PC, Yu CJ, et al. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression tumor angiogenesis patients survival and timing of relapse in non-small-cell lung Am J Respir Crit Care Med. 2000; 162: 1957-1963.
- Luca MS, Huang JE, Gershenwald RK, et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP- 2 activity and increases tumor growth and metastasis. Am J 1997; 151: 1105-1113.
- Kassim SK, El-Salahy EM, Fayed ST. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem. 2004; 37: 363-369.
- Giuntoli RL, Webb TJ, Zoso A, et Ovarian cancer- associated ascites demonstrates altered immune environment implications for antitumor immunity. Anticancer Res. 2009; 29: 2875-2884.
- Shahzad A, Knapp M, Lang I, et al. Interleukin 8 IL-8-a universal International Archives of Medicine. 2010; 3: 11.
- Davidson B, Nesland JM, Goldberg I, et Caveolin-1 expression in advanced-stage ovarian carcinoma a clinicopathologic study. Gynecol Oncol. 2001; 81: 166-171.
- Merritt WM, Lin YG, Spannuth WA, et Effect of interleukin-8 gene silencing with liposome-encapsulated small interfer-ing RNA on ovarian cancer cell growth. Journal of the National Cancer Institute. 2008; 100: 359-372.
- Savage SA, Abnet CC, Steven D, et Variants of the IL8 and IL8RB Genes and Risk for Gastric Cardia Adenocarcinoma and Esophageal Squamous Cell Carcinoma. Cancer Epidemiol Biomarkers Prev. 2004; 13: 2251.
- Dumitrescu T, Nicoli R, Serban SS, et al. IL-8 rs4073 T>A Polymorphism is not correlated with colorectal Annals of RSCB. 2012; 18.
- Aledson VF, Tiago DS, Celia AP, et al. Interleukin-8 gene polymorphism and susceptibility to gastric cancer in a brazilian population. Biol Res. 2012; 45: 369-374.
- Schultheis AM, Lurje G, Lenz Polymorphisms and Clinical Outcome in Recurrent Ovarian Cancer Treated with Cyclophosphamide and Bevacizumab. Clin Cancer Res. 2008; 14: 7554-7563.
- Andia DC, de Oliveira NFP, Letra AM, et al. Interleukin-8 Gene Promoter Polymorphism rs4073 May Contribute to Chronic APJ Periodontol. 2011; 82: 893-899.
- Hull J, Thomson A, Kwiatkowski Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000; 55: 1023-1027.
- Kamali-Sarvestani E, Aliparasti MR, Atefi S. Association of interleukin-8 IL-8 or CXCL8- 51T/A and CXCR2 +1208C/T gene polymorphisms with breast cancer. Neoplasma. 2007; 54: 484-489.
- Wei Y, Lan Y, Tang R, et Single nucleotide polymorphism and haplotype association of the interleukin-8 gene with nasopharyngeal carcinoma. Clinical Immunology. 2007; 125: 309-317.
- Wacharasint P, Nakada TA, Boyd JH, et AA genotype of IL-8 -251A/T is associated with low PaO(2)/FiO(2) in critically ill patients and with increased IL-8 expression. Respirology. 2012; 17: 1253-1260.
- Selvaraj PS, Prabhu Anand MS, Jawahar G, et al. Promoter polymorphism of IL-8 gene and IL-8 production in pulmonary Current Science. 2006; 90: 10.
- Ahn MH, Byung-Lae Park BL, Shin-Hwa Lee SH, et A promoter SNP rs4073T>A in the common allele of the interleukin 8 gene is associated with the development of idiopathic pulmonary fibrosis via the IL-8 protein enhancing mode. Resp Res. 2011; 12: 73.
- Hull J, Ackerman H, Isles K, et Unusual haplotypic structure of IL8 a susceptibility locus for a common respiratory virus. Am J Hum Genet. 2001; 69: 413-419.
- Hacking D, Knight JC, Rockett K, et al. Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease susceptibility. Genes Immun. 2004; 5: 274-282.