The Role of Mutations Genes NPM1, FLT3, DNMT3A, CEBPA, IDH1& IDH2 in Cytogenetically Normal Acute Myeloid Leukemia
Author'(s): Saeed Heidarisani, Mohammad Yazdani, Hediyeh Hosseini and Shahin Asadi*
Division of Medical Genetics and Molecular Pathology Research, Harvard University, Boston Children's Hospital, Boston.
*Correspondence:
Shahin Asadi, Medical Genetics-Harvard University. Director of the Division of Medical Genetics and Molecular Optogenetic Research, Boston.
Received: 11 Jul 2022; Accepted: 22 Aug 2022; Published: 27 Aug 2022
Citation: Heidarisani S, Yazdani M, Hosseini H, et al. The Role of Mutations Genes NPM1, FLT3, DNMT3A, CEBPA, IDh3& IDH2 in Cytogenetically Normal Acute Myeloid Leukemia. Cancer Sci Res. 2022; 5(3): 1-5.
Abstract
Cytogenetically normal acute myeloid leukemia (CN-AML) is a type of hematopoietic tissue (bone marrow) cancer called acute myeloid leukemia. In normal bone marrow, early blood cells called hematopoietic stem cells develop into several types of blood cells: white blood cells (leukocytes) that protect the body against infection, red blood cells (erythrocytes) that carry oxygen and platelets (thrombocytes) play a role in blood coagulation, in acute myeloid leukemia, the bone marrow produces a large number of abnormal and immature white blood cells called myeloid blasts. Instead of becoming normal white blood cells, myeloid blasts turn into blood cancer cells.
Keywords
Overview of Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
Cytogenetically normal acute myeloid leukemia (CN-AML) is a type of hematopoietic tissue (bone marrow) cancer called acute myeloid leukemia. In normal bone marrow, early blood cells called hematopoietic stem cells develop into several types of blood cells: white blood cells (leukocytes) that protect the body against infection, red blood cells (erythrocytes) ) that carry oxygen and platelets (thrombocytes) play a role in blood coagulation, in acute myeloid leukemia, the bone marrow produces a large number of abnormal and immature white blood cells called myeloid blasts. Instead of becoming normal white blood cells, myeloid blasts turn into blood cancer cells. A large number of abnormal cells in the bone marrow interferes with the production of functional white blood cells, red blood cells, and platelets [1].
Clinical Signs and Symptoms of Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
People with CN-AML have a lack of mature blood cells: a lack of white blood cells (leukopenia) leads to increased susceptibility to infections, a low number of red blood cells (anemia) causes fatigue and weakness, and loss of appetite. A low platelet count (thrombocytopenia) can lead to easy bruising and abnormal bleeding. Other symptoms of CN-AML may include fever and weight loss [1].
The age of onset of CN-AML ranges from childhood to late adulthood. CN-AML is said to be an intermediate-risk cancer because it has a variable prognosis: some people with it respond to standard treatment, while others may need stronger treatments. The age of onset of the disease and its prognosis are influenced by certain genetic factors that are involved in this disease [1].
Etiology of Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
CN-AML is classified as "normal cytogenetic" based on the type of genetic changes involved in its development. Normal cytogenetics refers to the fact that this form of acute myeloid leukemia is not associated with major chromosomal abnormalities. About half of people with acute myeloid leukemia have this form of the disease. The other half have genetic changes that alter large regions of specific chromosomes. These changes can be detected by a test known as cytogenetic analysis. CN-AML is associated with smaller genetic alterations that cannot be seen by cytogenetic analysis [1,2].
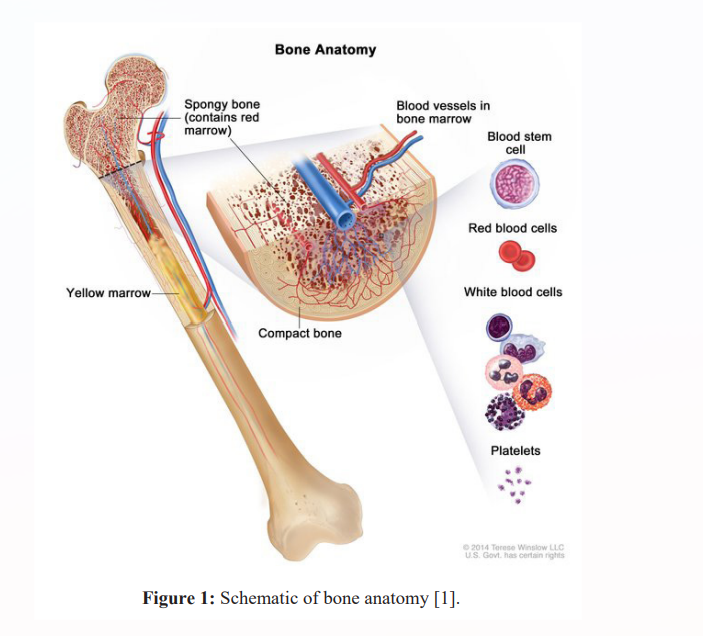
Mutations in a large number of genes have been found in people with CN-AML. Common genes involved in this disease include NPM1, FLT3, DNMT3A, CEBPA, IDh3 and IDH2. The proteins produced from these genes have different functions in the cell. Most of them are involved in the regulation of processes such as growth and division (proliferation), maturation (differentiation) or survival of cells. For example, the protein produced from the FLT3 gene stimulates cell proliferation and survival. Proteins produced from CEBPA and DNMT3A genes regulate gene activity and help control the time of cell division and how they mature. The NPM1 gene encodes a protein that is likely to be involved in the regulation of cell growth and division. Mutations in any of these genes can disrupt one or more of these processes in hematopoietic stem cells, leading to the overproduction of abnormal and immature white blood cells that characterize CN-AML. Although the proteins produced by the other two genes involved in CN-AML, IDh3 and IDh3, are not normally involved in cell proliferation, differentiation, or survival, mutations in these genes lead to the production of proteins with novel functions. These changes lead to a disruption in the differentiation of hematopoietic stem cells, which leads to CN-AML [1,2].
CN-AML is a complex condition influenced by multiple genetic and environmental factors. Typically, the mutation involves more than one gene. For example, people with NPM1 gene mutations often have mutations in the FLT3 gene, both of which are likely to contribute to cancer development. In addition, environmental factors such as smoking or exposure to radiation increase a person's risk of developing acute myeloid leukemia [1,3].

AML is not usually inherited, but is caused by genetic changes in the body's cells that occur after conception. Rarely, an inherited mutation in the CEBPA gene causes acute myeloid leukemia. In these cases, the disease follows an autosomal dominant inheritance pattern, meaning that one copy of the altered CEBPA gene in each cell is sufficient to cause the disorder. These cases are called familial acute myeloid leukemia with mutated CEBPA CN-AML [1,3].
Frequency of Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
Acute myeloid leukemia occurs in approximately 3.5 of 100,000 people each year. 40-50% of people with acute myeloid leukemia have CN-AML [1,4].
Diagnosis of Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
Cytogenetically normal acute myeloid leukemia (CN-AML) can be diagnosed based on the clinical and clinical findings of patients and some pathological tests. The most accurate method of diagnosing this disease is molecular genetic testing for the relevant genes in order to check the presence of possible mutations [1,4].
Treatment Options for Cytogenetically Normal Acute Myeloid Leukemia (CN-AML)
The treatment and management strategy of CN-AML is symptomatic and supportive. There is no effective treatment for this disease and all clinical measures are aimed at reducing the suffering of the sufferers. Genetic counseling is necessary for parents who want a healthy child [1,5].
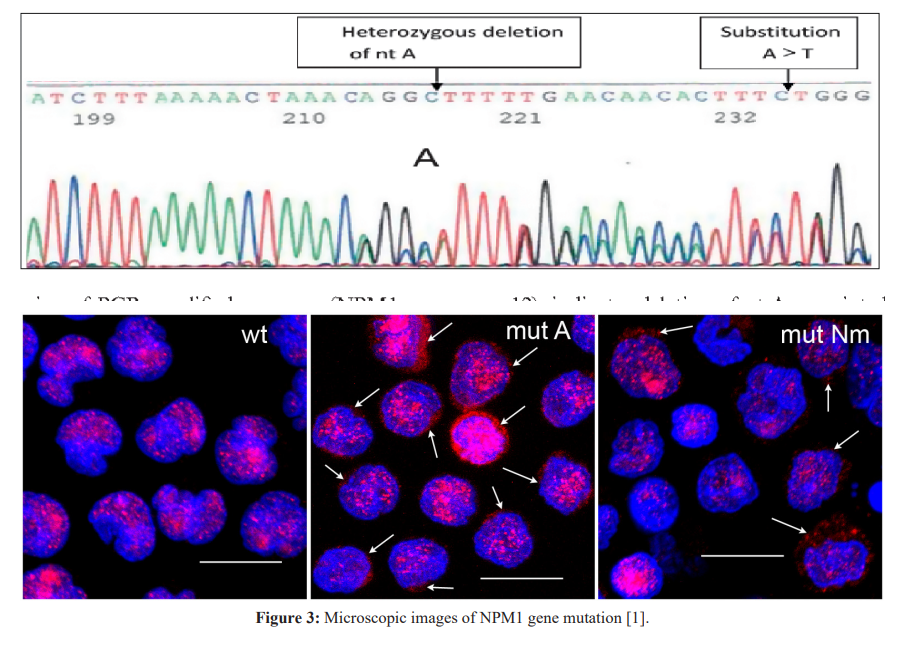
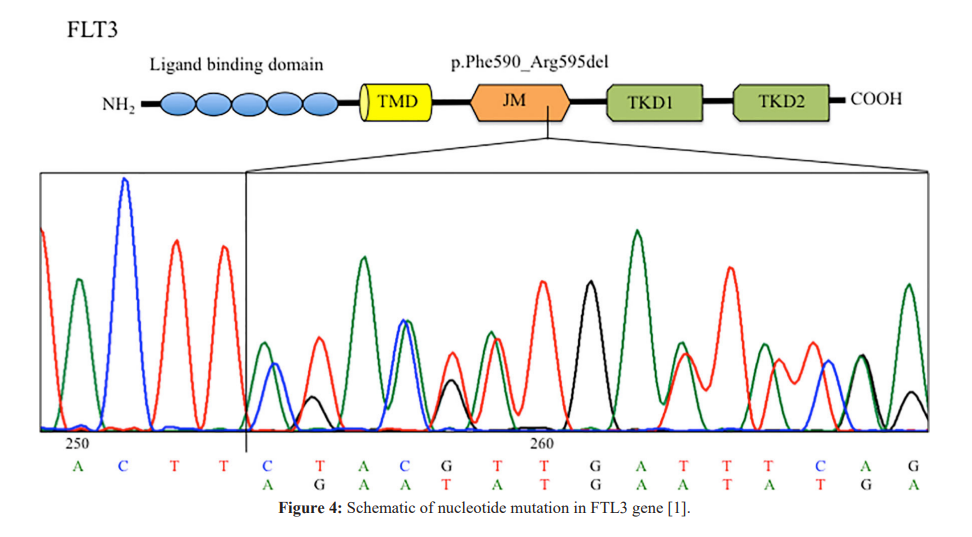
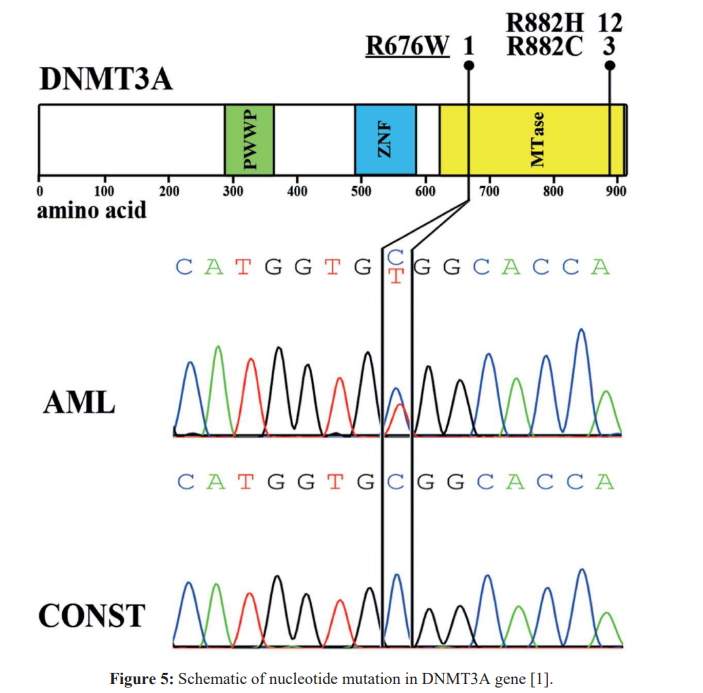
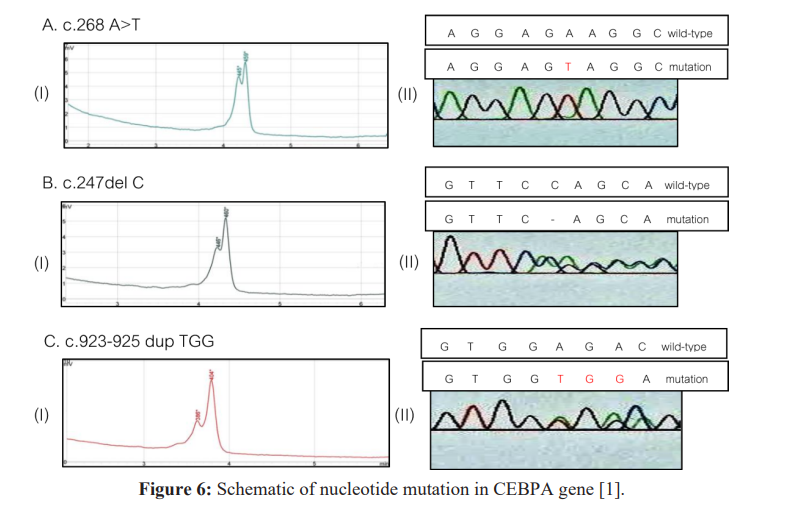
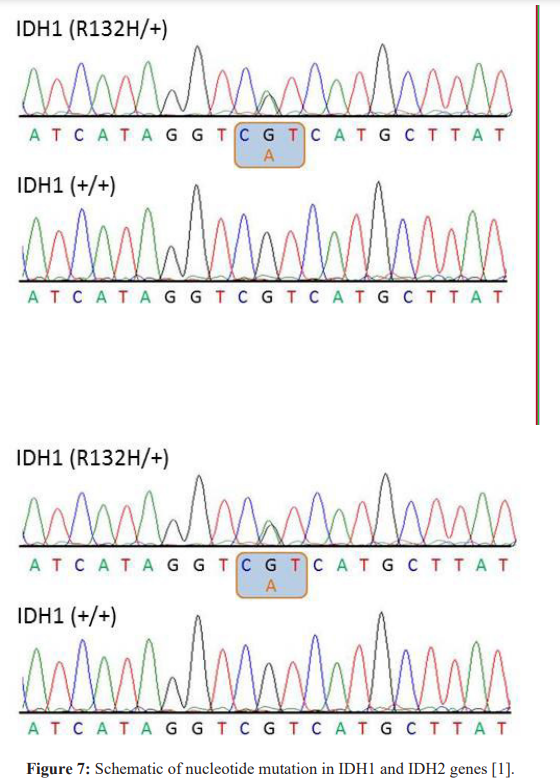
Conclusion
People with CN-AML have a lack of mature blood cells: a lack of white blood cells (leukopenia) leads to increased susceptibility to infections, a low number of red blood cells (anemia) causes fatigue and weakness, and loss of appetite. CN-AML is classified as "normal cytogenetic" based on the type of genetic changes involved in its development. Normal cytogenetics refers to the fact that this form of acute myeloid leukemia is not associated with major chromosomal abnormalities. About half of people with acute myeloid leukemia have this form of the disease. The other half have genetic changes that alter large regions of specific chromosomes. These changes can be detected by a test known as cytogenetic analysis. The treatment and management strategy of CN-AML is symptomatic and supportive. There is no effective treatment for this disease and all clinical measures are aimed at reducing the suffering of the sufferers [1-5].
References
- Asadi The Book of Hematopathology. Amidi Publications. Iran. 2022.
- Marcucci G, Haferlach T, Döhner H. Molecular genetics of adult acute myeloid leukemia prognostic and therapeutic implications. J Clin Oncol. 2011; 29: 475-486.
- Mawad R, Estey EH. Acute myeloid leukemia with normal cytogenetics. Curr Oncol Rep. 2012; 14: 359-368.
- Sanders MA, Valk PJ. The evolving molecular genetic landscape in acute myeloid leukaemia. Curr Opin Hematol. 2013; 20: 79-85.
- Welch JS, Ley TJ, Link DC, e The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012; 150: 264-278.