Thermal Analgesia, Exploring the Boundary Between Pain Relief and Nociception Using A Novel Pulsed Heating Device
Author'(s): Charles Chabal1*, Peter Dunbar2 and Ian Painter3
1Chief Science Officer Soovu Labs Inc. Seattle WA, USA.
2Chief Medical Officer Soovu Labs Inc. Seattle WA, USA.
3Department of Health Services, University of Washington, Seattle,WA, USA.
*Correspondence:
Charles Chabal, MD, Soovu Labs Inc., 2439 8TH Ave N, Seattle WA. USA, Tel: 98109-2212; Fax: 206-579-4910.
Received: 27 August 2020; Accepted: 22 September 2020
Citation: Chabal C, Dunbar P, Painter I. Is Thermal Analgesia, Exploring the Boundary Between Pain Relief and Nociception Using A Novel Pulsed Heating Device. Anesth Pain Res. 2020; 4(2): 1-7.
Abstract
Rational: Heat is a well-accepted pain reliever, but questions remain about significant fundamentals such as optimal temperature, time of onset and duration of effect. This study compared the delivery of two levels of thermal energy to a control condition and examined onset, analgesia, and duration.
Objectives: A randomized blinded controlled three arm trial compared the analgesic response to heat delivered via pulses at 4 pulses/minute at 45°C (N=30) versus heat delivered via pulses at 2 pulses/minute at 45°C (N=49) to steady heat at 37°C (N=51) in subjects with longstanding low back pain. Treatment lasted 30 minutes with followup out to four hours. The hypothesis was that the highest energy group (4 pulses/minute) would receive improved analgesia compared to the other groups. Time of onset and duration of effect was also measured.
Findings: Reduction in pain was greater for the both the Initial Group (2 pulses per minute) and the High Energy Group (4 pulses per minute) as compared to the control group (steady heat). The High Energy group (reduced pain for 180 minutes as compared to the Initial Study group that reduced pain for 120 minutes.
Conclusion: High level pulsed heat 45°C at 4 pulses per minute produced significantly longer analgesia as compared to pulsed heat 45°C at 2 pulses per minute, and steady heat at 37°C. Pain relief was rapid, with an onset of analgesia < 5 minutes. The results suggest that there is a dose relationship between thermal energy delivered and duration of analgesia with the upper limit likely defined as the analgesic nociceptive boundary. The results provide some important insights into the analgesic effect of heat in humans.
Keywords
Introduction
Humans have used heat to provide analgesia and comfort for thousands of years yet there are many aspects that remain poorly understood. In particular, the effectiveness of heat for chronic pain has surprising gaps for such an established therapy. French,at al. [1] offered that understanding the clinical effect of heat and pain is limited by the methodologies of many studies. In addition, while some studies have examined the effect of heat in acute and subacute pain, few have focused on chronic pain [2-6] and questions remain over thermal analgesia’s mechanism of action (MOA). For example, little is known about the optimal temperature and duration of heat. A better understanding of the MOA could potentially lead to more effective thermal based analgesia, a method that has few if any systemic side effects.
The need for a better understanding of thermal analgesia is also emphasized by the recent opioid crisis. New clinical guidelines recommend the use of nondrug treatments as one of the first options in pain management, with recommendations from the American College of Physicians listing superficial heat as the initial treatment [7]. Yet, as French et al. [1] pointed out, much of what we know about thermal analgesia is based on methodologically limited studies and focused primarily on acute or subacute back pain. Simultaneously, pain sufferers are faced with a host of treatments ranging from unregulated nutritional supplements through invasive injections and surgical implants. This underscores the need to provide both clinicians and patients better data surrounding the effectiveness and limitations of thermal analgesia in a condition as common as low back pain.
In an attempt to better understand the effectiveness of heat in the treatment of chronic low back pain, a previous report looked at the effectiveness of 30 minutes of high-level pulsed heat (45°C) compared to 30 minutes of low level steady heat (37°C) in subjects with chronic low back pain [8]. The mean duration of low back pain in these subjects was over 10 years. The study was designed with a rigorous methodology in a double blinded, randomized controlled manner with the low level steady heat functioning as the control condition. The results showed that the experimental condition of pulsed heat (45°C) produced statistically significant more analgesia than the control condition. The analgesic onset was very rapid (< 5 minutes) and lasted for 2 hours after the 30 minute treatment session [8]. The thermal energy imparted to the skin was well within accepted safety parameters [9]. This study raised some fundamental questions. The initial study was an exploratory study that empirically chose 45°C pulses of heat at the rate of 2 pulsed/ minute. The reasoning for this starting point was primarily related to safety and is examined in subsequent sections. For the present study, the primary question was whether the delivery of greater thermal energy would increase the effectiveness of the analgesia as defined by a greater reduction of pain compared to the control and experimental condition in the initial experiment and the duration of pain relief after cessation of treatment? The hypothesis of this study was that increased delivery of thermal energy would increase the analgesic response as compared to the initial study. A secondary question was whether the increased energy would affect the duration of pain relief.
Methods and Materials
This study is an extension to an ongoing previous study but under a new IRB from the Western Institutional Review Board. To minimize any confusion the first study is termed the Initial Study and this supplementary study, the High Energy Study. The Initial Study was conducted using a rigorous randomized double blinded placebo controlled design followed Good Clinical Practice Guidelines [10-11], and was performed at the Northern California Research Inc., Sacramento, CA. In this study, subjects were told that the purpose was to identify the optimal heating temperature for pain relief and that the study devices were set at different temperatures, low, medium or high. Subjects were randomly assigned to the experimental condition where they received pulsed heat at 45°C for 30 minutes or to the control condition where they received steady heat at 37°C for 30 minutes. The experimental condition received 10 second pulses of 45°C heat at the rate of 2 pulses per minute. All subjects were followed for 4 hours and the primary outcome measure was the reduction in pain 30 minutes after the 30 minute heating session.
In this High Energy Study subjects were recruited and told the study was comparing various temperatures of heat (low, medium or high) for the treatment of chronic low back pain. In reality, all subjects in the High Energy Study, received the high temperature setting. In this High Energy Group, subjects received four 10 second pulses of 45°C heat at the rate of 4 pulses per minute. While the maximum temperature of both the Initial Study and High Energy Study experimental groups were the same (45°C), the group in the High Energy Study received twice the amount of thermal energy than the experimental group in the Initial Study, four 10 second pulses/minute at 45°C versus 2 pulses/minute. The pain assessments were done by a blinded research assistant that did not enter the subject into the study. Subjects were recruited from an existing database and through community outreach. Subjects were paid $150 for completion of the single study session. No study personnel met any study subject and images of source data sheets were forward to the study statistics consultant at the University of Washington.
Subjects in both studies were assigned the same test units created by Soovu Labs (Figure 1). The test unit consisted of two heating pods with one inch diameter metal heating plates. The heating pods were attached to the subjects’ low back over the paravertebral muscles at the lumbar 4/5 vertebral level using a medical grade adhesive. The units were controlled by study personnel with a phone app via Bluetooth connectivity

Figure 1: Image of heating units used in the study. The left image shows the side of unit that faces away from the skin. The right image shows the metal heating plate that is one inch in diameter. The heating plate rests on the skin over the area of pain.
Inclusion criteria of the study were
- Chronic low back pain. By definition chronic low back pain is a condition that has been present for at least 6 months on more days than not. If there was a radiating component of the low back pain the radiating component must have a pain rating less than the non-radiating component of the low back pain.
- Subjects must have pretreatment level of pain 4 or greater on the Numeric Pain Scale.
- Ages 22 through 70 inclusive. The age limits were determined to include adults and those most likely to be able to use a smart phone app. FDA guideline defines adults as 22 years of age and older [12].
- Pain medications can be used prior to the trial; however, none can be used during the approximate four-hour trial. Other non- pain medications are permitted as needed.
- Medications permitted during the trial include medications such as tramadol, codeine, NSIADs, gabapentin and acetaminophen.
- Subjects must have a cell phone for clinic contact and follow-up.
Exclusion criteria of the study were:
- Sciatica or radicular pain without non-radiating low back pain, cancer, radicular pain greater than the non-radiating component of low back pain, pregnancy, skin lesions such as open skin or sores, scar tissue, skins grafts, old burns over the treatment area
- Current use of opioids including oxycodone, hydromorphone, hydrocodone, fentanyl and methadone.
Upon entering the study, subjects had a baseline pain level assessed using the 0 to 10 Numeric Pain Scale (NPS) [13,14]. Active treatment occurred for 30 minutes while seated after which the units were removed, and subjects were allowed to move about for the next four hours. Pain was assessed at baseline and after 5, 10, 15, and 30 minutes of treatment. Post treatment pain was assessed at 15, 30, 45, 60 minutes after treatment and then every 30 minutes until subjects were four hours post treatment.
The skin was also assessed by study personal at baseline, after the treatment session and four hours post treatment. The skin assessment examined any erythema or pigment or color change at the heating site. In addition, any pain or discomfort at the site was noted.
Sample size/power calculation
Sample size calculations were based on results of two pilot trials comparing an early prototype of the pulsed heat device against Thermacare devices (results unpublished). A standard deviation of the change in NPS of 1.75 was assumed based on the results the pilot trials. Sample size was calculated using standard sample size calculation software for a two-sample t-test to achieve 80% power to detect an effect size of a 1.0 difference in change in NPS from baseline to end of treatment plus 30 minutes between the two groups at the alpha level of 0.05.
Statistical analysis
The primary outcome was change in pain score from baseline to 30 minutes after treatment ended. Linear regression was used to compare differences in primary outcome between the higher energy group (current study) and the treatment (initial study) and control groups (initial study) adjusting for initial pain level. Unadjusted comparisons are also presented. Change in pain scores at each other post baseline time point were similarly analyzed.
Results
After screening, 30 subjects entered the study, and all completed the single session study. There were 17 females and 13 males. The mean age was 48.3 years (range 31-66 years). The mean duration of LBP was 11.04 years (range 0.7 - 33 years). These demographics were similar to found in Initial Study (Table 1).

Compared to the control group, 30 minutes of High Energy produced pain that lasted 180 minutes post heat treatment compared to the 120 minutes in the Initial Study experimental group. Compared to the control group in the Initial Study, the experimental group in the High Energy Study had a significant reduction in reported pain scores at the primary outcome measure thirty minutes post treatment (Table 2) and throughout the study follow-up period. When comparing the reduction in pain in the experimental group of the Initial Study with the experimental group in the High Energy Study, the High Energy Study group showed a greater reduction in pain at the primary endpoint (30 minutes post treatment) but did not reach statistical significance (Table 3). The results are graphically represented in Figure 2.

Table 2: Overall reduction in pain compared to baseline in control group (Initial Study) versus experimental group (High Energy Study). The primary outcome measure at 30 minutes post treatment is indicated by the shaded cell. The High Energy Study experimental group received twice the amount of thermal energy as the Initial Study experimental group although the maximum temperature of both groups was 45°C. In the Initial Study Experimental Group pain was relieved for 120 minutes post treatment versus 180 minutes (210 minutes total - 30 minutes of active treatment) in the High Energy Group.
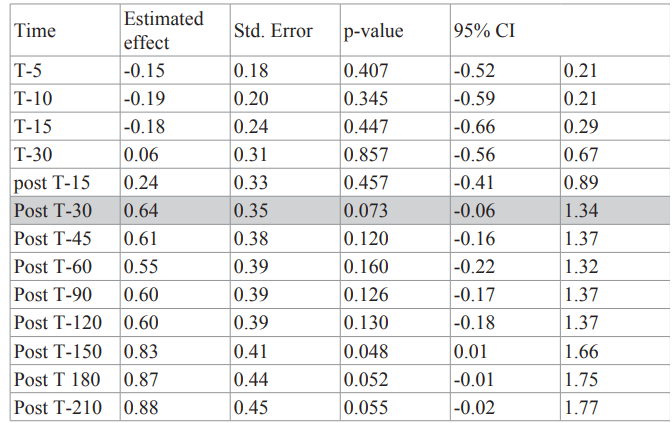
Table 3: Overall reduction in pain in experimental group (Initial study) versus experimental group (High Energy Study). The primary outcome measure at 30 minutes post treatment is indicated by the shaded cell. The High Energy experimental group received twice the amount of thermal energy as the Initial Study experimental group although the maximum temperature of both groups was 45°C. While there was a greater reduction of pain in the High Energy experimental group statistical significance was not reached.
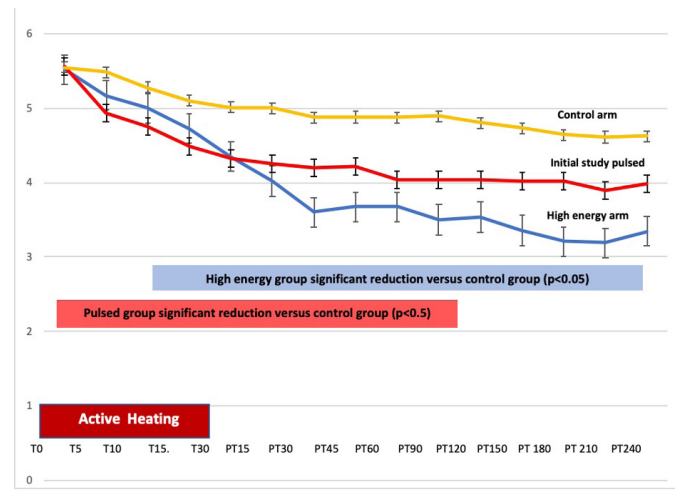
Figure 2: Reduction in pain from baseline for Initial Study control arm (yellow), Initial Study experimental arm (Red) and High Energy experimental arm (Blue). Active treatment is from Time 0 (T0) through Time 30 minutes (T 30). Follow-up interval from Post-treatment time 15 (PT15) through Post-treatment time (PT 240) minutes. X axis in minutes and Y axis pain level (0-10 scale). The High Energy experimental arm produced significantly longer reduction of pain versus the Initial Study experimental arm.
The skin was examined at the end of the 30 minute treatment session and prior to discharge with no skin damage noted and no subjects complaining of pain or skin discomfort.
Discussion
As noted earlier, while long used for comfort, many questions surround thermal analgesia in humans. Our previous randomized double blinded controlled study showed that pulsed heat at 45°C produced statistically better and faster analgesia compared to steady heat at 37°C in subjects with chronic low back pain [8]. In the Initial Study 45°C was chosen empirically based on animal data and safety profiles [9]. This follow up study (High Energy Study) was designed to further explore the boundaries of thermal analgesia. That boundary or ceiling effect is defined as the amount of thermal energy one can apply to the skin causing maximal analgesia likely from TRPV1 stimulation, versus the amount of energy that causes nociception or tissue damage. The results of this current study suggest that increased thermal energy produces more analgesia as compared to the first empirically designed study. Since no subject experienced discomfort from the heat nor suffered even minor skin changes it is likely that this algorithm did not reach a ceiling effect where analgesia is limited by nociception or tissue injury. This suggests that the peak of thermal analgesia was not reached in this study.
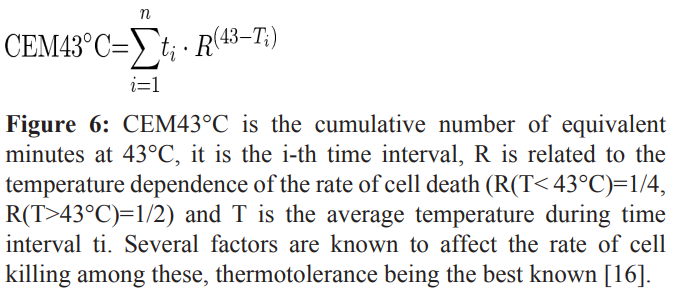
To better understand the parameters of the thermal analgesic boundary we used a standard pioneered in MRI (Magnetic Resonance Imaging) research [15,16]. MRIs produce significant amounts of radiofrequency energy that can injure underlying tissue. Tissue damage correlates with thermal dose and as a standard for comparison, the concept of cumulative equivalent minute 43 (CEM 43) was created[17,18]. CEM 43 is defined as a temperature exposure of tissue for a single minute at 43°C (CEM43) [16] and is mathematically represented in figure 6.
Different organs both within and between species show differing thresholds for damage with skin being one of the most resistant to thermal damage. Human skin has a threshold ranging from 240 - 600 CEM43 without resulting injury [19,20]. In addition to CEM43 considerations, data from human and porcine burn experiments by Moritz were also used [21]. In these actual thermal injury tests, heat exposure to over 240 CEM 43 in humans was without gross or histological skin damage [21]. Our initial experiment using pulsed heat to 45°C for 10 seconds with a 30 second off period produced 30 CEM43 equivalents, or 1/8 of the conservative 240 CEM 43 threshold [17,21].
This (High Energy) current study delivered twice as much thermal energy as the initial study. In this case pulsed heat to 45°C for 10 seconds with a 15 second off period produced 60 CEM43. This represented 1/4 of the 240 CEM43 threshold and 1/10 of the 600 CEM43 skin threshold [17,18]. The wider spread adoption and use of the CEM 43 measure for any thermal energy applied to the skin has advantages of standardization of heat treatment and offers better assessment of burn risk. While not generally used outside of the context of MRIs, adoption by both regulatory agencies such as the FDA and patient safety groups could offer a common standard to calculate thermal exposure and risk. For example, thermal exposure and risk are not calculated for common over-the-counter heating devices like heating pads despite reports of burns [22]. Future clinical studies should report thermal characteristics in terms of temperature (°C) and time as well as CEM43 units.
The results of this study raise some interesting questions. The initial study showed that with 30 minutes of heat treatment the duration of analgesia lasted for an additional 120 minutes post treatment. The present study showed similar results with an indication that the duration of action lasted 180 minutes post treatment (Table 2). While these results are somewhat surprising, others have reported sustained analgesic effects after treatments with chemical hot packs [4-6]. In these cases, the hot packs were about 40 degrees centigrade and were in place for at least eight hours. The actual duration of analgesia in these chemical hot pack experiments were not specifically reported but did last longer than the actual treatment time.
The presumed mechanism of action of thermal analgesia is likely related to the stimulation of subcutaneous receptors most likely TRPV 1 channels in the present temperature range of up to approximately 45°C [23-25]. Approximately is emphasized as in this experiment, 45°C was carefully measured at the skin/heating plate interface. In effect 45°C at that interface produces a three dimensional temperature gradient throughout surrounding volume of tissue that is affected by skin thickness, vascularity, melanin content, and subcutaneous fat with resulting temperatures within the accepted range of TRPV1 activation for near receptors but less so further from the interface. Alternatively, the heat may have directly stimulated C fibers [26] or Delta [27], again subject to the above-mentioned temperature gradient.
The increased thermal energy used in this present study (High Energy Study) may have caused a number of different type of reactions. The thermal energy spreading out over a 3 dimensional tissue volume may have recruited and stimulated more populations of TRPV1 receptors as compared to the lower energy Initial Study. In addition, the thermal energy may have caused a greater hyperpolarization with subsequent prolonged effects of the same receptors stimulated in our first study analogous to that seen with capsaicin [28].
Perhaps the hyperpolarization was responsible for the enhanced analgesia and longer duration of pain relief. The prolonged duration of analgesia after the 30 minutes of treatment could be a peripheral effect at the TRPV-1 receptor level or even C or A Delta fibers. In addition, the peripheral stimulation may have effected changes in the dorsal horn or associated pathways leading to prolonged analgesia even after the cessation of thermal stimulation.
With guidance from the CEM43 guidelines [19,20] and Moritz’s work [21], there is significant room to adjust and modify some of the thermal stimulation parameters while remaining on the safe side of the analgesic thermal boundary. These modifications may have beneficial effects depending on the mechanism of action. For example, a temperature of about 45°C is thought to stimulate TRPV-1 receptors [29]. At temperatures greater than 45°C perhaps more TRPV-1 receptors could be stimulated, or other types of receptors activated such as TRPM-3 or TRPA-1[30] and paradoxically traditional cold fibers [31]. Relying on the boundaries measured by CEM43 and Mortiz, it is possible that brief pulses of temperature significantly higher than 45°C may be delivered safely. One would speculate that if a mechanism of action for thermal analgesia involves activation of TRPV-1 receptors or recruiting additional types of TRPV receptors, greater analgesia could occur. A search of the literature has not revealed any previous study in humans exploring this possibility.
The present study was intended as an exploratory study examining the analgesic thermal boundary. This study was not powered to produced statistical significance but increasing the number of subjects to the Initial Study number of 50 would have likely achieved significance. Nevertheless, the results are highly suggestive of a greater analgesic response in the High Energy Group as compared to the lower energy Initial Study. It was hypothesized that greater thermal energy would produce greater analgesia and the results clearly suggest this.
The results coupled with our understanding of the standardized measurement of thermal energy and the potential for injury suggest that there is significant room to explore this boundary. In fact, using the CEM 43 formula as a guide, perhaps brief pulses of what has been traditionally thought of nociceptive temperatures ranging above 45C may produce additional analgesia by stimulating more receptors or perhaps even different population of receptors without causing pain or tissue injury.
The development and modification of the CEM43 standard used in this study offers a standard that can potentially be used for guidance in the development of future studies as it may help define the upper celling of thermal stimulation and allow for comparisons across different platforms.
It is hoped that the results of this study will spur more research into the mechanisms of thermal analgesia that may answer questions about the prolonged analgesic response to relatively brief thermal stimulation and whether recruiting more receptors or different populations of receptors can increase the analgesic response without causing tissue injury or pain. Another interesting possibility is that if thermal analgesia is due to at least in part stimulation of TRPV 1 channels perhaps adding stimulation of other TRPV channels such as TRPM 8 with a substance like menthol [32,33] could produce an additive or synergistic analgesic response. An improved understanding of these mechanisms may lead to important clinical advances in providing better nondrug analgesic options.
Conflict of interest
This study was funded by Soovu Labs Inc. via a contract to Northern California Research Inst., Sacramento, CA. Drs. Chabal and Dunbar are shareholders of Soovu Labs. Dr. Painter was paid as a consultant the project.
References
1.French SD, Cameron M, Walker BF, et al. Superficial heat or cold for low back pain. Cochrane Database Syst Rev. 2006; 25: 4750.
2.Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low- level heatwrap therapy for treating acute nonspecific low back pain. Arch Phys Med Rehabil. 2003; 84: 329-334.
3.Nadler SF, Steiner DJ, Petty SR, et al. Overnight use of continuous low-level heatwrap therapy for relief of low back pain. Arch Phys Med Rehabil. 2003; 84: 335-342.
4.Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low- level heat wrap therapy provides more efficacy than Ibuprofen and acetaminophen for acute low back pain. Spine. 2003; 27: 1012-1017.
5.Nuhr M, Hoerauf K, Bertalanffy A, et al. Active warming during emergency transport relieves acute low back pain. Spine. 2004; 29: 1499-1503.
6.Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005; 5: 395- 403.
7.Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017; 166: 514-530.
8.Chabal C, Dunbar PJ, Painter I, et al. Properties of thermal analgesia in a human chronic low back pain model. Journal of Pain Research. 2020; 13: 2083-2092.
9.Ye H, De S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burns. 2017; 43: 909-932.
10.FDA Regulations: Good Clinical Practice and Clinical Trials. Retrieved. 2019.
11.Guidelines for good clinical practice (GCP) for trials on pharmaceutical products. World Health Organization WHO. 1995.
12.FDA. Pediatric medical devices. 2019.
13.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011; 152: 2399-2404.
14.Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011; 41: 1073-1093.
15.van Rhoon GC. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia. 2016; 32: 5062.
16.van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43°C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol. 2013; 23: 22152227.
17.Pavel S. Yarmolenko, Eui Jung Moon, Chelsea Landon, et al. Thresholds for thermal damage to normal tissues: An update. International Journal of Hyperthermia. 2011; 27: 320-343.
18.Thrall DE, Rosner GL, Azuma C, et al. Using units of CEM 43 degrees C T90, local hyperthermia thermal dose can be delivered as prescribed. International Journal of Hyperthermia the Official Journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 2000; 16: 415-428.
19.Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Thermal Dose Requirement for Tissue Effect: Experimental and Clinical Findings. Proc SPIE Int Soc Opt Eng. 2003; 4954: 37.
20.Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: an update. Hyperthermia. 2011; 27: 320-343.
21.Moritz AR, Henriques FC. Studies of Thermal Injury: II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns. Am J Pathol. 1947; 23: 695720.
22.Barnett RL, Wingfield JR. On the Safety of Heating Pads Proceedings of the ASME 2013 International Mechanical Engineering Congress & Exposition IMECE. San Diego. 2013; 13-21.
23.Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006; 4: 115.
24.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007; 179: 155171.
25.Aghazadeh Tabrizi M, Baraldi PG, Baraldi S, et al. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med Res Rev. 2017; 37: 936983.
26.Campero M, Baumann TK, Bostock H, et al. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009; 587: 56335652.
27.Churyukanov M, Plaghki L, Legrain V, et al. Thermal Detection Thresholds of Aδ- and C-Fibre Afferents Activated by Brief CO2 Laser Pulses Applied onto the Human Hairy Skin. PLOS ONE. 2012; 7: 35817.
28.Chung MK, Campbell JN. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals (Basel). 2016; 9: 66.
29.Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin- receptor homologue with a high threshold for noxious heat. Nature. 1999; 398: 436441.
30.Wood J, Viana F, Voets T. Heat Pain and Cold Pain. In The Oxford Handbook of the Neurobiology of Pain. Oxford University Press. 2020.
31.Dodt E, Zotterman Y. The Discharge of Specific, Cold Fibres at High Temperatures. Acta Physiologica Scandinavica. 1952; 26: 358-365.
32.Andersen H, Olsen R, Møller H, et al. Lmenthol – a surrogate model of cold allodynia. EJP, 2014; 18: 315-325.
33.Pergolizzi JV, Taylor R, LeQuang JA, et al. The role and mechanism of action of menthol in topical analgesic products. J Clin Pharm Ther. 2018; 43: 313- 319.