Using A Novel, Non-Drug, Topical Pain-Relief Patch to Improve Pain and Function: Final Analysis of the PREVENT Study
Author'(s):Jeffrey Gudin1, Derek Dietze2, and Peter Hurwitz3*
1University of Miami School of Medicine, Miami, Florida USA.
2Metrics for Learning LLC, Queen Creek, Arizona, USA.
3Clarity Science LLC, Narragansett, Rhode Island, USA.
*Correspondence:
Peter Hurwitz, Clarity Science LLC 750 Boston Neck Road Suite 11,Narragansett, RI 02882, Tel +1917 757 0521, Fax +1855-891-8303.
Received: 02 Dec 2021; Accepted: 24 Jan 2022; Published: 30 Jan 2022
Citation: Gudin J, Dietze D, Hurwitz P. Using A Novel, Non-Drug, Topical Pain-Relief Patch to Improve Pain and Function: Final Analysis of the PREVENT Study. Anesth Pain Res. 2022; 6(1): 1-10.
Abstract
Background and Aims: The Global Burden of Disease Study 2016 confirmed that the high prevalence of pain and pain-related diseases is the leading cause of disability and disease burden globally. Pain is the most common reason patients consult primary care providers in the United States. According to recent literature, over 40% of adult Americans, an estimated 100 million people, live with chronic or recurrent pain. Of these, approximately 11 million adults in the US have high-impact chronic pain, or pain that lasts 3 months or longer and is accompanied by at least one major activity restriction. Ongoing research is needed to identify alternative strategies to existing treatment approaches as a means to reduce pain severity, pain interference with function, and to improve patients’ overall quality of life.
Optimal treatment strategies employ multimodal approaches that provide optimal benefit and minimal harm. Chronic pain is often categorized as either nociceptive (caused by damage to tissue or inflammatory stimuli) or neuropathic (damage to somatosensory nervous system). Mainstay therapies for pain include anti-inflammatory agents, opioids, and other oral and topical analgesics. Despite this approach, a large percentage of pain patients do not achieve adequate pain relief, and these traditional medication approaches are often associated with undesirable and potentially dangerous adverse effects. Alternative evidence-based multimodal approaches of pain management are therefore needed. Potential therapies include use of combination pharmacotherapies, which target both central and peripheral nociceptive mechanisms, and nonpharmacological interventions. Incorporation of micro and nanotechnologies into the development of novel treatment formulations has shown to have positive effects on patients. Topical analgesics, including pain relief patches, are a potentially valuable strategy in the management of a variety of conditions associated with musculoskeletal and various neuropathic pain disorders.
The purpose of this minimal risk, observational study was to evaluate patients with mild or moderate pain and evaluate perceptions of pain treatment and associated symptoms with the use of a novel, non-drug and micro/nanotechnology-based topical pain-relieving patch via validated scales over the course of 30 days.
Methods: This analysis of the PREVENT IRB-approved study evaluated the efficacy of a topical pain-relieving patch (Kailo Pain Relieving Patch (Pain Relief Technologies, USA) in reducing Brief Pain Inventory (BPI) scores in patients experiencing mild, moderate, or severe pain. The Treatment Group (TG) of 128 adult patients (89 females,39 males) with arthritic, neuropathic, or musculoskeletal pain received patches for 30 days. A Control Group (CG) of 20 adult patients (15 females, 5 males) did not initially receive the patch, and then crossed over to the Crossover Treatment Group (CROSSG), Surveys were administered to all patients at baseline, 14 days, and 30 days to assess changes in pain severity and interference by BPI Short Form. Changes in oral pain medication use, side effects and satisfaction with patch use were also assessed.
Results: Over 30 days, treatment group mean BPI Severity score decreased 61% (4.9 to 1.9/10?P< .001) and mean BPI Interference score decreased 61% (3.8 to 1.5/10?P< .001) The control group showed an increase in both BPI Severity of 23% (3.0 to 3.7/10) and BPI Interference Score of 58% (1.2 to 1.9/10). After crossing over to treatment, patients in the crossover group reported a decrease in BPI Severity score of 76% (3.7 to .9/10) and a decrease in BPI Interference score of 79% (1.9 to .4/10). No side effects of treatment were reported. After 30 days, 91% of patients reported “less” or “a lot less” usage of oral medications. 86% of patients were very/extremely satisfied with the patch and preferred the pain-relieving patch to oral medications. Results also showed improvements in Quality of Life (QoL), mood, and relations with other people, sleep, walking ability, and enjoyment of life.
Conclusions: Study results indicate that this novel, micro/nanotechnology-based topical pain-relieving patch can reduce BPI pain severity and interference scores and related pain for adult patients with arthritic, neuropathic, and musculoskeletal pain. Options such as this should be considered by healthcare clinicians and patients as part of a multimodal treatment approach. Patient outcomes further encourage more research to be conducted to confirm these results.
Keywords
Introduction
In the United States, pain is the most common reason patients consult primary care providers and is a leading cause of disability [1]. Targeted and alternative treatments to address various acute and chronic pain conditions are needed [2].
Current approaches to address pain include non-invasive and non-pharmacological approaches, such as cognitive and behavioral therapy, medication, physical therapy, and surgery [3-6]. Other topical interventions may include topical analgesic pain patches, sprays, or creams [7,8]. Over the last decade, use of pharmacological approaches like opioids and non-opioid drugs, such as NSAIDS, have significantly increased [9-11]. Many of these drugs are associated with bothersome and dangerous side effects, including bleeding, GI toxicity, addiction, abuse, and even death [12-15]. Due to these side effects, there has been an effort and focus on identifying targeted, non-invasive multi-modal approaches that minimize the reliance on pharmaceutical agents- leading to a reduction in side effects and improved quality of life [16]. Current pain guidelines encourage a multimodal approach to addressing pain that includes topical, non-invasive, and non- pharmacological approaches [17]. In fact, recent guideline updates from the American Academy of Family Physicians (AAFP) and the American College of Physicians (ACP) have recommended topical treatments as first line therapy for musculoskeletal pain, before consideration of other approaches. This is in addition to other Medical Associations, like the American College of Rheumatology (ACR) that also recommends topical therapies as a first line treatment approach [18].
Advancements in new technologies, delivery systems, and approaches have the potential to address acute and chronic painful conditions, reduce patient suffering, and improve outcomes [2]. The integration of novel micro- and nanotechnologies into analgesic therapies have resulted in the development and approval of products such as topical analgesic therapies [2,19,20]. Topical, non-invasive and non-pharmacological approaches have been shown safe and effective for chronic pain patients and have the potential to minimize side effects associated with traditional medication or interventional therapies. Topical analgesic treatment can reduce pain, improve function, and improve quality of life [7,8].
The prevailing hypothesis is that these technologies may play a role in influencing known pain pathways [12]. It is theorized that incorporation of very small micro- and nanomaterials into patch systems allows for the activation or inhibition of ion channels that produce an analgesic effect [12,21,22].
Micro- and nanotechnology incorporates materials with dimensions ranging from several micrometers (one micrometer is one millionth of a meter) to a few nanometers (one nanometer is one billionth of a meter). It is known that these materials have unique electrical, chemical, and magnetic properties that allow for the interaction with cells and tissues at a molecular level [23-25]. Micro technology has been utilized in peripheral and spinal electrical neuromodulation devices and other neurological applications such as nerve repair. A focus of this manuscript is to support existing data suggesting that certain metals can be utilized at a micro- and nanoparticle level for therapeutic conditions including pain management [2,23,26-28].
Action potentials rely on the proper functioning of ion channels and are responsible for transmission of signals within the neuronal circuits of peripheral nerves, spinal cord, and brain [29,30]. It is well known that influencing the body’s electromagnetic field may elicit changes in voltage-gated ion channels (i.e. calcium) within cells [31]. Alternative therapies incorporating electromagnetic therapy have emerged as a safe and effective option for chronic pain patients across various clinical settings [32].
This Pain Relief: Experiencing and Validity: Evaluating NanoTechnology (PREVENT) study included patients with mild, moderate, and severe pain and evaluated their overall perceptions of pain treatment and associated symptoms with the use of a proprietary non-invasive and non-drug topical patch. The 2.75 x 5.5-inch patch with adhesive contains copper, silver, and silicon and is printed on a laminate film. Through the combination of the conductive and semi-conductive material, the patch is designed to provide analgesia through micro-and nano-sized capacitors that emit and absorb electromagnetic radiation/energy, potentially influencing ion signals involved in nociception.
[33] noted following use of this non-drug patch. Patient-reported changes in Brief Pain Inventory short form (BPI) pain severity and pain interference scores, change in the use of pain medications 14- and 30-days following treatment, and differences between the treatment group, a control group, and a crossover group of patients are highlighted.
Methods Study Design
This study was a prospective, Institutional Review Board-approved Observational Study aimed at evaluating patients’ experiences and/or perceptions and analgesic response following use of a non- pharmacological, OTC topical pain-relieving patch (Kailo Pain Patch®, Pain Relief Technologies, Salt Lake City, Utah, USA). For those patients initially receiving the active patch, the Treatment Group (TG), analysis evaluated patient answers to baseline, day 14-, and day 30 surveys from validated pain measurement and symptom scales (e.g., Brief Pain Inventory (BPI)) as well as additional survey questions regarding pain medication use, patient satisfaction, patient quality of life, and resumption of their normal activities. In addition, evaluation of a Control Group (CG) of patients (no patch) at enrollment, and a crossover group of patients (CROSSG) who received the active patch after 30 days of being in the control group, were also included in the analysis.
1) ages 18 to 65 years, inclusive; 2) ability to provide written informed consent; 3) received the study patch from their treating clinician; and 4) had been diagnosed with a mild, moderate, or severe pain condition. Patients who had had a history of use drug or alcohol abuse, patients who had an implantable pacemaker or defibrillator, or patients who were pregnant, were ineligible to participate in the study.
Each site provided patients an identification number, and a confidential file containing the informed consent forms and patient identification numbers were kept and maintained in a secured cabinet only accessible to the principal investigator and authorized personnel. Patient survey responses were provided with no identifying patient information.
Patients could withdraw from this study at any time with the assurance of no unfavorable impact on their medical care. All diagnostic tests and treatment decisions were made at the discretion of clinicians, with no tests, treatments, or investigations performed as part of this study.
The study protocol was approved by IntegReview institutional review board and was performed in full accordance with the rules of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the principles of the declaration of Helsinki and the international council of Harmonisation/GCP. All patients gave informed and written consent. Patients who met the eligibility criteria and who were treated with the pain-relieving patch comprised the study’s treatment group (TG). Patient survey responses were used to evaluate pain relief by comparing answers to validated pain measurement scales (e.g., BPI) as well as other questions that assessed patient satisfaction.
Topical Intervention
The non-drug patch contains a composite of 2 conductive elements (copper and silver) and 1 semi-conductive element (silicon) and contains no drug or energy source. There is a removable/ replaceable adhesive backing. Patients in the treatment group were instructed to wear their patch as needed and for as long as needed. (SEE Picture 1 and 2)

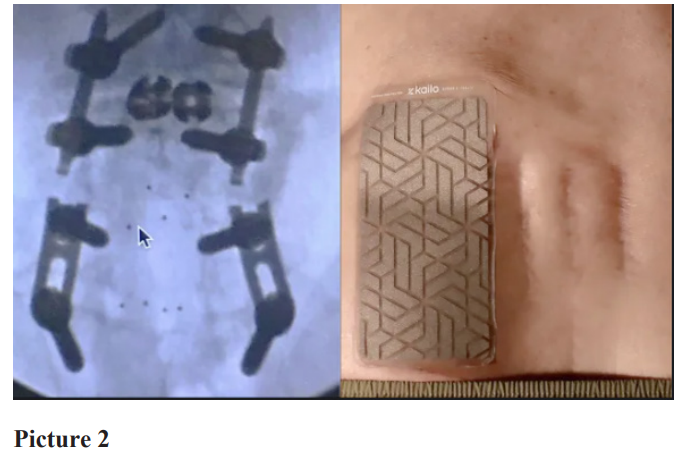
Study procedures and assessments
Following enrollment, patients were asked to complete surveys at baseline and follow-up on days 14 and 30 of the study period. The baseline and follow-up surveys were comprised of questions to address and document the nature and location of the primary pain complaint of the patient, which included: 1) arthritis; 2) neuropathy or radiculopathy; 3) myofascial or musculoskeletal pain or spasm; or 4) other. (Locations included hands, feet, hips, knees, neck, shoulders, and back, among others). All groups (TG, CG, CROSSG) indicated only one pain complaint/location, which was the intended patch area for the active treatment arms.
Patients completed the BPI as part of each survey. The BPI is often used as a measure of pain for a wide range of conditions including cancer, musculoskeletal disorders, depressive conditions, and surgical pain. The BPI is commonly recommended for use in clinical trials of patients with chronic pain and has adequate internal consistency, acceptable-to-excellent test-retest reliability, satisfactory-to-good construct validity, criterion validity, and is sensitive to change [34-38]. Ratings on the BPI are based on a 0-10 numerical scale. For the questions about pain severity, 0 is “no pain” and 10 is “pain as bad as you can imagine.” For the questions about pain interference with activities of daily living, 0 is “does not interfere” and 10 is “completely interferes.” Patient responses to questions regarding pain severity (4 questions) and pain interference (7 questions) were compiled to yield the overall score for pain severity and pain interference.
Patients were asked to indicate any other medications that they had been taking for pain relief at the time of the baseline, day 14, and day 30. Categories of medications that patients could choose included OTC agents (e.g., ibuprofen, naproxen, acetaminophen, aspirin, and other pain medications such as creams, gels, roll-ons, sprays, patches or rubs), prescription NSAIDs, prescription opioids, skeletal muscle relaxants, (e.g., methocarbamol, cyclobenzaprine, metaxalone) or prescription anticonvulsants (e.g., gabapentin or pregabalin). Patients could indicate use of more than one type/ class of analgesic medication.
Study end points
The primary endpoints included changes in patient Brief Pain Inventory (BPI) overall severity and interference scores among and between the treated groups and the control groups for the primary pain complaint, as well as changes in the use of prescription and OTC medications. We also assessed patient satisfaction with patch treatment and any side effects reported by patients during the trial.
Statistical analysis
For all variables, descriptive statistics were calculated, including frequencies and percent for categorical variables and means with standard deviation (SD) for continuous variables. The maximum sample size available was used for each statistical analysis.
Changes from baseline to day 14, and to day 30, in BPI mean pain severity and pain interference scores were analyzed using the paired t test to identify any statistically significant differences within the treatment group.
Each survey collected the numbers and types of prescription and OTC oral/topical medications being used for pain relief; statistically significant differences in the use of these types of medications from baseline to day 30 were determined using the McNemar test and X2 test for binomial paired and unpaired data respectively. Descriptive statistics were used to determine patient satisfaction with the pain-relieving patch within those treated. Descriptive statistics were also used to report any side effects experienced by patients.
A two-tailed alpha was set to 0.05 for all statistical comparisons. SPSS v. 27 was used for all analyses.
Results Baseline demographic and clinical characteristics of patients
A total of 148 patients at 3 US investigator sites were enrolled in the study and completed the baseline, day 14, and day 30 surveys. Of these, 128 patients (89 females,39 males) were in the Treatment Group (TG), and 20 adult patients (15 females, 5 males) were initially enrolled into the Control Group (CG) before crossing over to the Crossover Treatment Group (CROSSG).
Demographic results were similar for gender and age at the baseline survey for all groups of patients. Of the patients in the Treatment Group (TG), 39 (30.5%) were male and 89 (69.5%) were female. The mean age at baseline was 47.0 years. For the Control Group (CG), 5 (25.0%) were male and 15 (75%) were female. The mean age at baseline was 45.9 years.
The primary pain complaint for the patients was recorded at baseline for all groups. (Table 1). For the TG, myofascial/ musculoskeletal pain was the most prominent pain complaint indicated by 59/128 (46.1%) of patients. Neuropathy/radiculopathy was the next most common pain complaint for 39/128 (30.5%) of patients, and arthritis was the third most prominent primary complaint indicated by 30/128 (23.4%) of patients. For the CG, myofascial/musculoskeletal pain was the most prominent pain complaint indicated by 14/20 (70%) of patients, arthritis was the
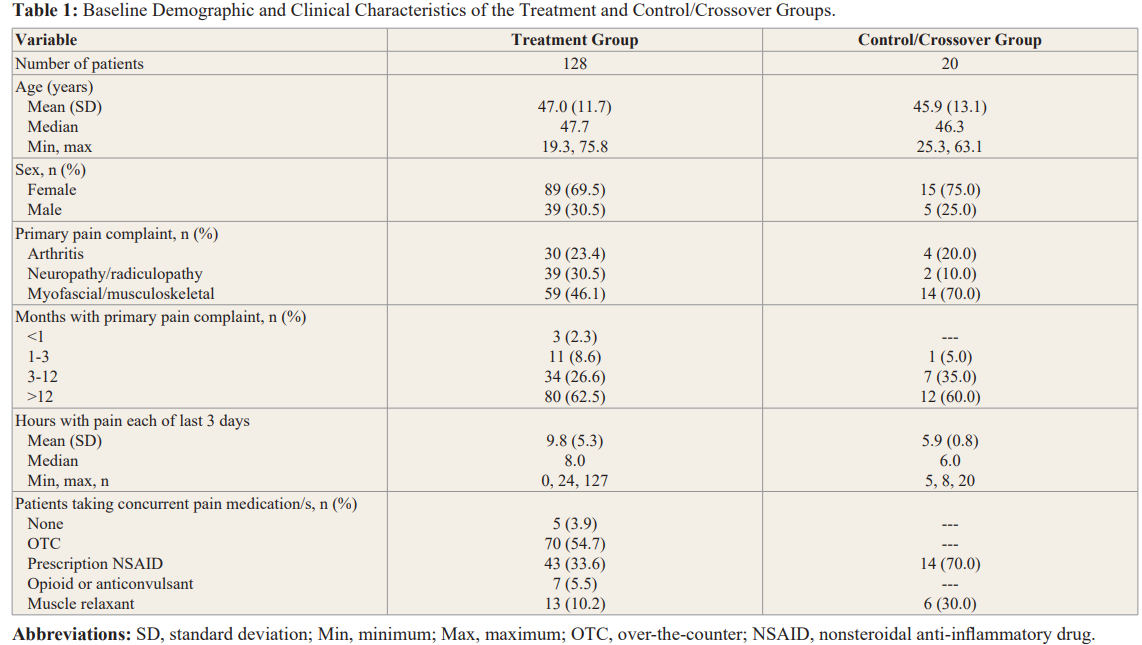
next most common pain complaint for 4/20 (20%) of patients, and neuropathy/radiculopathy was the third most prominent primary complaint indicated by 2/20 (10%) of patients.
Treatment Group (TG)
At baseline, of the 59 study participants who indicated myofascial/ musculoskeletal pain as their primary complaint, 83% noted that their back and lower extremities was the most common location of pain (n=49). Of the 39 patients who indicated neuropathy/ radiculopathy as their primary pain complaint, 82% noted their back was the most common location of their pain (n=32). Of the remaining 30 patients in the TG who indicated arthritis as their primary pain complaint, 80% noted their lower extremities (knee and foot) was the most common location of their pain (n=24).
Almost 27% of patients reported having their pain for 3 months to one year (34/128) and over 62% reported having pain for more than one year (80/128).
Control and Crossover Group (CG, CROSSG)
At baseline, all the 14 CG study participants who indicated myofascial/musculoskeletal pain as their primary complaint, noted that their lower extremities were the most common location of pain. The 4 patients who indicated arthritis and 2 patients who indicated neuropathy/radiculopathy as their primary pain complaint noted their lower extremities was the most common location of their pain.
Approximately 35% of patients reported having their pain for 3 months to one year (7/20)) and 60% reported having pain for more than one year (12/20).
Results
Treatment group paired data were collected. Over 30 days, treatment group mean BPI Severity score decreased 61% (4.9 to 1.9/10;P< .001) and mean BPI Interference score decreased 61% (3.8 to 1.5/10;P< .001) The control group showed an increase in both BPI Severity of 23% (3.0 to 3.7/10) and BPI Interference Score of 58% (1.2 to 1.9/10). After crossing over to treatment, patients in the crossover group reported a decrease in BPI Severity score of 76% (3.7 to .9/10) and a decrease in BPI Interference score of 79% (1.9 to .4/10) (Figure 2). No side effects of treatment were reported. After 30 days, 91% of patients reported “less” or “a lot less” usage of oral medications. 86% of patients were very/ extremely satisfied with the patch and preferred the pain-relieving patch to oral medications. Results also showed Quality of Life (QoL) improvements in mood, relations with other people, sleep, walking ability, and enjoyment of life.
Baseline BPI severity and interference scores
The mean BPI pain severity score for the Treatment Group at baseline was 4.9, with SD = 2.1, for the Control Group it was 3.0, with a SD = 0.6, and for the Crossover Group, it was 3.7, with a SD = 0.5, (Table 2). The baseline mean BPI interference score for the Treatment Group was 3.8, with SD = 2.6, for the Control Group, it was 1.2, with a SD = 0.3, and for the Crossover Group, it was 1.9,with a SD = 0.4. (Table 2).
Changes from baseline to day 14 and day 30 in mean BPI pain severity scores
Treatment Group
Patients in the Treatment Group showed a 41% decrease (4.9 to 2.9, 95% CI, -2.2 to -1.7, p < .001) from baseline to day 14 and a 61% decrease (4.9 to 1.9, 95% CI, -3.2 to -2.6, p < .001) from baseline to day 30 in mean BPI severity scores after using the pain patch. See Figure 1A
For each of the 4 questions which comprise the BPI pain severity score (pain at its worst and least in the last 24 hours, pain right now, and how much pain on average), see Table 2, mean scores from baseline to day 14 and to day 30 all decreased statistically significantly (p < .001). The amount of decrease from baseline to day 30 was greater than the decrease from baseline to day 14 for each of the 4 questions.
Control Group
For the Control Group, patients showed a 3% decrease (3.0 to 2.9, 95% CI, -0.5 to 0.3, p < .480) from baseline to day 14 and a 23% Increase (3.0 to 3.7, 95% CI, 0.3 to 1.0, p =.001) from baseline to day 30 in mean BPI severity scores. See Figure 1A.
For each of the 4 questions which comprise the BPI pain severity score (pain at its worst and least in the last 24 hours, pain right now, and how much pain on average), see Table 2, changes in mean scores from baseline to day 30 all increased.
Crossover Group
For the Crossover Group, patients showed a 27% decrease (3.7 to 2.7, 95% CI, -1.3 to -0.6, p < .001) from baseline to day 14 and a 76% decrease (3.7 to 0.9, 95% CI, 3.2 to -2.4, p < .001) from baseline to day 30 in mean BPI severity scores after starting active treatment with the pain patch. See Figure 1A.
For each of the 4 questions which comprise the BPI pain severity score (pain at its worst and least in the last 24 hours, pain right now, and how much pain on average), see Table 2, changes in mean scores from baseline to day 30 all decreased statistically significantly (p < .001). The amount of decrease from baseline to day 30 was greater than the decrease from baseline to day 14 for each of the 4 questions.
Changes from baseline to day 14 and day 30 in mean BPI pain interference scores
Treatment Group
In the TG, the BPI pain interference scores (the mean of the component scores for general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life) decreased statistically significantly (p <. 001) from baseline to day 14 and day 30. At day 14, patients had a 40% decrease (3.8 to 2.3, 95% CI, -1.7 to -1.3, p < .001) that improved to a 61% decrease (3.8 to 1.5, 95% CI, -2.6 to -2.0, p < .001) at day 30 (see Figure 1B). The amount of decrease in mean pain interference scores
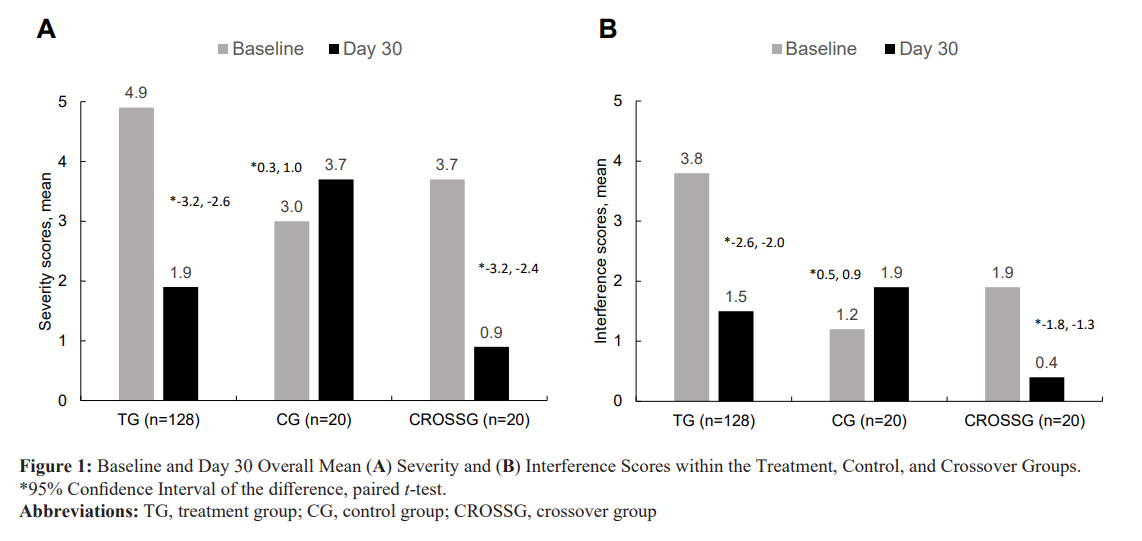
from baseline to day 30 was statistically significantly greater than from baseline to day 14 (95% CI, -2.3 to -1.5, p < .001).
Control Group
In the CG, the BPI pain interference scores either remained the same or increased from baseline to day 14 and day 30. At day 14, patients had a 33% increase (1.2 to 1.6, 95% CI, 0.6 to 0.1, p = .004) that continued to increase to almost 60% (1.2 to 1.9, 95% CI, 0.5 to 0.9 p < .001) at day 30 (Figure 1B).
Crossover Group
In the CROSSG, the BPI pain interference scores decreased statistically significantly (p < .001) from baseline to day 14 and day 30. At day 14, patients had a 32% decrease (1.9 to 1.3, 95% CI, -0.9 to -0.4, p < .001) that improved to a 79% decrease (1.9 to 0.4, 95% CI, -1.8 to -1.3, p < .001) at day 30 (Figure 1B).
Changes in self-perceived pain relief from medications
One of the BPI questions (not part of the pain severity or interference scores) asks the patient how much pain relief (in increments of 10% from 0% = no relief to 100% = complete relief) they have experienced from treatments or medications within the last 24 hours. At baseline, TG patients reported a mean of 33% pain relief from current treatment or medications; by day 14 they reported 73% pain relief, and by day 30 they reported 81% pain relief. The change in mean percent relief from baseline to day 14 was statistically significant (95% CI, 34.4 to 46.4, p < .001) and was
Changes from baseline to day 14 and baseline to day 30 in the use of concurrent pain medications
Treatment Group
In each survey, patients indicated the type of medication they were taking for pain relief including OTC pain medications, prescription anti-inflammatory medications, opioids or anticonvulsants, or muscle relaxants. At baseline, there were 55% of patients (70/128) taking an OTC product for their pain, 34% of patients (43/128) taking a prescription NSAID, 10% (13/128) taking a muscle relaxant, and 6% (7/128) taking an opioid or anticonvulsant. Four percent of patients (5/128) indicated that they were not taking any concurrent medications at baseline.
There was a statistically significant decrease in the number of patients using one or more OTC pain medications from baseline to day 14 (70 to 64 patients, p = 0.031, McNemar Test) Ibuprofen was the highest reported OTC pain medication in use at baseline (47/128, 37%). Acetaminophen was the second most common OTC pain medication used by 39/128 (31%) of patients. As far as prescription anti-inflammatory medication, diclofenac was reported most often 23/128 (18%). All, except one patient in the Treatment Group reported that they discontinued their prescription NSAID by day 14 and all patients discontinued prescription NSAIDs by day 30 (a statistically significant decrease of p <.001 for each, McNemar test).
Although a minority of patients reported using opioids or anticonvulsants at baseline (7, 6%), all but 2 patients in the TG discontinued their prescription opioids and anticonvulsants by day 14 which persisted through day 30 (not a statistically significant decrease p = 0.125 for each, McNemar test).
Separate from indicating use of specific medications for pain, patients were asked how their use of oral pain medications had changed (scale: 1 = A lot more, 2 = More, 3 = No change, 4 = Less, 5 = A lot less). At day 14, 84% reported “less” or “a lot less.” At day 30, 91% reported “less” or “a lot less.”
Crossover Group
At baseline, 70% of patients (14/20) indicated that they were taking a prescription NSAID for their pain, and 40% of patients (6/20) were taking a muscle relaxant. 100% of patients (20/20) reported that they were using “a lot less” oral pain medications after using the patch.
Satisfaction with use of the pain patch
In the TG, day 14 satisfaction ratings related to specific aspects of use of the pain relief patch (scale: 1 = Strongly disagree, 2 = Disagree, 3 = Neutral, 4 = Agree, 5 = Strongly agree) were 4.6 for “convenient”, 4.5 for “easy to apply” and 4.3 for each of “preferred over other topical pain-relieving treatments,” and “preferred over pills/oral medication.” At day 30, the mean ratings were 4.7 for each of “easy to apply” and “convenient,” and 4.6 for each of “preferred over pills/oral medication” and “preferred over other pain-relieving treatments.” At day 30, overall satisfaction was 4.5 out of 5 (scale: 1 = not at all, 2 = Not very, 3 = Somewhat, 4 = Very, 5 = Extremely).
Use of the Patch
At day 14, 102/128 (80%) of patients reported that they kept the patch on ‘almost all of the time.’ The remaining 21/128 (16%) of patients reported that they used the patch ‘until the pain was gone, then again when the pain came back.’ At day 30, 85/128 (66%) of patients reported that they kept the patch on ‘almost all of the time.’ Of the remaining, 37/128 (29%) of patients reported that they used the patch ‘until the pain was gone, then again when the pain came back.’
At the first follow-up data collection point at day 14, 76/128 (59%) of patients reported that they felt pain relief in less than 5 minutes after applying the pain relief patch. 35% of patients (45/128) reported that it took longer than 10 minutes to feel pain relief. At day 30, 104/128 (81%) of patients reported that they felt pain relief in less than 5 minutes after application and 15/128 (12%) of patients reported pain relief after 10 minutes. At day 14, out of the 6 patients for whom it took longer than 10 minutes to achieve pain relief after initial application, 6/6 (100%) readjusted the location of their patch. At day 30, out of the 5 patients who did not experience pain relief within 10 minutes, 5/5 (100%) reported readjusting the location of the patch. On average, at day 14, 68/128 (53%) of patients reported attempting only one location before experiencing pain relief and 44/128 (34%) of patients reported attempting 2 locations before they experienced pain relief. Fifteen patients reported attempting more than 3 locations before finding pain relief. At day 30, 104/128 (81%) of patients reported attempting one location before experiencing pain relief and 16/128 (13%) attempted 2 locations prior to experiencing pain relief.
Duration of Pain Relief
At day 30, patients were asked how long it took for the pain to return once they removed the patch. 14% of patients (18/128) reported that their pain did not return after they removed the patch; 34% of patients (44/128) reported that it took longer than one day for the pain to return after patch removal, and 58/128 (45%) of patients reported that pain returned within 2 hours of removing the pain patch.
Safety
Patients reported no adverse skin reactions, adverse or serious adverse events while being treated with the pain relief patch.
Discussion
Here we report results of the PREVENT study, a prospective, non- randomized study of patients presenting with mild, moderate, or severe arthritic, neurological, and musculoskeletal pain. Patients indicated their utilization of pharmacological treatments for pain at baseline, 14 days, and 30 days. Treatments included OTC agents, prescription NSAIDs, opioids, anticonvulsants, or a combination of those four classes. BPI scores indicated that patients in the treatment group were experiencing mild (6%; 7/128), moderate (39%; 50/128), or severe pain (56%; 71/128).
In this final analysis, changes in BPI pain severity and pain interference scores and use of concurrent pain medications from baseline to day 14, and to day 30, were evaluated to assess the safety and analgesic efficacy of the Kailo Pain Patch®. Although BPI pain severity and interference scores and use of concurrent medications were reported and showed a decrease for those patients in the Crossover Group, due to the limited number of patients in this arm of the study, the impact and true significance of these data is not known. This analysis showed that patients reported positive results after utilizing the topical, non-drug, pain-relieving patch that led to a statistically significant reduction in mean BPI pain severity and interference score. There were no side effects reported with the topical pain-relieving patch.
The application of engineering technologies to healthcare promises a revolution in the way we diagnose, monitor, and treat diseases including pain. Although there remains a paucity of data on exactly how some emerging technologies work, understanding the pathophysiology of pain may provide plausible theories to explain the analgesic results noted above. Noxious stimuli are transduced into electrical signals in free nerve endings [39]. It is theorized that the mechanisms of analgesic effect of the metallic patch comes from the discharges of billions of capacitors when encountering the body’s natural ambient energy or energy from nociceptive-related electrical charges. By placing the patch along the pain pathway, it is thought to modulate nociceptive signals; the patch innovators hypothesize that the capacitors influence dysfunctional axons and act as a ‘bridge’ over the portion of the pain pathway where electrical disruption is occurring. It is known that with advancing age and in cases of injury, the body’s ability to transmit signals along the nervous system pathway are negatively affected [40]. A persistent analgesic benefit has been shown to persist in some patients even after the patch is removed; whether the treatment dampens peripheral sensitization or promotes the body’s natural healing process is unknown.
There is an unmet need for alternative treatment options for patients with pain that lack the adverse effects of conventional systemic analgesics. Considering the bothersome and dangerous adverse effects that occur with NSAIDS, acetaminophen, opioids and adjuvant analgesics, the incorporation of novel, topical, non- pharmacological treatment options will add important safe and effective options to a clinician’s armamentarium [41-46].
Results reported here from this IRB-approved observational study suggest that the non-pharmacological micro/nanotechnology based topical pain patch studied may provide a viable treatment alternative to pharmacological based therapies. Further analyses and formal controlled trials are planned to confirm these results.
Limitations
This was an observational study based on a sample of patients attending diverse clinical settings for the treatment of arthritic, neurological, and musculoskeletal pain who consented to participate in this study. This analysis reported on a group of patients who were treated with the study patch, including a small number of Control Group patients who did not initially receive the patch, but then moved into the CROSSG Group and received the patch 30 days after enrollment. Due to the small number of patients in the CG and CROSSG Groups, these results may not be as impactful and warrant further study. Further, limitations on Control Group enrollment were hampered by the onset of the global COVID-19 pandemic. Generalizability of the findings may be limited to the treatment groups only because those patients received topical therapies.
The data of those patients who did not complete the follow up surveys after baseline were removed from evaluation. Although patients were given instruction on proper patch placement and timing of use, patients individually decided patch placement and used the patch on an ‘as needed’ basis. This may limit the consistency in patch utilization and overall results among all patient groups as each patient utilized the patch according to their own needs. Although the FDA has provided guidance of the value of incorporating and reporting on this type of Real-World Evidence data [47], in addition to utilizing validated pain scales for data collection, there may be a limitation on the interpretability and validity of these results due to lack of consistency among patient data as is collected in a randomized and formal clinical trial.
Conclusion
Study results indicate that this novel, non-pharmacological formulated topical analgesic pain-relieving patch can reduce BPI pain severity and interference scores and related pain for adult patients with arthritic, neuropathic, and musculoskeletal pain. The results further support the use of this OTC pain patch as a first-line non-pharmacological treatment option and should be considered by healthcare clinicians and patients as part of a multimodal treatment approach. Patient outcomes further encourage more research to be conducted to confirm these results.
Acknowledgments
This IRB-approved study administered by Clarity Science LLC and was funded by Pain Relief Technologies, the distributors of the Kailo Pain Patch®.
Disclosure
Jeffrey Gudin MD has received compensation from Clarity Science LLC for his role as principal investigator and for providing protocol- required services for the study. Peter L Hurwitz is President of Clarity Science LLC, Derek T Dietze received compensation for study statistical analyses.
References
1.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018; 64: 832-840.
2.Chen J, Jin T, Zhang H. Nanotechnology in Chronic Pain Relief. Front Bioeng Biotechnol. 2020; 8: 682.
3.Volkow N, Benveniste H, McLellan AT. Use and misuse of opioids in chronic pain. Ann Rev Med. 2018; 69: 451-465.
4.Ambrose KR, Golightly YM. Physical exercise as non- pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol. 2015; 29: 120-130.
5.Ducic I, Mesbahi AN, Attinger CE, et al. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plastic Reconst Surgery. 2008; 121: 908- 914.
6.De Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochr Data Syst Rev. 2012; 11: 7407.
7.Gudin JA, Brennan MJ, Harris ED, et al. Changes in pain and concurrent pain medication use following compounded topical analgesic treatment for chronic pain: 3-and 6-month follow-up results from the prospective, observational Optimizing Patient Experience and Response to Topical Analgesics study. J Pain Res. 2017; 10: 2341-2354.
8.Gudin JA, Dietze DT, Hurwitz PL. Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study. J Pain Res. 2020; 13: 1557-1568.
9.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. PAIN. 2013; 154: 94- 100.
10.Ballantyne JC, Sullivan MD. Intensity of chronic pain—the wrong metric. N Engl J Med. 2015; 373: 2098-2099.
11.Kaye AD, Cornett EM, Hart B, et al. Novel pharmacological nonopioid therapies in chronic pain. Curr Pain Head Rep. 2018; 22: 31.
12.Zhuo M. Neural mechanisms underlying anxiety–chronic pain interactions. Trends Neurosci. 2016; 39: 136-145.
13.Pohl M, Smith L. Chronic pain and addiction: challenging co-occurring disorders. J Psych Drugs. 2012; 44: 119-124.
14.Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015; 156: 569-576.
15.Volkow ND, McLellan AT. Opioid abuse in chronic pain— misconceptions and mitigation strategies. New Eng J Med. 2016; 374: 1253-1263.
16.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010; 7: 482-493.
17.Cuomo A, Bimonte S, Forte CA, et al. Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019; 12: 711-714.
18.Sharon L Kolasinski, Tuhina Neogi, Marc C Hochberg, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020; 72: 149-162.
19.Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. Pharmacy Therapeutics. 2017; 42: 742-755.
20.Kumar A, Tan A, Wong J, et al. Nanotechnology for Neuroscience: Promising Approaches for Diagnostics, Therapeutics and Brain Activity Mapping. Adv Funct Mater. 2017; 27: 1700489.
21.Tang Z, Zhao P, Ni D, et al. Pyroelectric nanoplatform for NIR-II-triggered photothermal therapy with simultaneous pyroelectric dynamic therapy. Mat Horiz. 2018; 5: 946-952.
22.Zhang C, Bu W, Ni D, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew Chem Int Ed. 2016; 55: 2101-2106.
23.Bleeker EAJ, de Jong WH, Geertsma RE, et al. Considerations on the EU definition of a nanomaterial: science to support policy making. Regul Toxicol Pharmacol. 2013; 65: 119-125.
24.Taniguchi N. On the basic concept of ‘nano-technology. Presented at Proc Int Conf Prod Eng. Tokyo, Part II, Tokyo. 1974.
25.Siddhartha Shrivastava, Debabrata Dash. Applying Nanotechnology to Human Health: Revolution in Biomedical Sciences. Journal of Nanotechnology. 2009. https://doi.org/10.1155/2009/184702.
26.Siavash Beiranvand, Mohamad Masud Sorori. Pain management using nanotechnology approaches. Artificial Cells, Nanomedicine, and Biotechnology. 2019; 47: 462-468.
27.Chang Wesley, Kliot Michel, Sretavan David. Microtechnology and nanotechnology in nerve repair. Neurological research. 2009; 30: 1053-1062.
28.Brian A Simpson. Challenges for the 21st Century: The Future of Electrical Neuromodulation. Pain Medicine. 2006; 7: 191- 194. https://doi.org/10.1111/j.1526-4637.2006.00134.x.
29.Spillane J, Kullmann DM, Hanna MG, et al. J. Neurol.Neurosurg. Psychiatry. 2016; 87: 37.
30.Pancrazio JJ. Nanomedicine. 2008; 3: 823.
31.Pall ML. Electromagnetic fields act via activation of voltage- gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013; 17: 958-965.
32.Arneja AS, Kotowich A, Staley D, et al. Electromagnetic fields in the treatment of chronic lower back pain in patients with degenerative disc disease. Future Sci OA. 2016; 2: 105.
33.Gudin JA, Dietze DT, Hurwitz PL. Using Nanotechnology to Improve Pain and Function with a Novel, Drug-Free, Topical Pain-Relief Patch: An Interim Analysis. Anesth Pain Res. 2020; 4: 1-10.
34.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008; 9: 105-121.
35.Erdemoglu AK, Koc R. Brief pain inventory score identifying and discriminating neuropathic and nociceptive pain. Acta Neurol Scand. 2013; 128: 351-358.
36.Keller S, Bann CM, Dodd SL, et al. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004; 20: 309-318.
37.Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified brief pain inventory short form in patients with osteoarthritis. Eur J Pain. 2006; 10: 353-361.
38.Tan G, Jensen MP, Thornby JI, et al. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004; 5: 133-137.
39.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010; 120: 3760-3772.
40.Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med. 2012; 13: 27-36.
41.Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009; 103: 1227-1237.
42.Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009; 8: 669- 681.
43.John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol. 2009; 62: 505-515.
44.Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009; 69: 51-69.
45.Scarpignato C, Hunt RH. Nonsteroidal anti-inflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010; 39: 433-464.
46.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011; 342: 7086.