Abnormal Uterine Bleeding
Author'(s): Dr. Asanka Gunasena G. G*, MBBS, MD (Obs & Gyn), MRCOG (UK)
Diploma in Advanced Gynaecological Endoscopy (France), Consultant Obstetrician and Gynaecologist, District Base Hospital, Rikillagaskada, Sri Lanka.
*Correspondence:
Asanka Gunasena G.G, Diploma in Advanced Gynaecological Endoscopy (France), Consultant Obstetrician and Gynaecologist, District Base Hospital, Rikillagaskada, Sri Lanka, Tel: +94777704780; E-mail: asankagunasena@gmail.com.
Received: 06 November 2017 Accepted: 13 December 2017
Citation: Asanka Gunasena G.G. Abnormal Uterine Bleeding. Gynecol Reprod Health. 2017; 1(4): 1-8.
Keywords
Introduction
Abnormal uterine bleeding (AUB) may be defined as any variation from the normal menstrual cycle. It includes changes in regularity and frequency of menses, duration of flow, or amount of blood loss [1]. Among women of reproductive age, AUB is a common cause of visits to the emergency department and/or health care provider. Heavy menstrual bleeding (HMB) is the most common presentation of AUB.
Abnormal uterine bleeding affects up to 30% of women throughout their reproductive lifetime [2]. These disorders may significantly affect quality of life; result in time off work and lead to surgical intervention including endometrial ablation and hysterectomy. AUB ultimately has a significant impact on the health care system including financial concerns [3].
The investigation and management of AUB has been hampered by the confusing and inconsistently applied nomenclature. There is also a paucity of standardized methods for investigation and categorization of the potential causes [4]. Therefore, it is essential to have a standardized universal terminology for AUB to improve communication among practitioners and to guide research and education on this topic.
As a result, the FIGO Menstrual Disorders Working Group (FMDG) has developed new guidelines for terminology related to this topic [5]. The suggested nomenclature for AUB aims to simplify descriptions of this clinical presentation and eliminate terminology such as menorrhagia, metrorrhagia, and dysfunctional uterine bleeding. This classification system is called PALM- COEIN FIGO classification.
Although the patient’s perception of the bleeding does not necessarily represent the actual blood loss, it is of utmost importance in the management of this problem. Ultimately, the woman’s experience and the impact on her quality of life determine the degree to which intervention may be required [1].
In the early 1990s, it was estimated that at least 60% of women presenting with HMB would have a hysterectomy to treat the problem, often as a first line [6]. Alternative effective treatments to hysterectomy are now available for women with HMB, particularly for those who have a normal uterus and no significant pathology such as large uterine fibroids. Therefore, the number of hysterectomies is decreasing rapidly particularly in the developed countries. However, in the low-resource setting, hysterectomy is still being offered frequently due to the unavailability of modern technology like endometrial ablation and umbilical artery embolization.
Definitions of terms used in the description of AUB
Menstrual bleeding is described mainly in relation to the volume, regularity, frequency and duration. The sense of ‘normal blood loss during a menstrual period’ for an individual woman is based on beliefs derived from personal experience, cultural, social and educational influences. However, there are some of the common terms used in the description of AUB (Table 1).
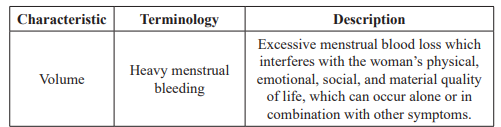
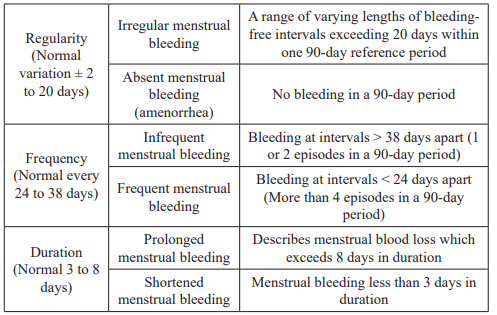
Table 1: Definitions of terms for AUB [1].
Acute, chronic, and intermenstrual AUB
Chronic AUB is defined as bleeding from the uterine corpus that is abnormal in volume, regularity, and/or timing, and has been present for the majority of the past 6 months. Chronic AUB does not necessarily require immediate intervention [7].
By contrast, acute AUB is defined as an episode of heavy bleeding that is of sufficient quantity to require immediate intervention to prevent further blood loss. Acute AUB may present in the background of existing chronic AUB or might occur without such a history [7].
Intermenstrual bleeding (IMB) occurs between clearly defined cyclic and predictable menses. Such bleeding may occur at random times or may manifest in a predictable manner at the same day in each cycle [7].
PALM-COEIN FIGO Classification for AUB
There are 9 main categories, which are arranged according to the acronym PALM-COEIN (Table 2). The components of the PALM group are discrete (structural) entities that can be measured visually with imaging techniques and/or histopathology, whereas the COEIN group is related to entities that are not defined by imaging or histopathology (non-structural). The acronym PALM-COEIN stands for Polyp, Adenomyosis, Leiomyoma, Malignancy and hyperplasia, Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified [7].
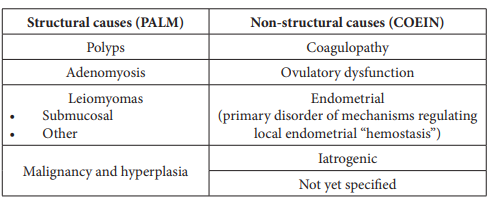
Table 2: PALM-COEIN Classification of AUB.
The entities like polyps and adenomyosis have only a primary classification which indicates their presence or absence. But leiomyomas have a secondary classification to distinguish those involving the endometrial cavity (submucosal) from others (intra- mural and subserosal) as submucosal lesions are the most likely to contribute to the genesis of AUB [7].
The term dysfunctional uterine bleeding (DUB) which was previously used as a diagnosis when there was no systemic or locally definable structural cause for AUB, is not included in the system. It was suggested during the agreement process that the term “DUB” should be abandoned [8]. In fact, the women who are classified as DUB actually have one or more of coagulopathy, disorders of ovulation, or primary endometrial disorder which are clearly described in the new classification system.
Some patients might have more than one cause for AUB. But caution should be taken with definable entities such as adenomyosis, leiomyomas, and endocervical/endometrial polyps as they are frequently asymptomatic and, therefore, not contributing to the presenting symptoms.
Malignant or premalignant lesions (e.g. atypical endometrial hyperplasia, endometrial carcinoma, and leiomyosarcoma) should be categorized as such within the PALM-COEIN system, but further detailing should be done according to the WHO and FIGO classification and staging systems [9,10].
Coagulopathy refers to systemic disorders of haemostasis and it is not clear how often these abnormalities cause or contribute to the genesis of AUB. However, recent evidence demonstrates that approximately 13% of women with HMB have biochemically detectable systemic disorders of hemostasis, most often von Willebrand disease [11]. Despite this fact, relatively few clinicians consider coagulopathy in the differential diagnosis of women with HMB [12].
Ovulatory disorders frequently occur at the extremes of reproductive age: adolescence and the menopause transition. Clinical manifestation may range from amenorrhea, through extremely light and infrequent bleeding, to episodes of unpredictable and extreme HMB. Ovulatory AUB tends to be regular and patients will often have premenstrual symptoms and dysmenorrhea. In contrast, anovulatory bleeding is often irregular, heavy, and prolonged and is more common near menarche and the perimenopause. Anovulatory AUB is more likely to be associated with endometrial hyperplasia and cancer [1].
Many cases of ovulatory disorders are secondary to endocrinopathies (e.g. polycystic ovary syndrome, hypothyroidism, hyperprolactinemia) [1]. Mental stress, obesity, anorexia, weight loss, or extreme exercise can also result in ovulatory disorders due to disturbances in hormonal pathways.
Primary disorder of local endometrial “hemostasis” is responsible for AUB in some patients who have predictable and cyclic menstrual bleeding (typical of ovulatory cycles) with no definable causes to be identified. High-quality evidence has demonstrated deficiencies in local production of vasoconstrictors such as endothelin-1 and prostaglandin F2α, and/or accelerated lysis of endometrial clot because of excessive production of plasminogen activator [13]. There is also evidence showing an increased local production of substances that promote vasodilation, e.g. prostaglandin E2 and prostacyclin (I2) [14,15]. Endometrial inflammation or infection (e.g. chronic endometritis) may also play a role in the genesis of AUB but a consistent relationship between histopathologic diagnosis and presence of AUB has not been demonstrated [16]. Therefore, endometrial disorders should be determined by exclusion of other identifiable abnormalities.
The iatrogenic causes of AUB include medicated or inert intrauterine systems and pharmacologic agents that directly impact the endometrium, interfere with blood coagulation mechanisms, or influence the systemic control of ovulation [1]. Gonadal steroid therapy (estrogens, progestins, and androgens) can produce unscheduled endometrial bleeding which is known as “breakthrough bleeding (BTB)” and this accounts for majority of patients with iatrogenic AUB. Many women experience unscheduled vaginal spotting/bleeding in the first 3-6 months of use of the levonorgestrel-releasing intrauterine system (LNG-IUS) [17].
Systemic agents that interfere with dopamine metabolism have the potential to cause AUB by interfering the ovulation. Examples include antidepressants (selective serotonin reuptake inhibitors and tricyclics), and antipsychotics (first generation and risperidone). Tamoxifen, corticosteroids and herbs (e.g. ginseng) are also implicated in the genesis of AUB. Finally, AUB is relatively common with the use of anticoagulant drugs such as warfarin, unfractionated heparin, and low molecular weight heparin [1].
Poorly defined and less studied entities like arteriovenous malformations and myometrial hypertrophy have been placed in a category termed “not yet classified.” This will accommodate any future entity as well.
Evaluation of AUB
A thorough history and physical examination will often indicate the cause of abnormal uterine bleeding and direct the need for further investigation and treatment. If there is any possibility of pregnancy, a sensitive urine or serum pregnancy test should be performed. Women with both acute and chronic AUB should be evaluated for anemia preferably with a full blood count including platelets.
A structured history can be used as a screening tool with 90% sensitivity for the detection of bleeding disorders [18]. Testing for coagulation disorders should be considered only in women who have a history of heavy menstrual bleeding since menarche, personal history of abnormal bleeding (postpartum hemorrhage, surgical-related bleeding, bleeding associated with dental work, bruising or epistaxis 1-2 times per month) or family history of bleeding symptoms. Such tests should include assays for von Willebrand factor. Thyroid function tests are not indicated unless there are clinical findings suggestive of thyroid disease [1].
Imaging Studies
Imaging studies are indicated when clinical examination suggests structural causes for bleeding, conservative management has failed, or there is a risk of malignancy. Transvaginal ultrasound scan (TVUS) is an appropriate and an adequate screening tool for most cases of AUB. TVUS may assist in the diagnosis of endometrial polyps, adenomyosis, leiomyomas, uterine anomalies, and generalized endometrial thickening associated with hyperplasia and malignancy. In addition, pathologies of the cervix, tubes, and ovaries can also be assessed at the same instance. But, TVUS is not 100% sensitive because polyps and other small lesions may elude detection even in ideal circumstances [19].
Saline infusion sonohysterography (SIS) is a more sensitive technique especially in cases of endometrial polyps and submucosal leiomyomas as it provides accurate details about the location and relationship to the uterine cavity [20]. SIS is a useful imaging modality as it is readily available than hysteroscopy and in some occasions, it can also obviate the need for more expensive imaging techniques like MRI.
MRI is rarely used to assess the endometrium in patients who have menorrhagia. Hysteroscopy provides direct visualization of pathologies of uterine cavity and facilitates directed biopsy. The main advantage over “blind” dilation and uterine curettage is directed biopsies under direct vision. Hysteroscopy can be performed in an office setting with or without minor anaesthesia or in the operating room with regional or general anaesthesia. Operating hysteroscopy has the ability to treat certain pathologies at the first sight (e.g. endometrial polyps). The risks of hysteroscopy include perforation of the uterus, infection, cervical lacerations, creation of false passages, and fluid overload [1].
Evaluation of the endometrium
Malignant and premalignant conditions (atypical hyperplasia or endometrial carcinoma) may result in AUB and hence pathologic assessment of the uterine cavity may be required in women at risk. A combination of factors like age, personal and genetic risk factors, and TVUS screening for endometrial thickness are used to determine which patients should undergo endometrial sampling. The average age for women with endometrial cancer is 61 years, but 5% to 30% of cases occur in premenopausal women [21]. Therefore, most suggest that endometrial sampling be considered for all women over a certain age, usually 45 years [6]. The other risk factors for women under the age of 50 include obesity, diabetes, nulliparity, history of PCOS, and family history of hereditary non- polyposis colorectal cancer (HNPCC) [21]. Women with HPNCC have a lifetime risk for endometrial cancer and colorectal cancer of 40% to 60% and a 12% risk for ovarian cancer [1]. Younger women with these risk factors should be triaged for endometrial assessment. Regardless of these risk factors, persistent AUB that is unexplained or not adequately treated requires endometrial sampling.
Several techniques can be used to perform endometrial sampling, but it is important that an adequate sample be obtained before the patient can be considered at low risk for a malignant neoplasm. Office endometrial biopsy is a minimally invasive option for endometrial evaluation in women at risk of malignancy. Endometrial sampling detects over 90% of endometrial cancers [22]. The sampling is blind and therefore will miss a focal lesion. Hysteroscopic directed sampling is recommended in the situation of a focal lesion which was found on ultrasound sacning. Dilatation and curettage (D&C) is no longer the standard of care for the initial assessment of the endometrium. It is a blind procedure, with risks of complications similar to hysteroscopy [23]. But it has been useful in low resource setting in the absence of hysteroscopy, especially in situations where office endometrial biopsy is difficult (nulliparous women and women with previous caesarean sections / cone biopsies).
Pharmaceutical Treatment for AUB
Pharmaceutical treatment should be considered where no structural or histological abnormality is present, or for fibroids less than 3 cm in diameter which are causing no distortion of the uterine cavity [6]. Any underlying medical disorder which could be the cause or a contributing factor for AUB (e.g. hypothyroidism, coagulopathy) should be treated first.
Excessive bleeding occurring with regular menstrual cycles (predictable bleeding) may not be due to hormonal imbalances. Disturbances of local endometrial haemostasis, such as increased fibrinolytic activity or increased prostaglandin levels could be the culprit in these patients. Leiomyomas also typically present with regular heavy periods. These patients can be successfully treated with both hormonal and non-hormonal options.
Hormonal imbalances in the hypothalamo pituitary ovarian endometrial axis result in anovulatory cycles, which are particularly common at the time of menarche and around the menopause. These patients experience unpredictable (irregular) and often heavy bleeding. They can be most effectively treated with hormonal options that regulate cycles, decreasing the likelihood of unscheduled and potentially heavy bleeding episodes.
When selecting a specific medical therapy, it is essential to adopt a patient-centered approach regardless of the type of abnormal bleeding. A discussion about the patient’s preference, desire for fertility or contraception, underlying medical conditions or contraindications, presence of dysmennorrhea, and severity of the bleeding should take place before prescription of medication.
Non-hormonal Treatments
It is recommended to start non-hormonal therapy (either tranexamic acid or NSAIDs) if pharmaceutical treatment is required while investigations and definitive treatment are being organized [6].
Non-steroidal anti- inflammatory drugs (NSAIDs)
Heavy menstrual bleeding can be associated with elevated prostaglandin levels in the uterine tissues [24]. NSAIDs reduce total prostaglandin production through the inhibition of cyclooxygenase, shifting the balance between prostaglandins and thromboxanes towards promotion of uterine vasoconstriction. NSAIDs can reduce the menstrual blood loss by 33% to 55% without a significant difference in adverse effects. NSAIDs also have shown an added benefit of improving dysmenorrhea in about 70% of patients [25], thus should be preferred to tranexamic acid when HMB coexists with dysmenorrhea. Although mefenamic acid and naproxen are the most extensively studied, ibuprofen, diclofenac and indomethacin have all been shown be effective in treating HMB. They should be taken regularly from the onset of bleeding, or just before, until heavy loss has abated. NSAIDs should not be used where it is thought that HMB is caused by bleeding disorders. Side effects such as gastrointestinal upset are unlikely to be significant as therapy continues only for a few days each month.
Antifibrinolytics
Women with heavy menstrual bleeding have been found to have elevated endometrial levels of plasminogen activators, with more local fibrinolytic activity than women with normal menstrual losses [26]. Tranexamic acid is an antifibrinolytic agent which reduces the breakdown of fibrin in a pre-formed clot thus reducing the menstrual blood loss. Placebo- controlled trials have shown an overall reduction in menstrual blood loss between 40% and 59% from baseline [27]. Randomized trials have also demonstrated superior results of using tranexamic acid over luteal-phase progestins [28] and NSAIDs [29]. But tranexamic acid does not treat dysmenorrhea.
There is no evidence that tranexamic acid affects platelet numbers or aggregation or has any effect on coagulation within healthy blood vessels. Thus, it does not appear to increase the overall rate of thrombosis [6]. Regardless of the lack of evidence, many still caution against the use of antifibrinolytics among patients with a past history of thromboembolism.
Ongoing use of NSAIDs and/or tranexamic acid is recommended for as long as they are found to be beneficial by the patient but the therapy should be discontinued if it does not improve symptoms within three menstrual cycles [6].
Hormonal Treatments Combined oral contraceptives
Combined oral contraceptives (COCs) contain estrogen and progestogen in combination. The progesterone component suppresses ovulation and inhibits ovarian steroidogenesis to create endometrial atrophy, while estrogen provides support to the endometrium to reduce the likelihood of unscheduled breakthrough bleeding. One RCT of COCs on short-term outcomes found a reduction of menstrual blood loss of 43% [30]. COCs are generally used for 21days followed by a 7day break (or iron supplements for 7 days), during which time withdrawal bleeding will occur which is physiologically different from the bleeding that occurs after a natural ovulatory cycle. Continuous use of COCs reduces both the amount of blood loss per cycle and the number of bleeding episodes per year and this method is particularly helpful for women with dysmenorrhea and pelvic pain. Care should be taken when COCs are prescribed about absolute and relative contraindications.
Oral Progestin’s
Progesterone is a physiological hormone produced during the luteal phase of the menstrual cycle and is responsible for the secretory transformation of the endometrium. Luteal phase progestin (for 7 - 11 days) can achieve menstrual regularity but alone is not an effective treatment for regular heavy menstrual bleeding [31]. But long-cycle, high-dose oral progestins (e.g. norethisterone 15 mg daily from day 5 to day 26 of the cycle) have been shown to reduce menstrual losses for women with heavy menstrual bleeding by 83% [32]. Common side effects from oral progestins include breast tenderness, water retention, weight gain, headaches, and acne, thus their clinical usefulness may be limited by tolerability.
Injected progestin’s
Depot medroxyprogesterone acetate (DMPA) is often used in clinical practice for treating heavy menstrual bleeding in addition to its wide-spread use as an excellent contraception. There are no published trials investigating the impact of DMPA on abnormal uterine bleeding but more than 50% of women will experience amenorrhea after 1 year of use [33]. But unscheduled bleeding is common in the first few months which might lead to discontinuation of treatment.
The Levonorgestrel-Releasing Intrauterine System (LNG-IUS) The levonorgestrel-releasing intrauterine system (LNG-IUS) is an intrauterine progestogen-only contraception, which administers 20 μg of levonorgestrel directly to the endometrium each day. The device is licensed for 5 years of use and it induces endometrial atrophy and reduces mean uterine vascular density [34]. RCTs have shown a reduction in menstrual blood loss ranging from 71% to 96%, improving haemoglobin and serum ferritin levels in patients suffering from HMB. Many women will become completely amenorrheic, with reported rates in the range of 20% to 80% at one year [6]. But the full benefit of treatment may not be seen for 6 months after insertion. It has also been found to improve dysmenorrhea and pelvic pain due to endometriosis [34,35].
The LNG-IUS has been shown to be substantially superior to other medical treatments, including NSAIDS and tranexamic acid [1]. Still the efficacy of LNG- IUS has not been compared to that of COCs for abnormal uterine bleeding. Comparing LNG-IUS with surgical treatments for AUB, a Cochrane meta-analysis found that although endometrial destruction, and especially hysterectomy, more effectively reduce menstrual blood loss, the LNG-IUS provides an equivalent improvement in quality of life [36]. Economic modelling has shown that LNG-IUS creates more quality of life for patients with AUB at a lower cost than any other medical (hormonal and non-hormonal) or surgical treatment strategy [6]. This is a very important fact to be considered especially in low- resource settings.
Irregular bleeding after insertion is common, but typically resolves within 3 to 6 months; thus patients should be counseled accordingly. Other side effects include expulsion and perforation which are partially dependent on the skill of the provider. Minor degree of systemic absorption results in hormonal symptoms (breast tenderness, mood changes, and acne) which are usually mild and dissipate with time.
Other hormonal treatments for HMB
Danazol is a synthetic androgenic steroid with anti-estrogenic and anti-progestogenic activity which induces endometrial atrophy by inhibiting ovarian steroidogenesis. Although some studies have reported a reduction in menstrual loss by up to 80% [37], danazol is not recommended for routine use in the treatment of HMB [6] due to significantly more adverse effects than other medical therapies (weight gain, acne, and androgenic effects).
Gonadotrophin-releasing hormone (GnRH) agonists act by down-regulation of GnRH receptors on hypothalamus, creating a reversible hypogonadal state. This property of inhibition of oestrogen production is useful in the pharmaceutical treatment of estrogen-dependent lesions such as endometriosis and uterine leiomyoma. GnRH agonists have been shown to reduce uterine and leiomyoma volume by up to 60% [38], and their use could be considered prior to surgery or when all other treatment options for uterine fibroids, including surgery or uterine artery embolization (UAE), are contraindicated [6]. Patients should be warned of the possible temporary “flare” or exacerbation of symptoms immediately after administration of GnRH agonists due to initial increase in FSH and LH secretion. The effects are usually reversed once GnRH treatment ceases. Long-term use of GnRH agonists is limited by its significant adverse effects including bone pain, loss of bone density, and hypo-estrogenic effects (hot flashes, night sweats, and vaginal dryness). If treatment is to be used for more than 6 months or if adverse effects are experienced, “add-back therapy” with low-dose estrogen and progestogen is recommended [6].
Surgical Management
Although the options of both pharmaceutical and surgical interventions are available for the woman with AUB, it is unclear whether operative interventions should be used as the initial treatment. The decision requires a thorough evaluation of the underlying pathology and patient factors. As medical treatment of AUB is effective for many women, and treatment with the LNG- IUS may be comparable to surgical options for improving quality of life [36], it is advisable to offer surgical intervention to women with clear indications.
Common indications for surgical intervention include; failure to respond to medical therapy, inability to utilize medical therapies due to side effects or contraindications, significant anemia, impact on quality of life and concomitant uterine pathology (e.g. large uterine fibroids, endometrial hyperplasia) [1].
Surgical options for AUB depend on several factors including uterine pathology, patient’s expectations (fertility wishes, need for contraception, and acceptance of amenorrhoea) and availability. Newer techniques like endometrial ablation and uterine artery embolization are not widely available especially in the developing countries.
Dilatation and curettage
Although there might be a role in cases of severe acute bleeding refractory to medical therapy, dilatation and curettage should be used as a diagnostic technique when endometrial sampling or hysteroscopic evaluation is not possible.
Endometrial Ablation
Before the introduction of endometrial ablation in early 90s, hysterectomy was the only surgical option available for patients with AUB who failed to respond to medical therapy. The primary aim is to destroy or remove the endometrium along with the superficial myometrium to prevent the occurrence of further menstrual bleeding.
The first procedures to be developed (first generation) were transcervical resection of the endometrium (TCRE) and rollerball endometrial ablation (REA). Both techniques involve distending the uterine cavity with fluid. These methods are performed under direct visualization (hysteroscopy) and outcome is dependent on the skill and experience of the surgeon. Hysteroscopic ablative methods are highly effective in controlling bleeding in 87% to 97% of women while creating amenorrhea in 23% to 60% of women. Out of these patients, 6% to 20% will ultimately require further intervention (usually hysterectomy) in 1 to 5 years of follow-up [39].
The second generation methods were introduced later to overcome the relative difficulty in learning the technique. These methods are not performed under direct vision of the uterine cavity and they utilize different types of energy. They are safer to use and less time-consuming. This group includes thermal balloon endometrial ablation (TBEA), microwave endometrial ablation (MEA), hydrothermablation, bipolar radiofrequency endometrial ablation and endometrial cryotherapy. The second generation techniques have similar efficacy and patient satisfaction compared to first generation methods [1]. The limitations of second generation techniques include equipment problems and their inability to treat uterine pathologies such as polyps and submucosal fibroids.
Risks of endometrial ablation techniques include uterine perforation, infection, hemorrhage, and bowel or bladder injury. Risks specific to first generation techniques include fluid overload, especially with the use of hypotonic solutions, and resulting hyponatremia plus its sequelae [1]. Women undergoing second generation procedures are less likely to have fluid overload, uterine perforation, cervical lacerations, and hematometra [39]. Therefore, all women considering endometrial ablation should have access to a second-generation ablation technique in view of their higher efficacy and safety [6].
Most types of endometrial ablative techniques report an overall patient satisfaction rate of > 90%. Although hysterectomy is needed by up to 30% of women within 4 years of endometrial ablation, it is related to more risks for the patient [40]. Therefore, a less invasive option, such as ablation, would offer the patient quicker recovery and a lower risk of complications.
Endometrial ablation should be considered in women who have a normal uterus without underlying uterine pathology (e.g. hyperplasia or malignancy) and also those with small uterine fibroids (less than 3 cm in diameter) [6]. The procedure should be offered only if the patient does not want to conceive in the future and advice should be given regarding effective contraception.
When compared with the ablation, LNG-IUS appears to have similar efficacy for bleeding control in women with menorrhagia and an otherwise normal uterine cavity [41]. This is an important fact to consider in situations where endometrial ablation is not available.
Hysterectomy
Hysterectomy provides a definitive solution for women with AUB and has a high rate of patient satisfaction. It was once considered the only suitable surgical treatment for women suffering from AUB. However, a number of less invasive options have emerged as alternatives to hysterectomy, which should initially be considered in order to avoid the potential complications that hysterectomy can entail. Hysterectomy should be considered only when other treatment options have failed, are contraindicated or are declined by the patient.
If hysterectomy is required, the least invasive method should be offered to women to minimize morbidity and recovery time. However, an individual assessment is essential when deciding the route of hysterectomy; vaginal, abdominal or laparoscopic approach. When vaginal approach is not possible, laparoscopic- assisted hysterectomy carries less surgical morbidity and quick recovery time for the patient [42].
Oophorectomy at the time of hysterectomy is undertaken when disease requires the ovaries to be removed. But it is often carried out traditionally as a prophylactic procedure to reduce the risk of cancer. Prophylactic oophorectomy has been a subject of debate and it should be assessed on individual basis.
Other surgical interventions for uterine fibroids
Abnormal uterine bleeding may result if a part or entire fibroid is within the uterine cavity. Uterine artery embolization (UAE), myomectomy or hysterectomy should be considered in cases of HMB where large fibroids (greater than 3 cm in diameter) are present and bleeding is having a severe impact on a woman’s quality of life. UAE or myomectomy will potentially allow the patients to retain their fertility.
Uterine artery embolization (UAE) is an option for those who want to retain their uterus and/or avoid surgery. UAE involves blockage of both uterine arteries with particles injected through a catheter, and this procedure results in shrinkage of the fibroids. No permanent effects on the rest of the uterus are reported with UAE.
Myomectomy is the surgical removal of fibroids which can be performed by laparotomy, laparoscopically or hysteroscopically. The choice of route depends on size and location of the uterine fibroids, the size and shape of the vagina and the training and experience of the surgeon.
Conclusion
Abnormal uterine bleeding is a common gynaecological problem which requires both inward treatment and outpatient care. The need of a standardized nomenclature and classification of AUB has been fulfilled by the Palm-Coein FIGO classification system. Advanced diagnostic tools like hysteroscopy are gaining popularity in most of the less industrialized countries as well. Most of the patients with AUB can be managed medically and surgical management can be offered to carefully selected patients. The number of hysterectomies can be minimized by offering less invasive procedures like endometrial ablation and operative hysteroscopy. Where facilities are not available, levonorgestrel intra-uterine system is an effective alternative to endometrial ablation as it provides similar success rates at a lower cost.
References
- Sukhbir Singh, Carolyn Best, Sheila Dunn, et al. Abnormal Uterine Bleeding in Pre-Menopausal Women. J Obstet Gynaecol Can. 2013; 35: S1-S28.
- Market Opinion and Research International (MORI). Women’s health in [Research study conducted on behalf of Parke- Davis Laboratories]. London: MORI; 1990.
- Frick KD, Clark MA, Steinwachs DM, et al. Financial and quality-of-life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical Women’s Health Issues. 2009; 19: 70-78.
- Woolcock JG, Critchley HO, Munro MG, et Review of the confusion in current and historical terminology and definitions for disturbances of menstrual bleeding. Fertil Steril. 2008; 90: 2269-2280.
- Fraser IS, Critchley HO, Munro MG, et Writing Group for this Menstrual Agreement Process. A process designed to lead to international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding. Fertil Steril. 2007; 87: 466-476.
- Heavy Menstrual Bleeding. NICE Clinical Guidelines, No. 44 National Collaborating Centre for Women's and Children's Health (UK) London: RCOG Press. 2007. ISBN-13: 978-1- 904752-35-6.
- Munro MG, Critchley HOD, Broder MS, et al. FIGO classification system PALM-COEIN for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynecology and Obstetrics. 2011; 113: 3-13.
- Fraser IS, Critchley HO, Munro MG, et al. Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding? Hum Reprod. 2007; 22: 635-643.
- Tavassoli FA, Devilee P. World Health Organization Classification of Tumors: Pathology and Genetics of Tumours of the Breast and Female Genital Lyon: IARC Press; 2003.
- Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynecol Obstet. 2006; 95: S105-s143.
- Shankar M, Lee CA, Sabin CA, et Von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004; 111: 734-740.
- Dilley A, Drews C, Lally C, et al. A survey of gynecologists concerning menorrhagia: perceptions of bleeding disorders as a possible cause. J Womens Health Gend Based Med. 2002; 11: 39-44.
- Gleeson Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Am J Obstet Gynecol. 1994; 171: 178-183.
- Smith SK, Abel MH, Kelly RW, et A role for prostacyclin PGi2 in excessive menstrual bleeding. Lancet. 1981; 1: 522- 524.
- Smith SK, Abel MH, Kelly RW, et Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1981; 88: 434-442.
- Pitsos M, Skurnick J, Heller D. Association of pathologic diagnoses with clinical findings in chronic endometritis. J Reprod Med. 2009; 54: 373-377.
- Backman T, Huhtala S, Blom T, et al. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: A nation-wide study of 17, 360 users. BJOG. 2000; 107: 335-359.
- Kadir RA, Economides DL, Sabin CA, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998; 351: 485-489.
- Dueholm M, Lundorf E, Hansen ES, et al. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001; 76: 350-357.
- Farquhar C, Ekeroma A, Furness S, et al. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta Obstet Gynecol Scand. 2003; 82: 493-504.
- Soliman PR, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial Obstet Gynecol. 2005; 105: 575-580.
- Stovall TG, Photopulos GJ, Poston WM, et al. Pipelle endometrial sampling in patients with known endometrial carcinoma. Obstet Gynecol. 1991; 77: 954-956.
- Bettocchi S, Ceci O, Vicino M. Diagnostic approach of dilation and curettage. Fertil Steril. 2001; 75: 803-805.
- Willman EA, Collind WD, Clayton SC. Studies on the involvement of prostaglandins in uterine symptomatology and pathology. BJOG. 1976; 83: 337-341.
- Lethaby A, Augwood C, Duckitt K, et al. Nonsteroidal anti- inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007; 4: CD000400.
- Gleeson N, Devitt M, Sheppard BL. Endometrial fibrinolytic enzymes in women with normal menstruation and dysfunctional uterine bleeding. BJOG. 1993; 100: 76-81.
- Lethaby A, Farhuhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000; 4:
- Preston JT, Cameron IT, Adams EJ, et Comparative study of tranexamic acid and norethisterone in the treatment of ovulatory menorrhagia. BJOG. 1995; 100: 401-405.
- Bonnar J, Sheppard BL. Treatments of menorrhagia during menstruation: randomized controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ. 1996; 313: 579- 582.
- Fraser IS, McCarron G. Randomized trial of 2 hormonal and 2 prostaglandin-inhibiting agents in women with a complaint of Aust N Z J Obstet Gynaecol. 1991; 31: 66-70.
- Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol, and norethindrone in the treatment of objectively proven unexplained AJOG. 1993; 169: 1134-1139.
- Irvine GA, Campbell-Brown MB, Lumsden MA, et al. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. British Journal of Obstetrics and Gynaecology. 1998; 105: 592-598.
- Schwallie PC, Assenzo JR. Contraceptive use-efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 Fert Steril. 1973; 24: 331-339.
- Petta CA, Ferriani RA, Abrao MS. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with Hum Reprod. 2005; 20: 1993- 1998.
- Baldaszti E, Wimmer-Puchinger B, Loschke Acceptability of the long-term contraceptive levonorgestrel-releasing intrauterine system Mirena: A 3 year follow-up study. Contraception. 2003; 67: 87-91.
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2006; 2: CD003855.
- Dockeray CJ, Sheppard BL, Bonnar J. Comparison between mefenamic acid and danazol in the treatment of established menorrhagia. BJOG. 1989; 96: 840-844.
- Friedman AJ, Hoffman DI, Comite F, et al. Treatment of leiomyomata uteri with leuprolide acetate depot: a double- blind placebo-controlled, multicenter Leuprolide study group. Obstet Gynecol. 1991; 77: 720-725.
- Lethaby A, Hickey M, Garry R, et al. Endometrial resection/ ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009; 4: CD001501.
- Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007; 110: 1279-1289.
- Lethaby AE, Cooke I, Rees MC, et al. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015; 4:
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological Cochrane Database Syst Rev. 2009; 3: CD003677.