Autologous Adipose-Derived Stem Cell Treatment for Women with Genital Lichen Sclerosus
Author'(s): Nathan Newman M.D*, Nualla Rogowski R.N, B.S.N, Daniella Newman, and Dodanim Talavera-Adame M.D, Ph.D
Dr. Nathan Newman Stem cell Lift, Advanced Cosmetic Surgery, 9301 Wilshire Blvd. #303 Beverly Hills, CA 90210, USA.
*Correspondence:
Nathan Newman, MD 9301 Wilshire Blvd Ste 303 Beverly Hills, CA 90210, USA, Tel: (310) 273-3344, Fax: (310) 273-7651, E-mail: doctor@nathannewmanmd.com.
Received: 03 August 2018 Accepted: 27 August 2018
Citation: Nathan Newman, Nualla Rogowski, Daniella Newman, et al. Autologous Adipose-Derived Stem Cell Treatment for Women with Genital Lichen Sclerosus. Gynecol Reprod Health. 2018; 2(4): 1-4.
Abstract
Background: Lichen Sclerosus (LS) is a debilitating disease that causes chronic inflammation most commonly noted in the anogenital region. Many patients with genital Lichen Sclerosus report unsuccessful results with standard treatment options including steroids, calcineurin inhibitors, and/or hormone therapy. These patients presented to our clinic seeking autologous adipose-derived stem cell treatment (AASCT).
Methods: Data was collected through questionnaires regarding disease symptoms on a daily basis and quality of life indicators. Questionnaires were completed by patients before and three months post AASCT. One hundred and eleven consecutive women who presented to our clinic from 2011 to 2015 with symptomatic, clinically apparent or biopsy proven genital Lichen Sclerosus were given the questionnaires and 100 of these patients completed both questionnaires.
Results: The questionnaires were statistically analyzed to compare improvement in symptoms before and three months after AASCT. The improvement in every symptom was statistically significant (P<0.001). These symptoms included: itching, burning, pain, discomfort, blisters, ulcers, fusing, tearing, and fissures. Improvement in ability to carry out daily activities (such as voiding, bowel movements, exercising, intercourse, and wearing pants/underwear) also showed substantial improvement that was statistically significant (P<0.001).
Conclusion: Given the results, we can confidently say that AASCT is a promising new alternative treatment for patients suffering from Lichen Sclerosus. Additional AASCT may provide patients with further improvement in their symptoms. At present, long term follow-up is being conducted by our clinic to evaluate the course of the disease and the duration of improvements achieved from this treatment.
Keywords
Introduction
Lichen Sclerosus (LS) is a chronic inflammatory autoimmune disease with a strong predilection for the anogenital areas. The true incidence rate for LS is unknown, as this disease often goes undiagnosed [1], however, rates between 1 in 30 and 1 in 1,000 have been reported [2-4]. LS can affect men and women at any age, however, women are 6-10 times more frequently affected than men [5-7]. The onset of symptoms is usually associated with a period of increased stress, whether physical, emotional, or hormonal. Hormonal changes may exacerbate the disease and therefore the onset of symptoms is noted more frequently at menarche or in post-menopausal woman [5,8,9]. Regrettably, LS often is not recognized by many health care providers, leading to a significant delay in diagnosis and treatment. This delay can result in irreversible anatomical destruction of the genitalia as well as considerable physical, emotional, and sexual difficulties that negatively impact the patient’s quality of life [2,10-16].
Currently, the first line treatment for LS is Clobetasol Propionate 0.05%, a topical ultra-potent corticoid steroid ointment [17]. Treatment regimens vary, but Clobetasol is commonly prescribed to be applied twice a day for 3 months [5,18] and tapered as symptoms resolve. The steroid ointment can be very effective, some patients receive symptomatic relief or even temporary disease remission [1]. Second line treatment for patients who do not respond to Clobetasol have not shown to be as effective as first line treatment options [19]. These treatments include topical calcineurin inhibitors such as tacrolimus, either alone or in addition to the topical steroid [20]. In advanced cases of sclerotic disease, surgical intervention may be considered, however, many publications advise against this and only recommend surgery when malignancy is noted, or if the sclerotic changes interfere with daily activities [12,21-25]. The use of vaginal dilators may be needed when sclerotic changes affect the anatomy [14]. As discussed previously, LS has a bimodal pattern that follow hormone levels (most commonly noted at menarche and post-menopause), therefore, hormone modulation may also be beneficial in treating LS. Many of the aforementioned treatment regimens can help relieve symptoms, though it is usually only temporary. For this reason, among others, patients sought out AASCT [26-28].
Methods
111 consecutive women with LS, aged 20-76, who underwent AASCT at our clinic from 2011 to 2015 were asked to complete a questionnaire developed by our clinic. All patients included in the study had active LS that was clinically evident, biopsy proven, or symptomatically apparent. Any patient with active infection, bleeding disease, suspicious or active cancer, kidney, and/or liver disease were excluded from the study. Questionnaires were completed before AASCT and at 3 months after one treatment. Patient’s response from before the procedure as compared to 3 months after one AASCT were analyzed. The questionnaires were used to evaluate disease symptoms and quality of life indicators. Patients were asked to rank their symptoms on a scale of 0-5 (0 = no symptoms, 5 = most severe symptoms) as experienced on a daily basis. Physical changes from Lichen Sclerosus were defined as below. Fusing was defined as effacement and disappearance of the labia minora and/or prepuce. Tearing was defined as small, superficial cuts that healed within 2-3 weeks and were commonly found along the prepuce, labia minora, fourchette, peri-anal area, and gluteal fold. Fissures, which were found to commonly occur along the perineum and anal verge, were defined as deep, non- healing, linear cuts that did not go beyond the dermal layer. Patients were also asked to rank specific activities of daily living on a scale of 0-5 (0 = no difficulty with that activity, 5 = unable to perform that activity). Impedance with activities of daily living was more associated with pain, tearing, and/or fear of discomfort. Wearing pants/underwear and exercise was often an issue with patients as the friction caused irritation, pain, and often triggered a flare.
Bowel movements were associated with discomfort, tearing, and bleeding. Urination often caused severe burning upon contact with sensitive and inflamed tissues. All patients treated with AASCT underwent a lipo-harvesting procedure to extract viable adipose tissue. During this procedure, Klein’s tumescent solution was first injected into the marked areas on the trunk or lower extremities using a blunt infiltration cannula. After waiting 15-20 minutes, the adipose tissue was harvested using a 16g blunt harvesting cannula under low suction pressure. The harvested adipose tissue (40cc to 200cc) was mechanically, serially, and/or enzymatically separated to concentrate progenitor and stromal cells including mesenchymal stem cells. Most patients treated with AASCT were concomitantly treated with platelet rich plasma (PRP). This procedure entailed a 60cc blood draw, followed by centrifuge and collection of the PRP. On average, 5-7cc of PRP was obtained and mixed with local buffered anesthetic and calcium chloride. Prior to any injections, a topical numbing ointment with benzocaine, lidocaine and tetracaine was applied to the intended treatment areas and left on for approximately 40 minutes. PRP with 2-3cc of lidocaine with epinephrine 1:100,000 was injected into the dermis and subcutis using a 30G needle, followed by approximately 8-15cc of stem cell enriched adipose tissue through an 18G blunt cannula.
Results
A chart review was carried out on the 100 women whom completed both questionnaires and the results were statistically analyzed. Of the 100 patients whom completed the questionnaires from 2011- 2015, the average-age was 46.67 years, with a range from 20 to 76 years. The mean time between onset of symptoms and diagnosis was 5.24 years, with a range of 0 to 47 years, and a median of 2 years. A biopsy was carried out in 61% (61 out of 100) of our patient population.
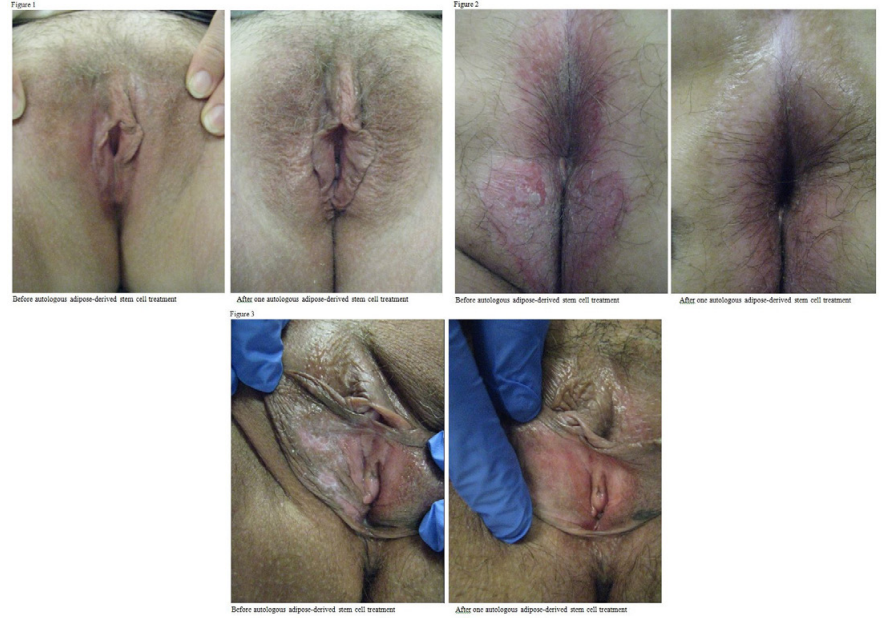
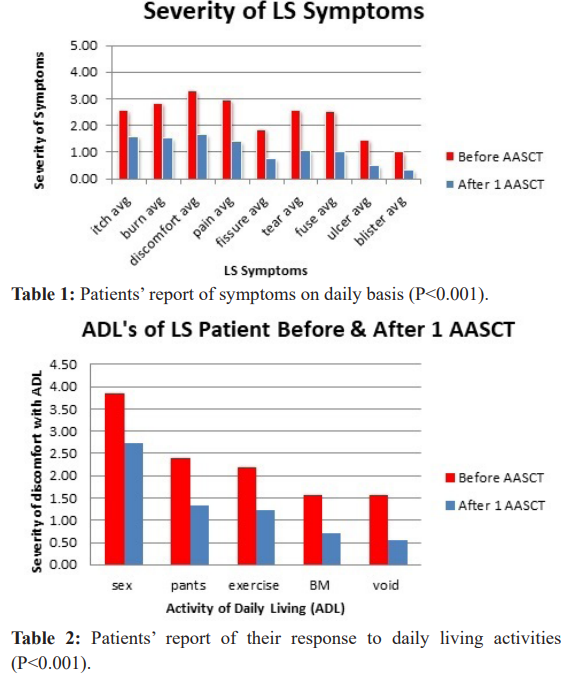
A statistically significant decrease in symptoms was found in patients subjected to AASCT after three months. The percentage of symptom improvement ranged from 38% to 68%. Some other symptoms such as discomfort, pain, fissures, tearing, and fusing were improved in the range of 46-66%. Improvement absence was not found (Table 1). Patients also reported their response to daily living activities with lowest improvement on intercourse (29%) and highest in voiding (64%). Middle improvement was reported in other daily activities such as bowel movement, wearing pants/ underwear, or exercising (Table 2). A statistically significant decrease in discomfort during daily activities was found three months after AASCT. Fortunately, the risks and complications associated with AASCT are very low.
The most common risks include: bleeding, bruising, edema, inflammation, and infection. None of the 100 patients in this study experienced any infections or complications associated with this procedure. The most common side effects noted with AASCT for LS were: swelling, bruising, burning, and discomfort. Prophylactic antibiotics were only given to patients with heart conditions or implants. The recovery time for patients was minimal, usually 1-2 weeks, and most patients tolerate AASCT with minimal discomfort, only requiring cool compress and Tylenol 500mg PO for two days to control post-operative discomfort. Some patients report an exacerbation of symptoms immediately following the procedure. After healing from the AASCT for LS, all 100 patients at our clinic, who failed to respond to traditional treatment options, reported significant improvement as mentioned before.
Conclusion
The concept of using autologous stem cell therapy in the treatment of autoimmune skin diseases, such as LS, is based on the reported effectiveness in treating other autoimmune conditions such as graft versus host [29] and sick cell disease [30]. The stem cells have an immunmodulating ability [31] to help heal the damaged tissues [32] and stop the autoimmune process. Mesenchymal stem cells have unique properties that allow them to regenerate tissue while inhibiting immune responses, allowing them to escape immune recognition while exerting anti-inflammatory effects [33,34]. These distinctive characteristics are believed to help put autoimmune diseases, such as graft-versus-host disease, into remission [34].
Autoimmune diseases such as multiple sclerosis, system sclerosis, rheumatoid arthritis, systemic lupus erythemateosus, juvenile idiopathic arthritis, and autoimmune cytopenia have been successfully treated with autologous hematopoetic stem cell transplation [35]. Advances in medicine allow us to use our bodies natural healing capacities to control inflammation and stop autoimmune diseases from progressing [36]. Studies have shown that adipose tissue derived multipotent stromal cells have higher immunomodulatory effects compared to bone marrow derived [37]. It has been reported that adipose tissue contains the most concentration of adult stem cells of any tissue in the body [38], approximately 500 fold more than found in the bone marrow [37]. This large number of cells allows for effective autologous treatment to be carried out at the bedside without the need for cultivation in the laboratory.
Studies have reported that Platelet Rich Plasma (PRP) works synergistically with autologous adipose derived stem cells in various ways [38]. Cervelli hypothesized that this benefit is because the growth factors produced from PRP help improve and regulate events related to cell-cell, cell-matrix, and proliferation of stem cells [39]. Patients with Lichen Sclerosus sought out AASCT in our clinic for many reasons, the most common were: inadequate/no relief from traditional treatment options, losing faith in healthcare providers because the significant delay in diagnosing Lichen Sclerosus, decreased quality of life (despite using traditional treatment options), wanting a long term solution, and desiring early intervention to prevent disease progression [26]. Many patients reported that their physicians may have failed to diagnose their disease resulting in a delay in diagnosis and progression of signs and symptoms. Even when diagnosed with LS, many patients do not find adequate relief from standard treatment options readily available and therefore presented to our clinic wishing to undergo AASCT.
The results achieved with one treatment of AASCT for Lichen Sclerosus dramatically improved patient symptoms, quality of life, and even restored structural anatomy in some. Patients were able to engage in intercourse, comfortably wear jeans and underwear, and reported an overall decrease of symptoms. Immune modulation from AASCT may help patients achieve remission from their autoimmune disease, and early intervention may help prevent damage and/or restore normal anatomy. Further research is currently being carried out at our clinic regarding the benefit of additional treatments with AASCT. We hope to use AASCT to not only alleviate symptoms but to modulate the autoimmune response and induce remission in our LS patients.
Acknowledgment
We acknowledge our patients for participating in filling out the questionnaires to enable us to conduct this research.
References
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004; 140: 709-712.
- Chi C-C, Kirtschig G, Baldo M, et al. Systematic review and meta- analysis of randomized controlled trials on topical interventions for genital lichen sclerosus. J Am Acad Dermatol. 2012; 67: 305-312.
- Leibovitz A, Kaplun V, Saposhnicov N, et al. Vulvovaginal examinations in elderly nursing home women residents. Arch Gerontol Geriatr. 2000; 31: 1-4.
- Tasker GL, Wojnarowska F. Lichen sclerosus. Clin Exp Dermatol. 2003; 28: 128-133.
- Cooper SM, Gao X-H, Powell JJ, et al. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004; 140: 702-706.
- Canady J, Karrer S, Fleck M, et al. Fibrosing connective tissue disorders of the skin: molecular similarities and distinctions. J Dermatol Sci. 2013; 70: 151-158.
- Powell J, Wojnarowska F. Lichen sclerosus. Lancet. 1999; 353: 1777-1783.
- Pugliese JM, Morey AF, Peterson Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007; 178: 2268-2276.
- Thomas RHM, Ridley CM, Mcgibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity-a study of 350 women. Br J Dermatol. 1988; 118: 41-46.
- Kunstfeld R, Kirnbauer R, Stingl G, et al. Successful treatment of vulvar lichen sclerosus with topical tacrolimus. Arch Dermatol. 2003; 139: 850-852.
- Dalziel Effect of lichen sclerosus on sexual function and parturition. J Reprod Med. 1995; 40: 351-354.
- Wedel N, Johnson L. Vulvar Lichen Sclerosus: Diagnosis and Management. J Nurse Pract. 2014; 10: 42-48.
- Gurumurthy M, Morah N, Gioffre G, et The surgical management of complications of vulval lichen sclerosus. Eur J Obstet Gynecol Reprod Biol. 2012; 162: 79-82.
- Thorstensen KA, Birenbaum Recognition and management of vulvar dermatologic conditions: lichen sclerosus, lichen planus, and lichen simplex chronicus. J Midwifery Womens Health. 2012; 57: 260-275.
- Vulvar lichen sclerosus may be treated effectively with topical corticosteroids, but long-term surveillance is required. Drugs Ther Perspect. 2010; 26: 16-19.
- Chi C-C, Kirtschig G, Baldo M, et al. Topical interventions for genital lichen sclerosus. Cochrane database Syst Rev. 2011; 12:
- Smith Y, Haefner H. Vulvar lichen sclerosus. Am J Clin Dermatol. 2004; 140: 28-31.
- McPherson T, Cooper S. Vulval lichen sclerosus and lichen planus. Dermatol Ther. 2010; 23: 523-532.
- Patsatsi A, Kyriakou A, Vavilis D. A therapeutic approach for female, relapsing genital lichen sclerosus: a single-center study. J Dermatolog Treat. 2013; 24: 336-339.
- Wehbe-Alamah H, Kornblau BL, Haderer J, et al. Silent no more! The lived experiences of women with lichen sclerosis. J Am Acad Nurse Pract. 2012; 24: 499-505.
- Torres A. Fitzpatrick’s Dermatology in General Medicine, 5th Ed. Vol I. American Medical Association. 1999.
- August P, Milward T. Cryosurgery in the treatment of lichen sclerosus et atrophicus of the Br J Dermatol. 1980; 103: 667- 670.
- Sommer J, Renziehausen K, Neuhauser H, et al. Cryotherapy of vulvar precancerous conditions--results of 12 years’ treatment. Arch Geschwulstforsch. 1985; 55: 341-349.
- Kartamaa M, Reitamo S. Treatment of lichen sclerosus with carbon dioxide laser vaporization. Br J Dermatol. 1997; 136: 356-359.
- Abramov Y, Elchalal U, Abramov D, et al. Surgical treatment of vulvar lichen sclerosus: a review. Obstet Gynecol Surv. 1996; 51: 193-199.
- Newman N, Dolphin NN, Newman GR. Signs and Symptoms of Genital Lichen Sclerosus in Women Seeking Autologous Adipose- Derived Stem Cell Treatment. 2015; 3.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: An update. Am J Clin Dermatol. 2013; 14: 27-47.
- Danby CS, Margesson LJ. Approach to the diagnosis and treatment of vulvar pain. Dermatol Ther. 2010; 23: 485-504.
- Ringdén O, Uzunel M, Rasmusson I, et Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81: 1390-1397.
- Hsieh MM, Kang EM, Fitzhugh CD, et Allogeneic Hematopoietic Stem-Cell Transplantation for Sickle Cell Disease. N Engl J Med. 2009; 361: 2309-2317.
- Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007; 262: 509-525.
- Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005; 129: 118-129.
- Abdi R, Fiorina P, Adra CN, et al. Immunomodulation by Mesenchymal Stem Cells A Potential Therapeutic Strategy for Type 1 Diabetes. Diabetes. 2008; 57: 1759-1767.
- M Shi, Z-W Liu, F-S Wang. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164: 1-8.
- Hügle T, Daikeler T. Stem cell transplantation for autoimmune diseases. Haematologica. 2010; 95: 185-188.
- Zhao Q, Ren H, Han Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother. 2015; 2: 3-20.
- Sara M Melief, Jaap Jan Zwaginga, Willem E Fibbe HR. Adipose Tissue-Derived Multipotent Stromal Cells Have a Higher Immunomodulatory Capacity Than Their Bone Marrow-Derived Counterparts. Stem Cells Transl Med. 2013; 2: 455-463.
- Abdel L, Aly A, Menoufy HE, et Influence of Autologus Adipose Derived Stem Cells and PRP on Regeneration of Dehiscence-Type Defects in Alveolar Bone : A Comparative Histochemical and Histomorphometric Study in Dogs. Int J Stem Cells. 2011; 4: 61-69.
- Cervelli V, Gentile P, Angelis B De, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post- traumatic lower extremity ulcers. Stem Cell Res. 2011; 6: 103-111.