Cognitive Impairments Induced by Adolescent Binge-Like Ethanol in Rat: Neuroprotective Role of Argan Oil
Author'(s):EL Mostafi Hicham1*, Touil Tariq1,2, Laaziz Abderrahim1, Ouichou Ali1, Elhessni Aboubaker1 and Mesfioui Abdelhalim1
1Laboratory of Genetic, Neuroendocrinology and Biotechnology, Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco.
2Higher Institute of Nursing and Health Professions of Rabat, Morocco.
*Correspondence:
EL Mostafi Hicham, Laboratory of Genetic, Neuroendocrinology and Biotechnology, Department of Biology, Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco, Tel: +212662770696; E-mail: elmostafihicham@gmail.com.
Received:18 June 2018; Accepted:25 July 2018
Citation: EL Mostafi Hicham, Touil Tariq, Laaziz Abderrahim, et al. Cognitive Impairments Induced by Adolescent Binge-Like Ethanol in Rat: Neuroprotective Role of Argan Oil. Addict Res. 2018; 2(1): 1-12.
Abstract
Adolescent alcohol binge drinking constitutes a major vulnerability factor to develop cognitive disorders. However, the pathways of treatment or prevention against this susceptibility remain less explored. Argan oil (AO), commonly used in traditional Moroccan medicines, is rich in oleic and linoleic acids, polyphenols, sterols, and tocopherols. This composition gives it numerous beneficial pharmacological effects of mental health. In the current study, we evaluated the short-term and long-term AO effects on (i) memory and learning deficits induced by adolescent binge-like ethanol intoxication (ii) the oxidative status of the hippocampus (Hipp) and the prefrontal cortex (PFC) in Wistar rats. To model binge-like ethanol intoxication, every 2 days, rats received an ethanol injection (3.0 g/kg) for 2 consecutive days across 14 days either from postnatal day 30 (PND30) to 43th (early adolescence). Two weeks before the onset of ethanol intoxication (PND21), rats were daily administered by oral gavage with AO (1ml/100g/day), for 5 or 20 weeks. The Y-Maze, Object Recognition and Morris water maze tests were used to assess the working memory, recognition memory, spatial memory and learning performance in adolescents or adults animals. Also, the catalase and superoxide dismutase (SOD) activities, the lipid peroxidation and nitrite concentrations were measured using spectrophotometric methods. The AO pretreatment increased the performance of working memory, recognition memory and spatial memory in rats previously intoxicated by ethanol, regardless of the age of the animals. These behavioral improvements were accompanied by stress oxidative marked changes in Hipp and PFC. AO pretreatment produces also significant decrease of the lipid peroxidation and nitrite levels. On the contrary, AO increased the catalase and SOD activities in adolescents and adults animals. For the first time, our results suggest that AO pretreatment is capable of attenuating cognitive impairments and oxidative stress in the Hipp and PFC of Wistar rats. This indicates that AO may exhibit a neuroprotection against the toxicity of ethanol in brain adolescent rats.
Keywords
Introduction
Adolescence represents a critical period in terms of drugs of abuse and brain functioning. The adolescent brain is more susceptible toethanol-induced memory impairment [1] or brain damage [2] than the adult brain. Humans experiencing alcohol during adolescence, relative to those who started drinking at the age of 21 years, have more risk to develop addiction in their life [3].
Adolescent or young adult humans drink alcohol following a pattern called “binge drinking,” defined as rapidly drinking large amounts of alcohol [4], and young binge drinkers have impaired visual and spatial working memory and lack control of impulsivity [5,6]. Although such pattern of drinking becomes common in adolescents, the impact of only a few alcohol intoxications on learning and memory appears underestimated or even neglected when considering academic performance, revealing the need for a better understanding of both the short-term and long-term effects of a few binges on cognitive function [7].
At the preclinical level, adolescent rats are more resistant to hypnotic and ataxic alcohol-related effects [8] but are more sensitive to alcohol induced neurotoxicity and memory impairment [9]. Many studies had submitted laboratory animals to forced binge-like exposure to determine the effects on brain neuronal networks and animal behavior. Interestingly, most of these studies use a pattern of exposure based on a “2-days-on, 2-days-off” procedure during 2 to 3 weeks [10-16]. The results of numerous studies on animal models of binge drinking have shown that the adolescent brain is more vulnerable to the effects of alcohol compared to adulthood, particularly with the frontal cortex and the Hipp [2], followed by a decrease in cognitive performance [17]. Schulteis & al., demonstrated that adolescent rats exposed to alcohol intermittently in order to mimic binge drinking exposure exhibit memory deficits when they are young adults [18].
For the drug treatment of cognitive disorders in bing drinking context, there has been no progress in the search for effective pharmacotherapy [19]. Only one trial is concerned with rivastigmine in the case of korsakov syndrome, but with a disappointing result [20]. Other products that modulate the NMDA system - ketamine, lamotrigine, topiramate, memantine, glutamate, antioxidant N-acetyl-cyctin or having an effect on chronic neuro-inflammation etc., could have a protective action on the brain, but to date, little attention has been paid to this area of research. In this context, our laboratory (GNBL) demonstrated, by using animal models, that certain alternative treatments complementary to conventional pharmacology had possible beneficial effects against emotional and cognitive disorders. It is mainly a pretreatment with Argan oil (AO) [21,22], obtained from the seeds of Argania spinosa L., tree endemic to Morocco. This oil has been widely used in traditional medicine as a natural remedy for several centuries [23].
At present, the interest in AO and its physiological effects has increased considerably, which has resulted in a change in the status of this oil from a dietary food product to that of a product highly prized for these antioxidant and nutritional qualities [24,25]. It is characterized by a composition rich in sterols and antioxidants such as polyphenols [26] and more particularly tocopherols (62.0 mg / 100g) [27]. The high content of tocopherols in AO gives it some protective effects against neurodegenerative diseases. It has been shown that α-tocopherols (α-TCP) and its tocotrienol isomer (TRF) are powerful neuroprotective agents against glutamate- induced toxicity on neuronal SK-N-SH cells and astrocytes. This also indicates that TRF and α-TCP may play a key role as antiapoptotic agents with neuroprotective properties [28].
In addition, many studies have shown that the metabolism of ethanol contributes to the formation of reactive oxygen species (ROS) in the brain : superoxide anions (O 2 °-) leading, in the presence of hydrogen peroxide (H2O2) and iron, the formation of highly reactive hydroxyl radicals (OH ° ), and hydroxyethyl radicals (°CH2-CH2OH) originating from the attack of ethanol by hydroxyl radicals or formed directly by CYP2E1 [29]. Although, the exact molecular mechanisms associated with brain lesions caused by ethanol remain uncler. The involvement of ROS in the development of all alcoholic disorders and their impact on mental health has been demonstrated [30,31]. Antioxidants in AO [24] are expected to prevent or delay the onset of ROS [32]. The suppression or reduction of oxidative stress by the use of exogenous antioxidants could be a promising preventive (neuroprotective) or therapeutic intervention for patients with cognitive disorders linked to excessive and early alcohol consumption.
In the light of these data, the main proposal of this work was to investigate in Wistar rats the short-term (end of adolescence PND63 (Day Post Natal)) and long-term effect (adult PND163) of virgin AO supplementation on:
• cognitive impairments induced by Adolescent binge-like Ethanol Intoxication;
• oxidative status in Hipp and the PFC rats.
Material and methods Animals
The animals used in this study are rats from the Wistar strain (N=39). They were born, bred, and housed on a 12 hrs light/12 h dark cycle, 50-60% humidity and at a standard temperature 21°C ± 1°C. They were allowed free access to standard rat chow and water. The standard pellet diet (ALF SAHEL Society, El Jadida, Morocco) was a balanced diet containing protein 20.1%, fat 4.1%, carbohydrates 60.0%, fiber 5.8%, minerals 8%, and vitamins 2.0%. This work has been fully realized in Genetic, Neuroendocrinology and Biotechnology Laboratory located at Ibn Tofail University (Kénitra, Morocco) and all experimental procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals. Cognitive capacities were analyzed both at PND63 and PND163. The different cohorts are presented as follow:

AO: Oral Gavage Procedure
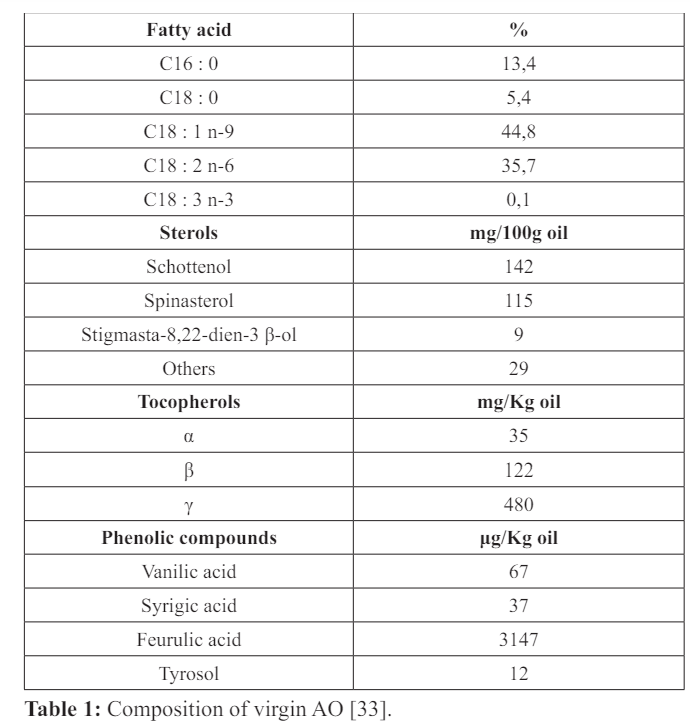
The AO used in this study had a composition similar to that used in human food (Table 1) [33], originating in southwestern Morocco (Agadir-Inzggan) and was extracted from fresh seeds by artisanal methods without any preliminary treatment (cf. Annex). Rats in the two groups (Adolescents & Adults) were supplemented by intragastric gavage (1ml of AO /100g of body weight/day, between 8:00 am and 10:00 am) one day after weaning (PND22) (34;35; 21) for 5 (PND63) or 20 weeks (P163). During the same period, the control animals received the equivalent water. At the same time, for 5 months, body weights were measured weekly.
Ethanol intoxications during early adolescence
The procedure was conducted as previously described [13,36-38] using a validated protocol which consisted of treating adolescent rats with 3g/kg ethanol (98% v/v) (SIGMA) intraperitoneal (i.p) injections (EtOH & Argan+EtOH groups) for 2 consecutive days at 48 hrs intervals during “early adolescence” from PND30 to PND43 (equivalent to 13-16 years in humans) [39]. At the end of the treatment, EtOH rats received a total of 8 ethanol injections. The injected ethanol was diluted in 0.9% saline to a final concentration of 20% (v/v). Concurrently, control rats (Control & Argan groups) received 0.9% saline i.p. injections following the same schedule.
Behavioral assays
Immediately at the end of AO supplementation (after 5 weeks for adolescents group or 20 weeks for Adults group), rats were submitted to selected behaviors tests to evaluation of cognitive impairment. In this current study, all animals were exposed to tree tests in the same sequence (Y-maze, Object recognition and Water maze tests). Just before each test, the animals were placed into the testing equipment under light (600Lux). All behaviors were recorded with a camera for subsequent analysis.
Y-maze spontaneous alternation
The Y-maze spontaneous alternation paradigm is based on the natural tendency of rodents to explore novel environment. When placed in the Y-maze, normal rat prefer to explore the least recently visited arm, and thus tend to alternate visits between the three arms. A rat with impaired working memory cannot remember which arm it just visited and thus show decreased spontaneous alternation [40].
The apparatus was a Y-maze made of wood and having 3 identical arms (50x15x30 cm) placed at 120° from each others. Each arm had walls with specific motifs allowing distinguish it from the others. Each rat was placed at the end of one of the three arms, and allowed to explore freely the apparatus for 5min, with the experimenter out of the animal’s sight. Alternations are operationally defined as successive entries into each of the three arms as on overlapping triplet sets (i.e., ABC, BCA...). The percentage of spontaneous alternations (%SPA) was calculated as index of working memory performance. The chance level is around 22% SPA.
Object recognition task
The object recognition (RO) test allows evaluation of recognition memory and is characterized by a rapid forgetting [41,42]. This test is based on the natural tendency of rodents to explore a novel object by comparison to a familiar one [43]. The object recognition task was performed in automated open fields (100cm x 100cm x 40cm). The open-fields were placed in a room homogeneously illuminated at 70 Lux at the level of each open field. The objects to be discriminated were a plastic balloon (12 cm diameter) and a glass cylinder (15 cm height). Animals were first habituated to the open-field for 10 min. The next day, they were submitted to a 10-minutes acquisition trial during which they were placed in the open-field in presence of an object A (glass cylinder). The time the animal took to explore the object A (when the animal’s snout was directed towards the object at a distance 1 cm) is manually recorded. A 10-minutes retention trial is performed 24h later. During this trial, the object A and another object B (plastic balloon) are placed in the open-field, and the times tA and tB the animal takes to explore the two objects are recorded. A recognition index (RI) is defined as (tB / (tA + tB)) x100. A recognition index of 50% corresponds to random, and higher the index, better is the performance.
Morris water maze
This test evaluates spatial learning and memory. In this situation rat are trained to escape from water by swimming to a hidden platform whose location can only be identified using distal extra-maze cues [44-46]. The water maze consisted of a white circular tank (1.50 m diameter) filled with opaque water. Pool temperature was adjusted to 21 ± 1°C. It is equipped with a retractable platform 20 cm in diameter. The procedure involves a learning phase in which the rats received 5 blocks of training trials over 5 consecutive days. Each rat was placed in the pool at one of 4 (N, O, S, E) randomized start positions, and allows locating the hidden platform. Trials lasted for maximum of 120s and were separated by 15-20 min interval; the latency to reach the platform is measured. Spatial learning performance was assessed during a probe trial 1h after training (5th day), and for which the target platform was removed from the pool. The pool is virtually subdivided into 4 equal quadrants: target quadrant, adjacent right and left quadrants, and the opposite quadrant. Performance is assessed by preference for the target quadrant, as well as by the number of times the rat crosses the theoretical platform location. The longer the rat spends time in the target quadrant and cuts off the theoretical location of the platform, the more it shows a good memory of the spatial location of the platform.
Biochemical analysis
After behavioral assays, animals were sacrificed by cervical dislocation and brains were immediately removed and cooled on dry ice. Then, the Hipp and the PFC was separated from the rest of the encephalon and homogenized in ice-cold 20 mM Tris-HCl buffer (pH 7.4) for determinations of lipid peroxidation level, nitrite content, catalase and superoxyde dismutase activities.
Nitrite content assay
An aliquot of crude homogenate (10%) for each structure was centrifuged at 4°C (800xg for 10min), and supernatant was used to analyze nitrite levels as described elsewhere [47]. Briefly, samples were incubated at room temperature for 20 min with Griess reagent (0.1% N-(1-naphthyl) ethylenediaminedihydrochloride; 1% sulfanilamide in 5% phosphoric acid; 1:1). The absorbance was measured at 550 nm and compared to that of standard solutions of sodium nitrite.
Lipid peroxidation (LPO) assay
Lipid peroxidation was evaluated by measuring the thiobarbituric- acid-reacting substances (TBARS) in homogenates, as previously described [48]. Briefly, an aliquot of crude homogenate of Hipp or PFC was centrifuged at 4°C (1000xg for 10 min), and supernatant was mixed with 1ml 10% trichloroacetic acid and 1ml 0.67% thiobarbituric acid. They were then heated in a boiling water bath for 15 min and butanol (2:1, v/v) was added to the solution. After centrifugation (800xg for 5 min), thiobarbituric-acidreacting substances were determined from the absorbance at 535 nm. The results above were expressed as nmol of malondialdehyde (MDA)/g wet tissue.
Determination of catalase activity
Catalase activity was measured by the method that uses H 2 O2 to generate H2O and O2 [49]. In a 3ml quartz tank, 1.95ml of 50mM phosphate buffer, 1ml of H2O2 (0.019 M) and 0.05 ml of the sample were added [50]. The absorbance was measured at 240nm at time 0 (T0) and after every 30 seconds for 2 min. Results are expressed as mmol/min/mg of protein.
Determination of total Superoxide-dismutase (SODt) activity
Determination of total Superoxide-dismutase (SODt) activity The SODt activity was evaluated by measuring its ability to inhibit the photoreduction of NBT (nitroblue tetrazolium) [51]. In an aerobic medium, the riboflafine / methionine / NBT mixture gave a blue coloration, the optical density of which was measured at 580 nm. One unit of SODt corresponds to the amount of protein necessary to inhibit photoreduction by 50%.
Statistical analyses
All values are expressed as means ± SEM for behavioral tests and biochemical analyses. Statistical comparison between groups was performed using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Fisher’s PLSD post-hoc test was used to compare data between two groups. The accepted level of significance set at p<0.05. All statistics were performed using GraphPad Prism 6 software.
Results
Effects of AO treatment on body weight gain, relative brain, Hipp and PFC weights in rats exposed during early adolescence to a binge-like ethanol intoxication
The body weight of all animals increased normally on all the groups before any type of treatment. In adulthood, 17 weeks following EtOH weaning, a very significant body weight loss was observed in the EtOH group compared to control (p = 0,0003). Moreover, at the end of adolescence, 2 weeks following EtOH weaning, the body weight loss in EtOH treated animals was also detected but the difference was not statistically insignificant compared to control (p=0,1858). However, no significant difference was observed between control and EtOH animals when these were chronically supplemented with AO (Argan+EtOH group), and this throughout the period of the experimentation, before and after bing-like ethanol intoxication (Figure 1).
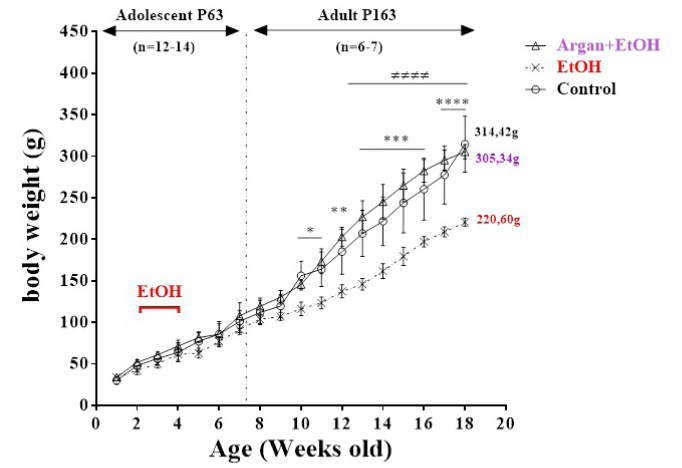
Figure 1: Effects of AO supplementation (1ml/100g/day) over a period of 20 weeks (from the 21th day of life) in the body weight gain in rats submitted during early adolescence to bing-like EtOH (3g/Kg/2day Off - 2day On). The results are expressed as mean ± SEM of the body weight with interval of 7 days (n = 10-12 animals per group). (*) P<0.05 compared to control group; (≠) P<0,05 EtOH compared to Argan+EtOH group (One-way ANOVA with repeated measures followed by Tukey’s test).
The estimation of the relative weight of the brain, Hipp and PFC (Table 2) showed that early EtOH intoxication induced a significant decrease on the brain weight in the EtOH group compared to control two weeks after the last ethanol dose (p=0,001). Early supplementation with AO, abolishes this difference (EtOH vs. Argan+EtOH; p=0,3503). Also, the intoxication with the EtOH reduces the relative weight of the Hipp and the PFC compared to control but this reduction remain non significant on both adolescent (p=0,1002, p=0,0681 respectively) and Adult group (p=0,2011, p=0,3103 respectively).
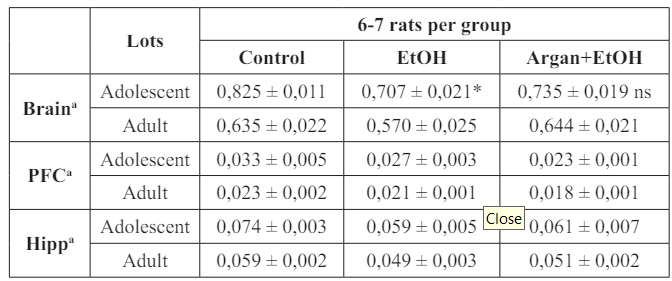
Table 2: Effect of early adolescence binge-like EtOH (3g/Kg/-2day Off, 2day On) and AO supplementation (1ml/100g/day) on relative weights of brain, Hipp and PFC in adults and adolescents rats.
The results are expressed as mean ± SEM of the brain, Hipp and PFC (g) weights based on the body weight of each animal (a). (*) P<0.05 compared to control group (One-way ANOVA with repeated measures followed by Tukey’s test). PFC : Prefrontal Cortex; Hipp : Hipp; (ns) results not significant.
Effect of Bing-like EtOH intoxication and AO pre-treatment on cognitive performance in adolescent and adult rats
Effects on working memory in the Y maze test: Y maze test is commonly used to evaluate the impairment on the working memory on animals. In both adolescent and adult animals, SPA is around 60%, well above random (22.22%). As shown in Figure 1A, the early EtOH intoxication on rats could lead to a significant decrease on working memory. On both adolescent and adult animals, the SPA is significantly decreased in the EtOH group compared to control (p=0,0063 and p<0,0001 respectively). Based on age, our results show that working memory is severely affected in adult rats compared with their adolescent counterparts (EtOH groups: Adolescent vs Adult p=0,0059). On the contrary, AO pre- treatment before EtOH intoxication increases the SPA regardless of the animals’ age. Thus, no significant difference was observed between the EtOH+Argan and control groups (EtOH+Argan vs Control; Adolescent p=0,0564; Adult p=0,0693) (Figure 2A).
Effects on the recognition memory in the object recognition test: Concerning the object recognition test, exploration duration of the familiar object during the acquisition trial was comparable between groups both in adolescent and adult ages (data no shown). When animals were allowed to explore both the familiar and a novel object during a retention trial, 24 hrs after acquisition, we can see that no difference was found between groups in object recognition performance (RI%) in adolescents rats. Nevertheless, in adult rats, a significant decrease in RI% was revealed for the EtOH group compared to control (EtOH vs Contol p=0,0012) (Figure 2B).
Also, intergroup analysis shows that at 24 hrs delay, only Control adolescent and adult groups showed recognition performance significantly above the chance level (p<0,05 one group t-test), while EtOH and Argan+EtOH adolescents tended to perform above the chance level (p=0,1079 and p=0,0504 respectively). In adulthood, EtOH rats perform significantly below the chance level (p=0,0500) exploring more the familial object that novel one. On the other hand, AO pretreated EtOH rats (Argan+EtOH group) show no difference in recognition performance compared to control group at both adolescent and adult age (p=0,0587 and p=0,0655 respectively) (Figure 2B).
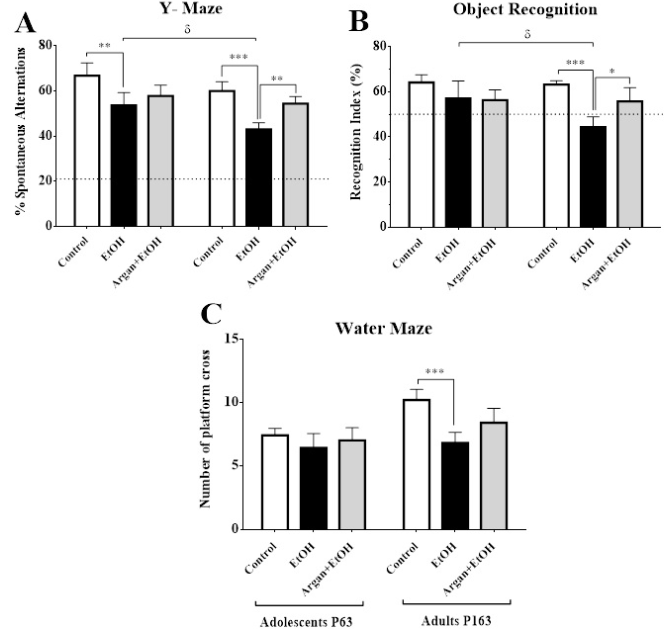
Figure 2: Effect of early adolescence binge-like EtOH (3g/Kg/-2day Off, 2day On) and AO supplementation (1ml/100g/day) on Cognitive abilities in adolescents and adults rats. A. Percentage of spontaneous alternation (SPA) in the Y maze. B. Object recognition index (RI) after 24h of delay. C. Number of platform crosses during the probe trial in Morris water maze. The dashed lines represent the chance levels of SPA 22.22% (A) and of RI 50% (B). (*) Treatment effect ; (δ) age effect (paired t-test).
Effects on learning and spatial memory performance in the Morris Water Maze Test: In Morris Water Maze, when the animals are trained for 5 days to reach the hidden platform using extra-labyrinth wall signs, the latency (Figure 3) to find it decreases significantly in both adolescent and adult groups. Yet, this parameter tended to be higher in EtOH groups, as compared to control counterparts (p<0,05), which suggests a learning delay induced by early bing-like EtOH intoxication.
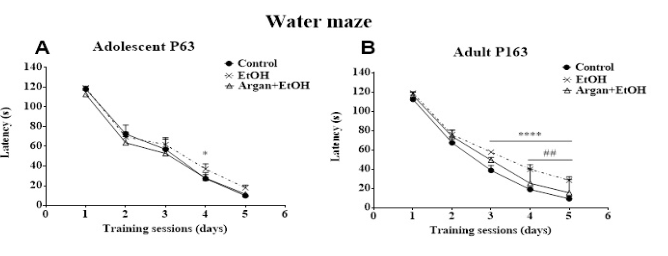
Figure 3: Effect of early adolescence binge-like EtOH (3g/Kg/-2day Off, 2day On) and AO supplementation (1ml/100g/day) on special learning performance. (A) and (B) latency to find the submerge platform during the five days of training in adolescents and adults rats successively. (*) EtOH vs Contrôle ; (#) Argan+EtOH vs EtOH. Test ANOVA (Tukey's multiple comparisons test).
In adolescent EtOH rats, the latency to reach the submerged platform during the 4th session, was significantly increased compared to control (p=0,0626). However, in adult animals, it appears that early ethyl intoxication seriously affects learning performance. Thus, the latency to find the hidden platform was significantly increased from the 3rd session until the 5th (Control vs EtOH: 3rd session p=0,0022; 4rd session p=0,012; 5rd session p=0,0002) (Figure 3).
On the contrary, no significative difference in learning performance was observed between control and Argan+EtOH groups, over the course of training. While in adult EtOH rats, AO pretreatment was significantly increases the learning performance compared to the EtOH group during the 4th and 5th sessions (Argan+EtOH vs EtoH: 4th sessions p=0,0456; 5th sessions p=0,0407). These results suggest that prolonged AO supplementation prevents animals against early bing-like EtOH intoxication (Figure 3).
When the platform was removed to test the strength of the learning during the probe test (1h after the 5th session), both adolescent and adult rats showed a significant preference for the target quadrant, with a highly significant difference between the control and treated groups (p<0,0001). Moreover, the percentage of time spent in the target quadrant decreased significantly in EtOH rats compared to control on both adult (p=0,0001) and adolescent (p=0,0034) groups (data no shown). The number of platform crosses (Figure 2C) was also significantly lower in EtOH adult rats compared to control (p=0,0011). No significative difference in this parameter was observed in adolescent rats (EtOH vs Contol p=0,1890). These data clearly suggest that adult rats under early EtOH intoxication showed special learning performance. In the other hand, AO pretreatment was slightly improve this parameter in adult rats but the results are not statistically significant (EtOH vs Argan+EtOH p=0,0516) (Figure 2C).
All together, results obtained from cognitive tests show that binge-like EtOH intoxication (3g/Kg/ 2 day Off, 2day On) during early adolescence (PND30-43) was seriously affects the cognitive abilities of animals, especially in adult age, after 4 months of the last dose of ethanol. However, early and prolonged AO pretreatment (10g/kg/day, 5 weeks for adolescent and 20 weeks for adult groups), induces a considerable improvement on the performances of animals. In adult rats, it significantly increases sensory, recognition and special memorization as well as learning performance. While in adolescent rats, it appears that AO supplementation for 5 weeks was insufficient to significantly improve those cognitive parameters.
Effect of Bing-like EtOH intoxication and AO pretreatment on oxidative status of the Hipp and PFC in adolescent and adult rats
Effect on NO and TBARS levels: As shown in Figure 4, lipid peroxidation and NO production was noticeably increased in EtOH group when compared to the Control group in both adolescent and adult rats, and these in Hipp and PFC. Thus, in adolescent rats the early bing-like EtOH induces a significant increase in the TBARS (Figure 4A & B) and NO levels (Figure 4C & D), in both Hipp (p=0,0108 and p<0,0001 respectively) and PFC (p=0,0121 and p<0,0001 respectively). Also, in adult rats, these parameters remain significantly increased in the EtOH group compared to control in the two structures studied (Hipp. TBARS p=0,0034; NO p=0,0071; PFC. NO p=0,0021) (Figure 4B, C & D). During that phase, TBARS production in PFC was not significantly altered in EtOH group in comparison with control group (p=0,2578) (Figure 4A).
According to age, our results show that only the NO level was significantly decreased among the adult EtOH group compared to adolescent EtOH group, in Hipp (p=0,0010) and PFC (p=0,0019) (Figure 4C &D). Though, no difference in TBARS concentration among the EtOH adolescents and their counterparts adults groups was detected in Hipp (p=0,0600) and PFC (p=0,3589) (Figure 4A & B) for both periods of evaluation. On the other hand, AO pretreated EtOH rats showed decrease in lipid peroxidation level and NO content when compared with the EtOH group, especially for adult group (after 20 weeks of supplementation) (EtOH vs Argan+EtOH: in Hipp. TBARS p=0,0301; NO p=0,0104; in PFC. TBARS p=0,7671; NO p=0,0037) (Figure 4). Nevertheless, in adolescent EtOH rats supplemented with AO for only 5 weeks (Argan+EtOH group), no difference was observed in TBARS level and NO content when compared to the EtOH group (in PFC. TBARS p=0,4269; NO p=0,0867; in Hipp. TBARS p=0,0530; NO p=0,0508) (Figure 4).
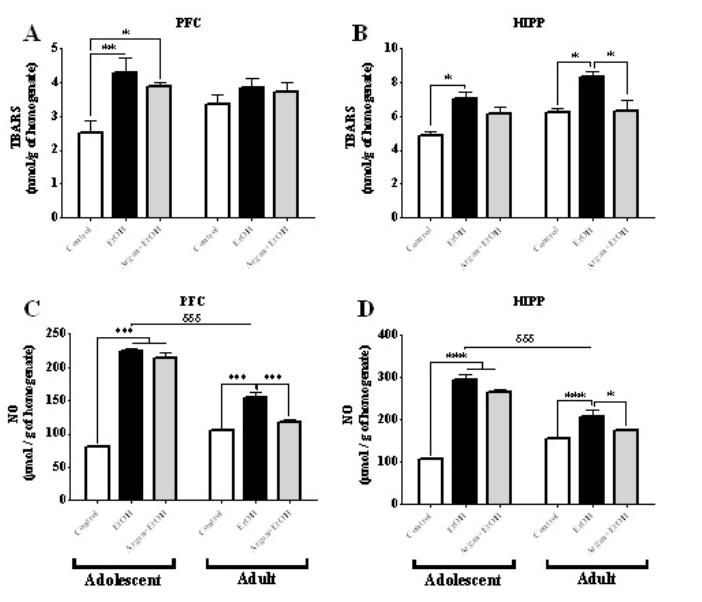
Figure 4: Effect of early adolescence binge-like EtOH (3g/Kg/-2day Off, 2day On) and AO supplementation (1ml/100g/day) on the pro-oxidant parameters in the Hipp and PFC. The results were expressed as means ± SEM of the TBARS level (μmol) per gram of homogenate after 3 weeks (Adolescent) and 17 weeks (Adult) of the last dose of ethanol on PFC (A) and Hipp (B), and NO concentration (μmol) per gram of homogenate after 3 weeks (Adolescent) and 17 weeks (Adult) of the last dose of ethanol on PFC (C) and Hipp (D). (*) Treatment effect ; (δ) age effect (paired t-test).
Effect on catalase (CAT) and total superoxide dismutase (SODt) enzymatic activities
In both of the Hipp and the PFC, CAT and SODt enzymatic activities was significantly decreased in EtOH group compared to control group, and these after 3 weeks of the last dose of ethanol (Adolescent group) [PFC. (CAT. p=0,0123; SODt. p=0,0011); Hipp. (CAT. p<0,0001; SODt. p=0,0246); Figure 5A & C].However, after 17 weeks of the last dose of ethanol (Adult group), the significant decrease of these two parameters in the EtOH group compared to Control group, was observed only in the Hipp [CAT. p=0,0084; SODt. p=0,0002; (Figure 5B & D)]. Yet, in PFC no difference was found between EtOH and Control groups for both of CAT and SODt enzymatic activities [(p=0,3265 and p=0,0620 respectively; (Figure 5B & D)].
Concerning the AO pretreatment, we can see that all parameters were increased in supplemented EtOH rats. Significantly, in adult age for Hipp structure [EtOH vs. Argan+EtOH : CAT. p=0,0428; SODt. p=0,0407; (Figure 5B & D)]. Yet, in PFC no significative difference was found in CAT and SODt enzymatic activities between EtOH and Argan+EtOH groups (p=0,0604; p=0,182 respectively). In adolescents group, the CAT enzymatic activity was increased significantly only in PFC (p=0,0236). Nevertheless, in the Hipp no significative difference was observed between EtOH and Argan+EtOH group, in CAT (p=0,0938) and SODt (p=0,0624) enzymatic activities (Figure 5B &D), same thing for SODt enzymatic activity in the PFC (p=0,0600) (Figure5C).
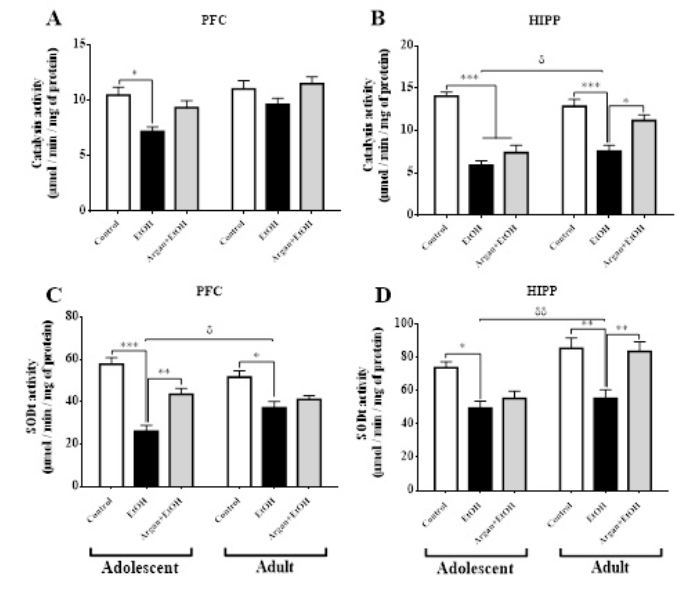
Figure 5: Effect of early adolescence binge-like EtOH (3g/Kg/-2day Off, 2day On) and AO supplementation (1ml/100g/day) on the Antioxidant parameters in the Hipp and PFC. The results were expressed as means ± SEM of the CAT enzymatic activity (μmol) per minute per milligram of protein after 3 weeks (Adolescent) and 17 weeks (Adult) of the last dose of ethanol on PFC (A) and Hipp (B), and SODt enzymatic activity (μmol) per minute per milligram of protein after 3 weeks (Adolescent) and 17 weeks (Adult) of the last dose of ethanol on PFC (C) and Hipp (D). (*)Treatment effect ; (δ) age effect (paired t-test).
In summary, the results of these experiment shows that early binge-like Ethanol intoxication is responsible in rats brain for the oxidative stress resulting from a disruption of the pro-oxidant/ antioxidant ratio. Thus, Bing drinking in early adolescence induces, at short term (late adolescence) and long-term (adult), for a hyperproduction of free radicals (NO and TBARS) and a decrease in anti-oxidant defense (Catalase and SODt). However, prolonged supplementation with AO, reduces the pro-oxidant parameter and increases the anti-oxidant enzyme activity in the Hipp and PFC. These data suggest improved oxidative status of the brain in adult rats previously intoxicated by ethanol and submitted to 20 weeks of AO supplementation.
Discussion
In the context of binge drinking alcoholism in adolescence, the majority of studies have highlighted the long-term consequences on cognitive functions of chronic exposure to massive quantities of ethanol. In this regard, the present study was aimed to evaluate the impact of acute intoxication (8 doses of EtOH/14j - 2days Off - 2days On) during early adolescence and AO pretreatment on cognitive abilities and oxidative status in the Hipp and CPF, after 2 weeks (63PND) or 17 weeks (163PND) of weaning.
Early adolescent bing-like EtOH, affects permanently the cognitive abilities of rats
Historically with Jellinek's classification of alcoholism in 1960, the concept of binge drinking currently used joins that of paroxysmal alcohol consumption or dipsomania [52]. At present, there are multiple definitions, including a massive and rapid consumption of alcohol followed by a period of abstinence [4]. Binge drinking is mostly found among 15-25 year olds [53,54], the period of adolescence during which the brain has not completed its maturation and is still undergoing important mechanisms of neuromaturation including synaptic "pruning" and myelination. Alcohol disrupts these processes of maturation, and the adolescent brain is much more sensitive to the toxic effects of alcohol compared to that of adults [9]. Evidence of this increased sensitivity is of many kinds and has been largely determined in animal models, especially in rodents (rats and mice).
Rodents also present a period of "adolescence" similar in many respects to that observed in humans. For example, adolescent rodents (adolescence covering the second month of life) [39] have a greater propensity to play, to interact socially and to be more impulsive at the physiological and behavioral level [55]. They tend to consume more alcohol and are particularly sensitive to the behavioral effects of ethanol (pleasant, sedative, hypnotic, disinhibiting) [56]. In addition, adolescent rodents also have many similarities in terms of development and brain maturation (decrease of the volume of the cerebral cortex, synaptic pruning…) [57,58]. Studies, have shown that a exposure to binge drinking alcohol inevitably leads to a significant loss of the volume of the cortical and subcortical regions including the Hipp, accompanied by cognitive deficits [59-61]. Surprisingly, abstinence from chronic or acute alcohol consumption is associated with recovery of brain volume and long-term improvement in cognition [62,63].
Structurally, the findings of our study are consistent with previous reports. Thus, the relative weight of the brain was significantly decreased in the adolescent animals (PND63) previously intoxicated with EtOH. Same results were found for the relative weights of the Hipp and PFC, one week after the last dose of EtOH, but, the losses are statistically insignificant. Nevertheless, in adulthood after 17 weeks of abstinence (PND163), a dramatic increase in relative weight in both the brain, the Hipp and PFC, was observed in rats submitted to bing-like EtOH during early adolescence. These structural changes are associated with cognitive deficits in different learning and memorizing tasks, whatever the age of the animals. Thus, the performances of sensory (%SPA), recognition (%IR), spatial memory (% of time spent in the target quadrant) and learning memories (latency to find the hidden platform), were significantly decreased under the EtOH intoxication.
In contrast to studies suggesting the association between improved cognition and recovery of brain volume after a prolonged period of abstinence [64,63], our study showed that the recovery of the relative weight of the Hipp and CPF observed after 17 weeks of abstinence, did not have any beneficial effects on the cognitive performance of the rats. In contrary, the study found an even greater decrease in memorization and learning performance in adult EtOH animals compared to their adolescent counterparts. This may be due to the model of ethyl intoxication used in this study, which consists in administering a high dose of EtOH (ip 3g of pure EtOH/2j×8) during a critical period of brain development which is early adolescence (PND30-PPND44). These results suggest lasting and irreversible changes in the neural circuits involved in memory and learning mechanisms. Indeed, exposure to ethanol during early adolescence may interfere with cellular processes of brain maturation, especially in the Hipp and the cortex. in particular, ethanol induces inhibition of the mediation of synaptic potentials and long-term potentiation (LTP) by N-methyl-D-aspartic acid (NMDA) on rat hippocampal slices, so that more important in adolescents than in adults [65]. These effects of ethanol present behavioral consequences, where it appears, that the deficits of acquisition of the spatial memory are more important and more lasting (presumed irreversible) in both of adolescents and adults rats [9,62,66,67].
The use of animal models exposed to a large amount of alcohol has shown that, in adults, the main ethanol-induced brain damage is localized in limbic structures, notably the Hipp and more particularly the gurus dentate [7]. Ethanol consumption mainly induces cellular degeneration by necrosis [68] and a reduction of neurogenesis [69,70], associated with cognitive deficits. Monti & al., 2005, have shown that binge drinking kills 2 to 3 more the neurons in the adolescent animal compared to an adult animal exposed to the same amount of alcohol [71]. Other studies have shown, in vitro that exposure to ethanol induces impaired ability of NSCs to proliferate, differentiate and survive [72,73]. Inhibition of NSCs proliferation as well as reduced survival of neuronal progenitors and dendritic arborization have also been reported in vivo [74-76]. All of these data indicate that ethanol inhibits neurogenesis and contributing to neurodegeneration by loss of production of new cells [77], explaining - at least in part - the irreversibility of the cognitive deficits associated with the relative weight reductions of the Hipp and PFC found in our study.
On the other hand, one of the key factors of alcohol-related toxicity is the oxidative stress it engenders, up to exceeding the capacity of the antioxidant system to eliminate reactive oxygen species (ROS), which leads to their accumulation in the cells. The ROS are chemical species derived from molecular oxygen, whose outer layer contains an unpaired electron. Since of this electron, these compounds react easily with their environment and particularly alter phospholipids and membrane proteins, proteins of the respiratory chain, and DNA [78]. In the brain, the production of ROS by ethanol comes mainly from its acetaldehyde catabolism by the enzymes ADH (alcohol dehydrogenase) and CYP2E1 (cytochrome P450) which stimulate the production of ROS. The production of ROS is amplified by the fact that ethanol stimulates the production of both ADH and CYP2E1 enzymes. Acetaldehyde facilitates the oxidation of membrane lipids in mitochondria, decreases the amount of antioxidant enzymes [79] and stimulates the production of ROS and NO via induction of NADPH oxidase/ Xanthine oxidase and inducible NO synthase systems [80].
In the case of bing drinking, as the consumption of ethanol is excessive and punctual (over a short period of time), the pro- oxidants/antioxidants balance is so disturbed, and the system is overloaded, so that the amount of antioxidant enzymes decreases, while that of the ROS increases considerably. This results a significant reduction in the ability of neurons to eliminate the excessive production of ROS [81]. These data correlate with our results, thus, evaluation of the oxidative status of the brain shows that ethyl intoxication under the mode of bing drinking (ip 3g EtOH / 2j x 8), induces a hyperproduction of free radicals (increase of NO and TBARS levels) and a decrease of the antioxidant defense (Catalase and SODt activities) in both of the Hipp and the PFC, after 2 weeks of abstinence. Also, this perturbation of the prooxidant / antioxidant ratio is revealed, in adulthood after 17 weeks of chronic abstinence, explaining - at least in part - the irreversibility and persistence of cognitive deficits observed in adult animals after 4 months of the last dose of EtOH.
AO pretreatment improves the cognitive abilities impaired by early adolescent bing-like EtOH
Diet is one of the environmental factors susceptible to develop or reduce susceptibility to mental diseases [82]. Argan oil is well known for its beneficial effect as hypolipemiant, hypotensive and antioxidant activities [83]. The protective effect of argan oil could be attributed to its interesting chemical composition. It is essentially characterized by the presence of unsaturated fatty acids and antioxidant compounds such as Vitamin E family (tocopherol) [24,25]. The results of the present study, show that prolonged supplementation with AO (5 or 20 weeks), induces an improvement in cognitive performance in different tasks of memorization and learning in rats previously intoxicated by EtOH. It appears that the %SPA in the Y maze test and the %IR during the retention phase in the object recognition test, were significantly increased in AO supplemented EtOH rats compared to EtOH rats not supplemented. In addition, they spend more time in the target quadrant during the Morris probe Test. Some animal performances were greatly changed by age. In adult rats, it significantly increases sensory and recognition memorization as well as learning performance. While in adolescent rats, it appears that the 5 weeks of supplementation with AO (10 days before, 14 during and 10 days after EtOH intoxication), was insufficient to significantly improve these cognitive parameters.
Thus, AO supplementation was induced long-term changes in the cognitive performance of rats, although to varying degrees in adolescents and adults ages, with predominant variations in adults, depending on the behavioral test used. The composition of argan can facilitate healthy brain by providing antioxidants, polyinsatured fatty acids and vitamins that would influence cellular functions involved in the synaptic transmission [83], neurite growth [84], membrane fluidity [85] and neurodegeneration [86]. Although ethanol has been described for many years as being able to alter the fluidity of membranes in the brain [87] and affects the phenomenon of neurogenesis [69], the beneficial effects of AO revealed in our study may have been more relevant here since argan supplementation begun in an early age (just after weaning 21PND) during which neural networks continue to settle. These results suggest that the therapeutic efficacy of AO could thus strongly depend on the duration, dose and treatment conditions.
The beneficial effects of argan oil in regulating the proxidant / antioxidant balance, revealed in this study, could explain, at least in part, by its rich chemical composition among other tocopherol, schottenol, spinasterol and ferulic acid. These products play a crucial role as antioxidants against oxidative damage [88]. Indeed, the mechanisms of action of antioxidants are diverse, including the capture of singlet oxygen, the deactivation of radicals by covalent addition reaction, the reduction of radicals or peroxides, the chelating of transition metals [89]. Some testimonies have reported that tocopherols and polyphenols are the most important antioxidants in argan oil thanks to their presence in large quantities and their antioxidant activity [90]. It has been shown that dietary argan oils exhibit more antioxidant activity compared to many other dietary vegetable oils [91,92].
α-Tocopherol is considered a powerful antioxidant against the oxidative stress that is at the root of nervous system disorders [93]. It acts not only as an effective antioxidant, lipophilic and scavenger of free radicals [93,92], but also stabilizes cell membranes [94]. Additionally, α -tocopherol plays a key role in maintaining neurological function [95] and oral supplementation of α-tocopherol affects cerebrospinal fluid and brain [96]. In addition, treatment with α-tocopherol alone or in combination is able to delay the progression of AD [97]. Indeed, our data establish that AO, rich in tocopherols, is able to counteract many side effects in PFC and Hipp induced by binge drinking intoxication in early adolescence: overproduction of ROS (NO and TBARS), reduction of enzymatic activity of endogenous antioxidants (CAT and SODt) and decrease of the relative weight of these structures. Thus, our results that appear inconsistent with the effects of the commonly described AO effects in literature may be due, on the one hand to the length of the period of supplementation (20 weeks) and on the other hand to the few studies that have specifically investigated the benefit of a dietary intake of AO on biochemical parameters.
Conclusion
In conclusion, the present study disassembled for the first time that the components of AO could have neuroprotective effects against cognitive disorders induced by adolescent binge-like alcohol intoxication. We have demonstrated that AO pretreatment is effective in increasing the cognitive abilities of rats in the short and long term by increasing sensory, recognition, spatial memory and learning performance. Also AO shows an ability to protect the brain against oxidative stress in the Hipp and PFC. Relative weight recoveries of these structures were also observed in EtOH rats receiving dietary 10 ml/Kg/day of AO, starting from weaning, for 5 or 20 weeks (Figure 6).
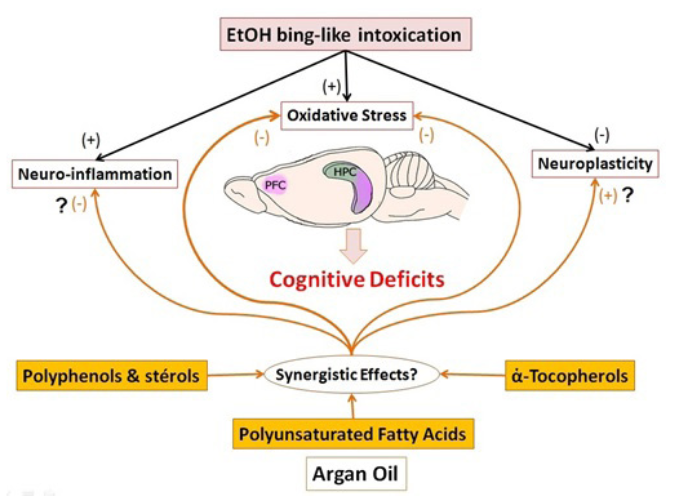
Figure 6: Schematic illustration of physiopathological hypothesis of the link between the consumption of ethanol and the cognitive deficits. Our results suggest that AO pretreatment improve the cognitive performance by synergistic effects of this compound, through increased hypocampal neurogenesis, reduction of oxidative stress, and modulation of inflammatory processes in the brain.
The neuroprotective effect of AO in cognitive impairments induced by adolescent binge-like ethanol may be attributed, at least in part, to its antioxidant activity and the likely modulation of hippocampal neurogenesis pathways in the brain. Knowing the interesting chemical composition of AO and the complexity of the pathophysiology of memory deficits, which involves nero- inflammatory pathways, neuroplasticity and oxidative stress among others; we can consider the possible synergistic effects of these compounds that would be more beneficial than the use of each (Figure 6). The current study suggests that the neuroprotective effect of AO against cognitive impairments induced by early binge-like ethanol in adult and adolescent rats, may be the result of modulation of enzymatic antioxidant activities and hippocampal neurogenesis pathways of the brain. This suggests that a AO- based diet may help prevent and / or treat, in the long term, the progression of cognitive disorders induced by excessive alcohol consumption in early adolescence.
References
1. White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol. 2005; 17: 161-76.
2. Crews FT, Braun CJ, Hoplight B, et al. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000; 24: 1712-1723.
3. Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006; 160: 739-746.
4. https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/NewsletterNumber3.pdf.
5. Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005; 29: 317-325.
6. Crego A, Rodriguez-Holguin S, Parada M, et al. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend. 2010; 109: 45-56.
7. Jeanblanc J, Sauton P, Jeanblanc V, et al. Face validity of a preclinical model of operant binge drinking: Just a question of speed. Addiction Biology. AB-12- 2017-0292.R2.
8. Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998; 22: 670-676.
9. Markwiese BJ, Acheson SK, Levin ED, et al. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998; 22: 416-421.
10. Roberto M, Nelson TE, Ur CL, et al. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002; 87: 2385-2397.
11. Roberto M, Nelson TE, Ur CL, et al. The transient depression of hippocampal CA1 LTP induced by chronic intermittent ethanol exposure is associated with an inhibition of the MAP kinase pathway. Eur J Neurosci. 2003; 17: 1646-1654.
12. Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005; 1048: 69-79.
13. Pascual M, Blanco AM, Cauli O, et al. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007; 25 : 541e550.
14. Alaux-Cantin S, Warnault V, Legastelois R, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013; 67: 521-331.
15. Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neurosci.2013; 254: 324-334.
16. Fleming RL, Li Q, Risher ML, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013; 37: 1154-1460.
17. Renoir T, Paizanis E, El Yacoubi M, et al. Differential long- term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild- type mice. Int J Neuropsychopharmacol. 2008; 11: 1149-1162.
18. Schulteis G, Archer C, Tapert SF, et al. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol 2008; 42: 459-467.
19. Rolland B, Naassila M. Binge drinking : current diagnostic and therapeutic issues.CNS Drugs. 2017; 31: 181-186.
20. Luykx HJ, Dorresteijn LD, Haffmans PM, et al. Rivastigmine in Wernicke-Korsakoff's syndrome: five patients with rivastigmine showed no more improvement than five patients without rivastigmine. Alcohol. 2008; 43: 70-72.
21. Bousalham R, Jahidi-Rhazali L, Harmouch A, et al. Does Argan Oil Supplementation Affect Metabolic Parameters and Behavior in Wistar Rats? Food and Nutrition Sciences. 2015; 6: 816-824.
22. Bahbiti Y, Ammouri H, Berkiks I, et al. Anticonvulsant effect of argan oil on pilocarpine model induced status epilepticus in wistar rats, Nutritional Neuroscience, 2016.
23. Charrouf Z, Guillaume D, Driouich A. The argan tree, an asset for Morocco. Biofutur. 2002; 220: 54-57.
24. Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids : from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009; 77: 937-946.
25. Drissi A, Girona J, Cherki M, et al. Evidence of Hypolipemiant and Antioxidant Properties of Argan Oil Derived from the Argan Tree (Argania spinosa). Clinical Nutrition. 2004; 23: 1159-1166.
26. Charrouf Z, Guillaume D. Phenols and polyphenols from Argania spinosa. Am J Food Technol. 2007; 2: 679-683.
27. Boukhobza M, Pichon-Prun N. L’arganier ressource économique et medicinale pour le Maroc. Phytothérapie. 1988; 27: 21-26.
28. Selvaraju TR, Khaza'ai H, Vidyadaran S, et al. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn J Basic Med Sci. 2014; 16: 195-204.
29. Zhu H, Jia Z, Misra H, et al. Oxidative stress and redoxsignaling mechanisms of alcoholic liver disease: updated expe-rimental and clinical evidence. J Dig Dis. 2012; 13: 133-142.
30. Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J Gastroenterol. 2014; 20: 17756-17772.
31. Oliveira GB, Fontes ED Jr, De Carvalho S, et al. Mynocycline mitigates motor impairments and cortical neuronal loss induced by focal ischemia in rats chronically exposed to ethanol during adolescence. Brain Res. 2014; 15: 23-34.
32. Pauly G, Henry F, Danoux L, et al. Cosmetic and/or dermopharmaceutical preparations containing leaf extracts of the plant Argania spinosa. US patent. 2006; 7871766 B2.
33. Khallouki F, Younos C, Soulimani R, et al. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur J Cancer Prev. 2003; 12: 67-75.
34. Berrougui H, De Sotomayor MA, Perez-Guerrero C, et al. Argan (Argania spinosa) Oil Lowers Blood Pressure and Improves Endothelial Dysfunction in Spontaneously Hypertensive Rats. British Journal of Nutrition. 2004; 92: 921- 929.
35. Mekhfi H, Belmekki F, Ziyyat A, et al. Antithrombotic Activity of Argan Oil : An in Vivo Experimental Study. Nutrition . 2012; 28: 937-941.
36. Pascual M, Boix J, Felipo V, et al. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009; 108: 920-931.
37. Pascual M, Do Couto BR, Alfonso-Loeches S, et al. Changes in histone acetylation in the prefrontal cortex of ethanol- exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012; 62: 2309- 2319.
38. Rodriguez-Arias M, Maldonado C, Vidal-Infer A et al. Intermittent ethanol exposure increases long-lasting behavioral and neurochemical effects of MDMA in adolescent mice. Psychopharmacology (Berl). 2011; 218: 429-442.
39. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000; 24: 417-463.
40. Nagahara AH, Brioni JD, McGaugh JL. Effects of intraseptal infusion of muscimol on inhibitory avoidance and spatial learning: differential effects of pretraining and posttraining administration. Psychobiology. 1992; 20: 198-204.
41. Dodart JC, Mathis C, Ungerer A. Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport. 1997; 8: 1173-1178.
42. Meziane H, Dodart JC, Mathis C, et al. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A. 1998; 95: 12683-12688.
43. Ennaceur A, Delacour J. A New One-Trial Test for Neurobiological Studies of Memory in Rats.1: Behavioral Data. Behavioural Brain Research. 1988; 37: 47-59.
44. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47-60.
45. Hodges H. Maze Procedures : The Radial-Arm and Water Maze Compared. Cognitive Brain Research. 1996; 3:167-181.
46. Owen AM, Evans AC, Petrides M. Evidence for a twostage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cerebral Cortex. 1996; 6: 31-38.
47. Green LC, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981; 212: 56-58.
48. Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products, Am. J. Clin. Nutr. 1993; 57: 779-785.
49. Chance B, Maehly A. Assay of catalases and peroxidases. Methods Enzymol. 1955; 2: 764-75.
50. Aebi H. Catalase in vitro, Methods Enzymol. 1984; 105: 121- 126.
51. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem. 1971; 44: 276-287.
52. Jellinek EM. The disease concept of alcoholism. New Haven, CT, US: Hillhouse Press. 1960.
53. Naassila M. Bases neurobiologiques de l'addiction à l'alcool. Presse Med. 2018. https://doi.org/10.1016/j.lpm.2017.12.001
54. Escapad. Estimation 2011 des consommations de produits psychoactifs à 17 ans, Note de synthèse, décembre 2011 - OFDT - Saint-Denis.
55. Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011; 1: 390- 403.
56. Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev. Psychobiol. 2010; 52: 236-243.
57. Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010; 44:15-26.
58. Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Critical Reviews in Clinical Laboratory Sciences . 2011; 48: 19-47.
59. Oscar-Berman M, MarinkoviÄ? K. Alcoholism and the Brain: An Overview. Alcohol Research & Health. 2003; 27: 125-133.
60. Oscar-Berman M, MarinkoviÄ? K. Alcohol: Effects on Neurobehavioral Functions and the Brain. Neuropsychol Rev. 2007; 17: 239-257
61. Beresford TP, Arciniegas B. Alfers J. Hippocampus Volume Loss Due to Chronic Heavy Drinking. Alcoholism : Clinical & Experimental Research. 2006; 30: 1866-1870.
62. Pfefferbaum A, Sullivan EV, Rosenbloom MJ, et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998; 55: 905-910.
63. Sullivan EV. Neuropsychological vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha, A.; Eckardt, M.J.; and Warren, K., eds. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism (NIAAA) Research Monograph No. 34. Bethesda, MD: NIAAA. 2000; 34: 473-508.
64. Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism : Clinical and Experimental Research. 1997; 21: 521-529.
65. Pyapali GK, Dennis T, Wilkie W, et al. Age and Dose- Dependent Effects of Ethanol on the Induction of Hippocampal Long-Term Potentiation. Alcohol, Oct 1999; 2: 107-111.
66. Harper C, Kril JJ, Daly J. Are we drinking our neurones away? Br Med J. 1987; 294: 534-536.
67. Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience 1997; 79: 983-998.
68. Obernier JA, Bouldin TW, Crews FT. Binge Ethanol Exposure in Adult Rats Causes Necrotic Cell Death . Alcohol Clin Exp Res. 2002; 26: 547-557.
69. Ehlersa CL, Liub W, Willsa DN, et al. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neurosci. 2013; 244: 1-15.
70. Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus journal of Neurochemistry. 2002; 83: 1087-1093.
71. Monti PM, Miranda RJr, Nixon K, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005; 29: 207- 220.
72. Hao HN, Zhao J, Thomas RL, et al. Fetal hematopoietic stem cells can differentiate sequentially into neural stem cells and then astrocytes in vitro. J. Hematother. Stem Cells Res. 2003; 12: 23-32.
73. Tateno M, Ukai W, Yamamoto M, et al. The Effect of Ethanol on Cell Fate Determination of Neural Stem Cells. ALCOHOLISM: CLINICAL AND EXPERIMENTAL RESEARCH. 2005; 29: 225S-229S.
74. Morris SA, Eaves DW, Smith AR, et al. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. J. Hippocampus. 2010; 20: 596-607.
75. He J, Nixon K, Shetty AK, et al. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. EJN. 2005; 21: 2711-2720.
76. Herrera DG, Yague AG, Johnsen-Soriano S, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc. Natl. Acad. Sci. 2003; 100: 7919-7924.
77. Nixon K. Alcohol and adult neurogenesis : Roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus 2006; Special Issue: Special Issue on Neurogenesis. 2006; 16: 287-295.
78. Deitrich R, Zimatkin S, Pronko S. Oxidation of Ethanol in the brain and its consequences. Alcohol Res Health. 2006; 29: 266-273.
79. Rintala J, Jaatinen P, Parkkila S, et al. Evidence of acetaldehyde–protein adduct formation in rat brain after lifelong consumption of ethanol. Alcohol Alcohol 2000; 35: 458-463.
80. Haorah J, Ramirez SH, Floreani N, et al. Mechanism of alcohol-inducedoxidative stress and neuronal injury. Free Radic Biol Med. 2008; 45: 1542-1550.
81. Allen RG, Tressini M. Oxidative stress and gene regulation. Free Radical Biol Med 2000; 28: 463-499.
82. Link A, Balaguer F, Goel A. Cancer Chemoprevention by Dietary Polyphenols: Promising Role for Epigenetics. Biochemical Pharmacology. 2010; 80: 1771-1792.
83. Anderson GH. Diet, Neurotransmitters and Brain Function. British Medical Bulletin. 1981; 37: 95-100.
84. Futerman AH, Banker GA. The Economics of Neurite Outgrowth—The Addition of New Membrane to Growing Axons. Trends in Neurosciences. 1996; 19:144-149.
85. Nealon JR, Blanksby SJ, Mitchell TW, et al. Systematic Differences in Membrane Acyl Composition Associated with Varying Body Mass in Mammals Occur in All Phospholipid Classes: An Analysis of Kidney and Brain. The Journal of Experimental Biology, 2008; 211: 3195-3204.
86. Ledesma MD, Martin MG, Dotti CG. Lipid Changes in the Aged Brain: Effect on Synaptic Function and Neuronal Survival. Progress in Lipid Research. 2012; 51: 23-35.
87. Chin JH, Goldstein DB. Effects of Low Concentrations of Ethanol on the Fluidity of Spin-Labeled Erythrocyte and Brain Membranes. Molecular Pharmacology. 1977; 13: 435-441.
88. Badreddine A, Karym E M, Zarrouk A, et al. An expeditious synthesis of spinasterol and schottenol, two phytosterols present in argan oil and in cactus pear seed oil, and evaluation of their biological activities on cells of the central nervous system. Steroids. 2015; 99 : 119-124.
89. Diallo A. Etude de la phytochimie et des activités biologiques de Syzygium guineense willd. (MYRTACEAE). Thèse de Doctorat. Mali. 2005.
90. López LC, Cabrera-Vique C, Venegas C, et al. Argan oil- contained antioxidants for human mitochondria. Nat Prod Commun. 2013; 8: 47-50.
91. El Kharrassi Y, Samadi M, Lopez T, et al. Biological activities of Schottenol and Spinasterol, two natural phytosterols present in argan oil and in cactus pear seed oil, on murine miroglial BV2 cells. Biochem Biophys Res Commun. 2014; 446: 798-804.
92. Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Molecular and cellular biochemistry. 2010; 345: 91-104.
93. Caligiani A, Bonzanini F, Palla G, et al. Characterization of a potential nutraceutical ingredient : pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum. Plant Foods Hum Nutr. 2010; 65: 277-283.
94. Ulatowski LM, Manor D. Vitamin E and neurodegeneration. See comment in PubMed Commons below Neurobiol Dis. 2015; 84: 78-83.
95. Sen CK, Khanna S, Roy S. Tocotrienol The Natural Vitamin E to Defend the Nervous System ? Ann N Y Acad Sci. 2004; 1031: 127-142.
96. Emilien G, Beyreuther K, Masters CL, et al. Prospects for Pharmacological Intervention in Alzheimer Disease. Arch Neurol. 2000; 57: 454-459.