Does Endometrial Injury Increase the Chance of Pregnancy during IVF among Patients with Recurrent Implantation Failures?
Author'(s): Kovacs Peter1*, Glenn Tanya2,3, O'Leary Kathleen2,3, Lindheim R Steven2
1Kaali Institute, IVF Center, Istenhegyi uy 54/a, 1125 Budapest, Hungary.
2Department of Obstetrics and Gynecology, Wright State University, Boonshoft School of Medicine, Dayton, OH, USA.
3Wright Patterson Medical Center, Dayton, OH, USA.
*Correspondence:
Peter Kovacs, Medical Director, Kaali Institute, IVF Center, Budapest, Hungary, Tel: +36-1-202-2802, E-mail: peterkovacs1970@hotmail.com.
Received: 27 December 2017 Accepted: 15 January 2018
Citation: Kovacs Peter, Glenn Tanya, O’Leary Kathleen, et al. Does Endometrial Injury Increase the Chance of Pregnancy during IVF among Patients with Recurrent Implantation Failures?. Gynecol Reprod Health. 2018; 2(1): 1-6.
Abstract
Objective: The objective of this research was to assess the impact of endometrial injury on pregnancy rates during IVF among patients with recurrent implantation failure (RIF) with an Endobiops sampler or hysteroscopic directed biopsy.
Methods: A retrospective matched cohort analysis was performed. IVF outcome was compared among 44 patients with ≥2 failed IVF cycles undergoing luteal phase endometrial injury in the cycle preceding the current IVF treatment (Group A) to 44 similar patients without intervention (Group B). Stimulation parameters as well as IVF outcome (pregnancy and implantation rates [PR; IR]) were compared using Student’s t-test and chi square tests.
Findings: The two groups were comparable in demographics. Overall ongoing PR (41% vs. 23%, p=0.06) and IR (27% vs. 15%, p=0.08) were higher in Group A, but the difference in ongoing PR was only significant in GnRH antagonist cycles (43% vs. 19%, p=0.04). No differences in the studied parameters were noted between Endobiops sampling versus hysteroscopic endometrial injury.
Conclusions: Endometrial injury improves pregnancy outcomes in subsequent IVF treatment among patients with RIF compared to patients who undergo repeat IVF treatment without. The implications of improved outcomes in GnRH-ant cycles and comparison of Endobiops biopsy to hysteroscopic injury remains to be clarified.
Keywords
Introduction
The process of implantation in the uterus results from a complex set of events as a result of highly orchestrated “cross talk” between a euploid blastocyst and a receptive endometrium. These occur through a series of coordinated genetic and hormonal events regulating intracellular signaling in both the host uterus and implanting blastocyst [1].
Despite the technical advances that have continued to evolve in the area of assisted reproduction, overall pregnancy and implantation rates have remained relatively low [2]. It is clear that the success of in vitro fertilization and embryo transfer (IVF-ET) cycles not only depends upon embryo quality but also on uterine receptivity as the transfer of euploid embryos following preimplantation genetic screening (PGS) still does not guarantee implantation [3]. The latter is likely to be the result of transferring embryos into a non- receptive uterus [4].
Implantation failure refers to the failure of an embryo(s) to produce detectable amounts of human chorionic gonadotropin (hCG) and/or to reach a stage when an intrauterine gestational sac can be recognized by ultrasonography [5]. Recurrent implantation failure (RIF) refers to the phenomena when the transfer of normal appearing embryos repeatedly fails to lead to the stage of recognizable intrauterine sac. There is no universally accepted definition and RIF has been defined as either two or three failed fresh IVF transfers or no implantation after the transfer of the total of 10 or more cleavage stage embryos or 4 or more blastocysts [5]. This is in contrast to recurrent IVF failure which has been referred to as the failure to achieve a pregnancy after several IVF attempts attributed to suboptimal embryo quality, advanced maternal age, and uterine factors [6].
The management of infertile couples with failed implantation during IVF is challenging especially in those who are young, good responders, and those who generate good quality embryos. Numerous controversial interventions have been tried including assisted hatching, blastocyst transfer, preimplantation genetic screening (PGS), acupuncture, medical therapies including aspirin, low-weight molecular heparin, intravenous gamma globulin (IVIG), donor gametes and gestational surrogacy [5,7-14].
The possibility of suboptimal endometrial receptivity has also been suggested as an etiology. Original work in the guinea-pig uterus revealed the finding that injury caused by scratching in the secretory phase provoked a rapid growth of endometrial cells similar to decidual cells of pregnancy [15]. In the human, Barash et al. were the first to show that endometrial biopsies performed in various stages of the cycle preceding the embryo transfer results in improved clinical outcome [16]. This was followed by several non-randomized and randomized clinical trials that revealed that mechanical manipulation of the endometrium with an endometrial biopsy or at the time of hysteroscopy preceding an IVF cycle favorably affected implantation (IR), clinical pregnancy (CPR) and live birth (LBR) rates [17-29]. On the other hand, there are reports that question the beneficial effect [30-32].
The primary aim of our analysis was to study the overall effect of endometrial injury on pregnancy outcomes during IVF among patients with history of previous failed IVF treatments, and secondarily to assess the impact of the Endobiops sampler vs. hysteroscopic injury. Finally, we compared the impact of luteal phase endometrial injury on IVF outcome separately in GnRH- agonist (GnRH-a) and GnRH-antagonist (GnRH-ant) cycles.
Materials & Methods
This was a retrospective matched cohort study. Due to the retrospective data collection IRB approval was not needed as per local regulations.
Data on all eligible fresh IVF cycles from August 2012 to August 2013 were considered for the analysis. Selected cases were IVF cycles in women who were 42 years old or younger with ≥2 IVF failures (negative pregnancy test) despite the transfer of morphologically good embryos, who then underwent luteal phase Endobiops biopsy or office hysteroscopy involving endometrial injury in the cycle preceding the current IVF treatment (Group A). Controls were selected from the same study period to match Group A for the following parameters: ≥2 IVF failures, age ±2 years, and at least 5 oocytes retrieved (Group B). Cycles in which donated oocytes were used, those undergoing preimplantation genetic diagnosis, uterine myoma(s) >3 cm, severe male factors (<1 million/cc or surgically obtained sperm), and those where embryos were electively cryopreserved were excluded from the analysis.
In those undergoing endometrial biopsy (n=31), an Endobiops endometrial biopsy sampler (Prince Medical, France) was introduced through the cervix up to the uterine fundus without prior cervical dilatation. The piston was withdrawn back to the end of the sheath to create a negative pressure and rotated and moved back and forth between the fundus and the internal os to ensure endometrial tissue had been obtained to create the endometrial ‘scratching’ effect. The biopsy was performed once between day 14-24 of the cycle preceding the IVF treatment or during contraceptive pill use if administered to those using the GnRH-ant protocol. In those where the assessment of the uterine cavity was required (n=13), office hysteroscopy was used. In these cases a 2.8 mm 30 degree diagnostic hysteroscope (Karl Storz GmbH & Co) was introduced into the uterine cavity during the late follicular, early luteal phase of the menstrual cycle. After proper visualization of the endometrial cavity and confirmation of no intracavitary pathology the instrument was gently pushed into the endometrium to disrupt it to induce an endometrial injury effect. Those patients who were found to have pathology during diagnostic hysteroscopy were scheduled to undergo operative hysteroscopy and were excluded from this analysis.
Ovarian stimulation using daily injections of 150-450 IU/day of recombinant follicle stimulating hormone (FSH) (Gonal-F, Merck Serono) or urinary human menopausal gonadotropin (hMG) (Menopur, Ferring) following either mid-luteal phase long GnRH-a (Suprefact, Sanofi Aventis) or GnRH-ant (Cetrorelix 0.25 mg subcutaneously daily; Merck Serono) flexible protocols were used. Ovarian stimulation protocol and starting gonadotropin dose were individually determined based on age, ovarian reserve markers, body mass index (BMI) and response to previous treatment when available. All cycles were monitored starting on stimulation day 6, and dosing was adjusted as needed based on serum hormone levels and transvaginal sonography. When the lead follicle reached ≥ 17 mm diameter in size, recombinant hCG 250 mcg (Ovitrelle, Merck Serono) was administered to induce final follicle maturation. Thirty-five to thirty-six hours later, the transvaginal oocyte retrieval was scheduled.
Retrieved eggs were fertilized by conventional IVF or intracytoplasmic sperm injection (ICSI) depending on the sperm parameters and reproductive history. Fertilization was checked 16- 18 hours later. Embryos were cultured in groups up to cleavage or blastocyst stage. One to three embryos were transferred transcervically three to five days post-retrieval, based on cleavage rate and morphology. An embryo with at least 6 cells and less than 20% fragmentation on day 3 was considered good quality. Cycles with >3 good quality day 3 embryos were considered for blastocyst culture. On day 5, embryos that reached the blastocyst stage and had a tight regular inner cell mass and outer cell layer were considered good quality.
Embryo transfers were performed using soft plastic catheters (Wallace, Smith Medical International Ltd., UK) and the afterload technique under ultrasound guidance. Surplus embryos in both groups were cryopreserved using vitrification. Pregnancy was confirmed by serum β-hCG 12 to 14 days following embryo transfer and ongoing pregnancy rates (PR) was defined as presence of gestational sac after 8 weeks of gestation, respectively.
Data collected were patient’s age, ovarian reserve markers (baseline FSH, estradiol [E2]) and cycle stimulation characteristics. These included GnRH-a or GnRH-ant use, total gonadotropin (Gn) dose, length of stimulation, semen parameters, endometrial thickness, number of oocytes retrieved, number of fertilized oocytes, number of available embryos, number of good quality embryos, day of transfer, number of embryos transferred, cryopreservation, and pregnancy outcome.
Statistical Analyses
Statistical analysis was performed using the SPSS statistical package 18.0 (SPSS Inc., Chicago, IL). Student’s t-test and chi square were performed to analyze the data. A p-value of <0.05 was considered significant.
Results
In total, data based on 88 cycles (44 in both groups) was analyzed. Baseline demographic parameters, cycle stimulation characteristics, and the number of transferred embryos were comparable between groups (Tables 1 and 2). Overall PR (48% vs 34%, p=0.19), ongoing PR (41% vs. 23%, p=0.06) and IR (27% ± 34% vs. 15% ± 26%, p=0.08) were higher in RIF with endometrial scratch than RIF without an endometrial scratch (Table 3).
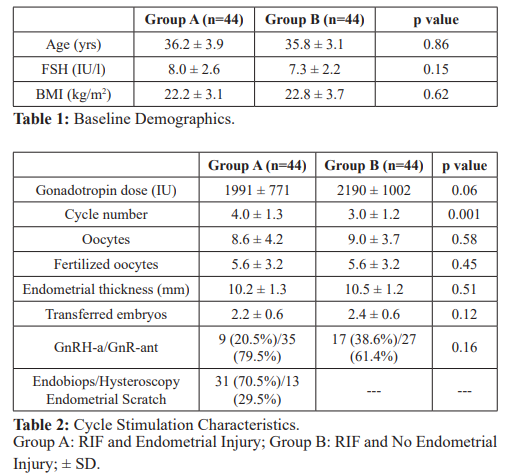
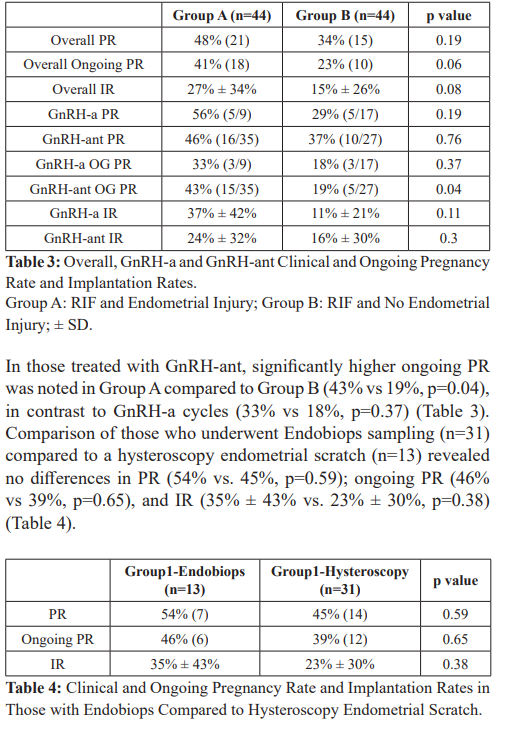
Conclusions
Our data supports existing reports that endometrial injury improves clinical outcome in subsequent IVF treatment among patients with RIF when compared to patients who undergo repeat IVF treatment without an intentional endometrial injury. While significant differences were noted in GnRH-ant cycles neither an Endobiops sampler nor hysteroscopic directed injury conferred benefit over the other
Barash and colleagues showed in 2003 that repeated endometrial biopsies in the cycle preceding the transfer cycle increased implantation, pregnancy and live birth rates among those with 1 or more previous failed IVF cycles [16]. Since then, multiple groups have reported on the impact of single or multiple endometrial biopsies or endometrial injury induced during hysteroscopy on IVF outcome performed in the cycle prior to the actual transfer cycle with conflicting results [17-32] including an RCT using endometrial injury in unselected patients undergoing their first IVF attempt also failed to find an effect on outcome [33].
In an attempt to provide further clarification, a meta-analysis by El-Toukhy showed that CPR was significantly improved after endometrial biopsy in both the randomized (RR: 2.63, 95% CI 1.39-4.96, p<0.01) and non-randomized studies (RR: 1.95, 95% CI 1.61–2.35, p<0.00001) [28]. In another meta-analysis by Potdar et al., endometrial injury prior to an IVF cycle was 70% more likely to result in a clinical pregnancy as opposed to no intervention. Moreover, CPR were twice as likely with biopsy/scratch (RR: 2.32, 95% CI 1.72-3.13) as opposed to hysteroscopy (RR: 1.51, 95% CI 1.30-1.75) [29]. Intentional endometrial injury has also been shown to have a positive impact in non IVF-treated women with unexplained infertility resulting in significantly higher cumulative pregnancy rates [34].
While the exact effect of endometrial injury is unclear, several potential mechanisms may explain the positive effects. Zhou et al., have shown that local injury to the endometrium resulted in differential expression of over 200 genes in the endometrium [21]. Gnainsky and colleagues have shown that the endometrial injury initiates a cascade of pro-inflammatory events that increase the chance of implantation. Tumor necrosis factor α (TNF α) is upregulated in response to the endometrial injury. TNF α increases endometrial stromal cell pro-inflammatory cytokine expression that recruits monocytes to the site of injury. These cells differentiate into dendritic cells and stimulate the endometrial stromal and epithelial cells to increase implantation associated gene expression to facilitate blastocyst implantation [35]. Liang et al., studied cytokine profiles in endometrial secretions aspirated immediately before the embryo transfer in IVF-ET cycles that were managed with endometrial injury in the preceding cycle. A significant impact on inflammatory cytokines (IFN-γ, IL-6, Il-8, Il-12, Il- 13, VEGF) was seen. IFN-γ and VEGF levels were significantly higher in cycles that resulted in pregnancy following the biopsy when compared to unsuccessful cycles after luteal biopsy [36].
However, many questions still exist. Do all patients or only those with RIF benefit from the intervention? If only RIF patients how do we define this group of patients as yet there is no universally accepted definition. Other areas in question include the optimal timing for the intervention including the luteal vs. follicular phase. One study by Karimzade et al. demonstrated that a biopsy done in the treatment cycle was associated with lower implantation and pregnancy rates probably due to disrupting the developing endometrium and therefore interfering with its receptivity [37]. Other questions included should one or several biopsies be performed to achieve maximum benefit? In most studies one biopsy was taken in the luteal phase but Barash et al. have done 4 biopsies in total on days 8, 12, 21, 26 [16]. What is the best technical approach (Pipelle biopsy vs hysteroscopic endometrial injury)? What is the ideal site of the biopsy? Some have described site-specific injury, on the posterior wall, midline, 10-15 mm from the fundus as most optimal [38].
We have shown that implantation and pregnancy rates can be improved in patients with RIF with endometrial injury. Moreover, pregnancy and implantation rates among RIF patients undergoing endometrial injury were comparable to that achieved in patients undergoing their 1st IVF cycle with similar baseline characteristics in the same study period (PR: 41% vs. 34%; IR: 27% vs 23%). This indicates that success rates in RIF patients can be restored to that expected among those undergoing their first IVF cycle. We found no difference between the effects of Endobiops biopsy vs. hysteroscopy. This finding is in disagreement with the findings of Potdar et al., who showed a greater improvement with biopsy when compared to hysteroscopy and more likely represents our small sample size [29]. Since the intervention is performed in the cycle preceding the actual stimulation the mechanism of action should be the same regardless of what treatment is followed subsequently. When the stimulation protocol was considered, a significant benefit of endometrial injury in GnRH-ant cycles was noted. The specific benefit in GnRH-ant is unclear and remains to be further clarified.
We acknowledge the limitations in our study including the retrospective nature. Moreover, the observed differences in our study could be due to differences in the patient populations. While patients were matched for age and ovarian reserve and had similar stimulation and laboratory outcomes, the possibility of selection bias could have an impact on the clinical outcome due to the non-randomized nature of the study. Given that women with RIF are desperate to seek treatment for this condition; further work is needed in a well-designed fashion to clarify the impact on endometrial injury in IVF cycles with specific focus on standardizing the timing and technique. The ongoing SCRaTCH study (NTR 5342), a multicenter randomized controlled trial where live birth rate after a 2nd fresh IVF/ICSI cycle in 900 women undergoing an endometrial scratch will hopefully provide further insight [39].
In conclusion, while direct comparisons have not been made, endometrial injury with Endobiops endometrial sampling and hysteroscopic directed biopsies both seem to be effective and induce an effect of similar magnitude particularly in GnRH-ant cycles. Further studies are ongoing to answer the questions raised about the full impact of endometrial injury on subsequent IVF pregnancy outcomes.
References
- Dominguez F, Pellicer A, Simón Paracrine dialogue in implantation. Mol Cell Endocrinol. 2002; 186: 175-181.
- Grifo SART Clinical Summary Report at NYU Fertility Center. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=1895
- De Rycke M, Belva F, Goossens V, et ESHRE PGD Consortium data collection XIII: cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum Reprod. 2015; 30: 1763-1789.
- Sharkey AM, Macklon The science of implantation emerges blinking into the light. Reprod Biomed Online. 2013; 27: 453-460.
- Coughlan C, Ledger W, Wang Q, et Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014; 28: 14-38.
- Ferraretti AP, La Marca A, Fauser BC, et ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011; 26: 1616-1624.
- American Society Reproductive The role of assisted hatching in in vitro fertilization: a review of the literature. Fertil Steril. 2008; 90: 196-198.
- Petersen CG, Mauri AL, Baruffi RL, et Implantation failures: success of assisted hatching with quarter-laser zona thinning. Reprod Biomed Online. 2005; 10: 224-229.
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, et Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016; 30: CD002118.
- Papanikolaou EG, Kolibianakis EM, Tournaye H, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in A systematic review and meta-analysis. Hum. Reprod. 2008; 23: 91-99.
- Zeyneloglu HB, Onalan Remedies for recurrent implantation failure. Semin Reprod Med. 2014; 32: 297-305.
- Stern C, Chamley L, Norris H, et al. A randomized, double- blind, placebo-controlled trial of heparin and aspirin for women with in vitro fertilization implantation failure and antiphopholipid or antinuclear antibodies. Fertil Steril. 2003; 80: 376-383.
- Harper J, Jackson E, Sermon K, et al. Adjuncts in the IVF laboratory: where is the evidence for 'add-on' interventions? Hum 2017; 32: 485-491.
- Datta AK, Campbell S, Deval B, et Add-ons in IVF programme - Hype or Hope? Facts Views Vis Obgyn. 2015; 7: 241-250.
- Loeb L. Uber die experimentelle erzeugung von knoten von deciduagewebein dem uterus des meerschweinchens nach stattgefundener copulation [On the experimental production of nodes of decidua tissue in the uterus of the guinea pig after copulation]. Zbl Allg Path Path 1907; 18: 563-565.
- Barash A, Dekel N, Fieldust S, et al. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro Fertil Steril. 2003; 79: 1317-1322.
- Raziel A, Schachter M, Strassburger D, et Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil. Steril. 2007; 87: 198-201.
- Karimzadeh MA, Ayazi Rozbahani M, Tabibnejad Endometrial local injury improves the pregnancy rate among recurrent implantation failure patients undergoing in vitro fertilisation/intra cytoplasmic sperm injection: a randomized clinical trial. Aust. N. Z. J. Obstet. Gynaecol. 2009; 49: 677- 680.
- Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF Reprod Biomed Online. 2004; 8: 590-594.
- Rama Raju GA, Shashi Kumari G, Krishna KM, et Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006; 274: 160-164.
- Zhou L, Li R, Wang R, et Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril. 2008; 89: 1166-1176.
- Makrakis E, Hassiakos D, Stathis D, et al. Hysteroscopy in women with implantation failures after in vitro fertilization: findings and effect on subsequent pregnancy rates. J Minim Invasive 2009; 16: 181-187.
- Narvekar SA, Gupta N, Shetty N, et Does local endometrial injury in the nontransfer cycle improve the IVF-ET outcome in the subsequent cycle in patients with previous unsuccessful IVF? A randomized controlled pilot study. J Hum Reprod Sci. 2010; 3: 15-19.
- Siristatidis C, Kreatsa M, Koutlaki N, et Endometrial injury for RIF patients undergoing IVF/ICSI: a prospective nonrandomized controlled trial. Gynecol Endocrinol. 2017; 33: 297-300.
- Hayashi T, Kitaya K, Tada Y, et Single curettage endometrial biopsy injury in the proliferative phase improves reproductive outcome of subsequent in vitro fertilization-embryo transfer cycle in infertile patients with repeated embryo implantation failure. Clin Exp Obstet Gynecol. 2013; 40: 323-326.
- Kumbak B, Sahin L, Ozkan S, et Impact of luteal phase hysteroscopy and concurrent endometrial biopsy on subsequent IVF cycle outcome. Arch Gynecol Obstet. 2014; 290: 369-374.
- Singh N, Toshyan V, Kumar S, et Does endometrial injury enhances implantation in recurrent in-vitro fertilization failures? A prospective randomized control study from tertiary care center. J Hum Reprod Sci. 2015; 8: 218-223.
- El-Toukhy T, Sunkara S, Khalaf Y. Local endometrial injury and IVF outcome: a systematic review and meta-analysis. Reprod Biomed Online. 2012; 25: 345-354.
- Potdar N, Gelbaya T, Nardo Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reprod Biomed Online. 2012; 25: 561-571.
- Baum M, Yerushalmi GM, Maman E, et al. Does local injury to the endometrium before IVF cycle really affect treatment outcome? Results of a randomized placebo controlled trial. Gynecol 2012; 28: 933-936.
- Tk A, Singhal H, S Premkumar P, et al. Local endometrial injury in women with failed IVF undergoing a repeat cycle: A randomized controlled trial. Eur J Obstet Gynecol Reprod 2017; 214: 109-114.
- Levin D, Hasson J, Cohen A, et al. The effect of endometrial injury on implantation and clinical pregnancy rates. Gynecol 2017; 33: 779-782.
- Yeung TW, Chai J, Li RH, et al. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled Hum Reprod. 2014; 29: 2474-2481.
- Gibreel A, Badawy A, El-Refai W, et Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res. 2013; 39: 680-684.
- Gnainsky Y, Granot I, Aldo P, et Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. 2015; 149: 75-85.
- Liang Y, Han J, Jia C, et al. Effect of Endometrial Injury on Secretion of Endometrial Cytokines and IVF Outcomes in Women with Unexplained Subfertility. Mediators Inflamm. 2015; 2015; 757184.
- Karimzade MA, Oskouian H, Ahmadi S, et al. Local injury to the endometrium on the day of oocyte retrieval has a negative impact on implantation in assisted reproductive cycles: A randomized controlled Arch Gynecol Obstet. 2010; 281: 499-503.
- Huang SY, Wang CJ, Soong YK, et al. Site-specific endometrial injury improves implantation and pregnancy in patients with repeated implantation Reprod Biol Endocrinol. 2011; 9: 140.
- Van Hoogenhuijze NE, Torrance HL, Mol F, et Endometrial scratching in women with implantation failure after a first IVF/ICSI cycle; does it lead to a higher live birth rate? The SCRaTCH study: a randomized controlled trial (NTR 5342). BMC Womens Health. 2017; 17: 47.