Factors Associated to Successful Homologous Artificial Insemination
Author'(s): Henry Aristóteles Mateo Sánez1*, Raphael Obiala Ezenwua2, Carmen Alexandra Moreno Moreno3, María Evarista Arellano García4, Daniela Mate Madrigal5
1Director of Hospital Santa Rosa de Lima, Ensenada, Baja California, Obstetrician-Gynecologist, Biologist of Human Reproduction, Mexico.
2Embryologist in charge of Santa Rosa de Lima Hospital, Ensenada, Baja California, Master of Science, Mexico.
3Physician in charge of the Research Department of Santa Rosa de Lima Hospital, Ensenada, Baja California, Mexico.
4Professor at the Autonomous University of Ensenada, Baja California, Faculty of Sciences, Master of Science, Mexico.
5Social Service in Research at the National Institute of Pediatrics in Mexico City. Course of specialty in medical genetics at the University of Valencia, Spain.
*Correspondence:
Henry Aristóteles Mateo Sánez, Director of Hospital Santa Rosa de Lima, Ensenada, Baja California, Obstetricians and Gynecologists, Reproductive Medicine, Mexico.
Received: 22 February 2018 Accepted: 16 March 2019
Citation: Henry AMS, Raphael OE, Carmen AMM, et al. Factors Associated to Successful Homologous Artificial Insemination. Gynecol Reprod Health. 2019; 3(2): 1-5.
Abstract
Goal: To describe the prevalence of a successful fertilization and pregnancy rate, and the influence of different factors in couples who undergo homologous artificial insemination.
Material and Methods: Retrospective cohort study, in patients with infertility that underwent a homologous artificial insemination procedure at the Fertility Clinic in Santa Rosa de Lima Hospital, Ensenada, Baja California, Mexico. Failure in achieving fertilization and pregnancy was associated to the presence of infertility factors from different origins: cervical factors, uterine, fallopian tube and peritoneum, endocrine-ovarian, masculine, or a combination of them. Measures of association were calculated and logistic regression was used to adjust for age and body mass index.
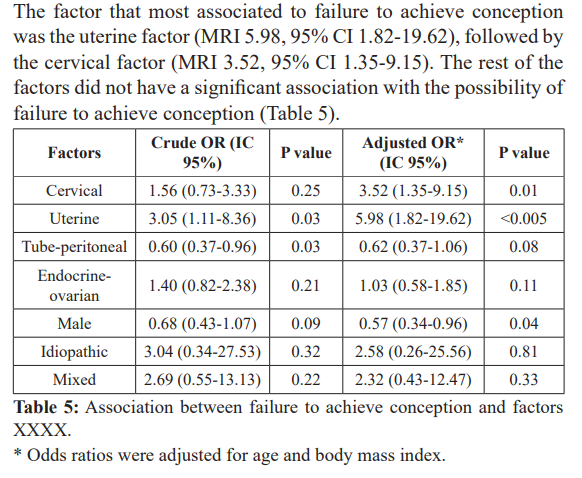
Results: 151 patients were studied. From all successful pregnancy cases, 63 were achieved in the first insemination cycle (17.9%), 35 in the second cycle (27.8%), 29 in the third cycle (36%) and 24 in the fourth cycle (42.8%). The uterine factor was strongly associated to failure in achieving pregnancy (OR 5.98, 95% CI 1.82-19.62), followed by the cervical factor (OR 3.52, 95% CI 1.35-9.15). The rest of the factors were not significantly associated to failure in achieving pregnancy.
Conclusion: Homologous artificial insemination.
Keywords
Background
Intrauterine insemination (IUI) together with controlled ovarian stimulation are low-complexity, low-cost and less invasive assisted reproductive techniques compared to highly complex techniques such as in vitro fertilization. Intrauterine insemination consists of depositing previously capacitated sperm in the uterine cavity as a treatment of infertility due to sperm alterations, ejaculation problems (sexual dysfunction), vaginismus, altered cervical factor, unexplained sterility and endometriosis grade I and II [1,2].
Commonly found pathologies are polycystic ovaries, blocked tubes and peritubular adhesions and endometriosis. The prevalence of polycystic ovarian syndrome (PCOS) in asymptomatic women is between 16-33%. The tube factor damage, usually, is secondary to pelvic inflammatory disease, surgical tubal sterilization and endometriosis.
Unexplained male infertility is present in 30-40% of men with normal sperm parameters. The potential explanations for male infertility are endocrine alterations of gonadal development during early pregnancy, due to environmental contamination and genetic factors [3]. Pregnancy rates according to the European Society of Human Reproduction and Embryology (ESHRE) reports 12.4% per cycle [4]. In Mexico, pregnancy rates reported by intrauterine insemination are up to 21.7% per patient and 13% per cycle [5,6]. Intrauterine insemination can be performed in a natural cycle or with help of controlled ovarian hyperstimulation by gonadotropins. This increases the amount of oocytes available for fertilization and places the capacitated semen inside the uterine cavity, which increases the likelihood of interaction and achieving pregnancy [7-9]. The objective of this study is to describe the prevalence of pregnancy and the influence of altered factors in infertile couples undergoing homologous intrauterine insemination at the Fertility Clinic of Baja California.
Materials and Methods
Retrospective cohort study, carried out at the Fertility Clinic of Santa Rosa de Lima Hospital, Ensenada, Baja California, Mexico. The evolution of a total of 352 patients who started their first cycle of homologous artificial insemination in the period from January 2015 to December 2017 was analyzed. The information was obtained from the clinical files, with authorization by the Ethics Committee of the Santa Rosa de Lima Hospital, at Ensenada, Baja California (Authorization No. 0015).
The inclusion criteria were: Couples with primary and secondary infertility, female ≤ 45 years, minimal permeability of one fallopian tube, 5 million progressive mobile sperm after sperm capacitation and patients who had signed the informed consent. A maximum of 4 inseminations was established. In patients who presented bilateral tubal obstruction, operative laparoscopy was performed, finding lax adhesions in 90% of them, which were released leaving at least one permeable and healthy fallopian tube.
Exclusion criteria were: patients who had not correctly followed the medication's dosage or who for some reason had been canceled before completing controlled ovarian hyperstimulation, hypo or hyperresponsiveness, or patients that left the treatment cycle, and those who for some reason did not take the blood sample for the hormonal profiles.
The altered factors studied were:
Cervical: colposcopy was performed and vaginal culture. Uterine: by endovaginal ultrasound analyzing the cavity for absence of fibroids.
Tube-peritoneal: hysterosalpingography and/or hysterosono- graphy.
Endocrine-ovarian: lab tests of prolactin, thyroid function, FSG, LH, estradiol, endovaginal Ultrasound.
Male: sperm analysis.
The comparative variables were the number of stimulation cycles, age, body mass index (BMI) and abortions.
Statical analysis
For the descriptive statistical analysis, measures of central tendency and dispersion were used. The comparison of means between two groups was performed with the Student's T test and the Analysis of Variance (ANOVA) was used to compare more than two means. The Chi square test was used to compare proportions and odds ratios were calculated as measures of association, which were adjusted by logistic regression to adjust for potential confounding factors. It was assumed that the numerical variables had a normal distribution based on the sample size of the study, taking as reference the Central Limit Theorem. All statistical analyzes were performed with STATA Version 12.
Sperm capacitation
Semen was obtained from the male partners of the patients who underwent artificial insemination. The sample was collected by masturbation in a sterile bottle with a sexual abstinence period of 3-7 days. After liquefaction at room temperature, the samples were examined under a microscope to define their characteristics in terms of concentration, motility and a Diff Quick stain for morphological analysis. Samples with an initial concentration of less than 5 million progressive motile sperm were washed with HTF+ hepes culture medium supplemented with 10% human serum albumin, in a 1:1 volume ratio in a conical tube and centrifuged at 1600 rpm for 10 minutes, then the supernatant is decanted and the button is resuspended with a volume of 0.7 ml.
Samples with an initial concentration greater than 5 million progressive mobile sperm were subjected to the density gradient training technique with PureSperm® (Nidacon, Gothenburg, Sweden) usually 2 layers of concentrations of 80% and 40%. The gradients were placed in a 15 ml conical tube, 1 ml of the 80% gradient medium is deposited at the bottom of the tube and carefully avoiding mixing, 1 ml of 40% gradient medium is placed, finally a non-selective volume greater than 2 ml of the seminal sample. Then centrifuged at 1600 rpm for 10 minutes, the supernatant is decanted and the button is resuspended with 4 ml human HTF+ hepes culture medium supplemented with 10% human serum albumin. Finally, centrifuged at 1600 rpm for 10 minutes, remove the supernatant and resuspend the button with 0.7 ml of medium.
Ovarian Stimulation Protocol and Luteal Phase Support
Ovarian stimulations were performed with subcutaneous administration of Gonal-f®, (75-100 IU/day) from the 3rd to the 12th day of the cycle, follicular follow-up with transvaginal ultrasound (General Electric, Voluson E10) on days 3, 8 and 12 of the cycle, getting at least one mature follicle between 17-22 mm, performing the shot with 250 μg of recombinant hGC (Ovidrel),performing a single intrauterine insemination (IUI) 36 hours after, with ultrasound guided to 1 cm of uterine fundus, remaining the patient in decubitus dorsal for 20 to 30 minutes.
All patients were indicated with progesterone as luteal phase support from the day of insemination until week 10 of pregnancy or menstruation at a dose of 400 mg daily orally or vaginally. Plasma β-hCG levels were measured 2 weeks after IUI. The detection of clinical pregnancy was defined with the transvaginal ultrasonographic visualization of intrauterine gestational sac.
Results
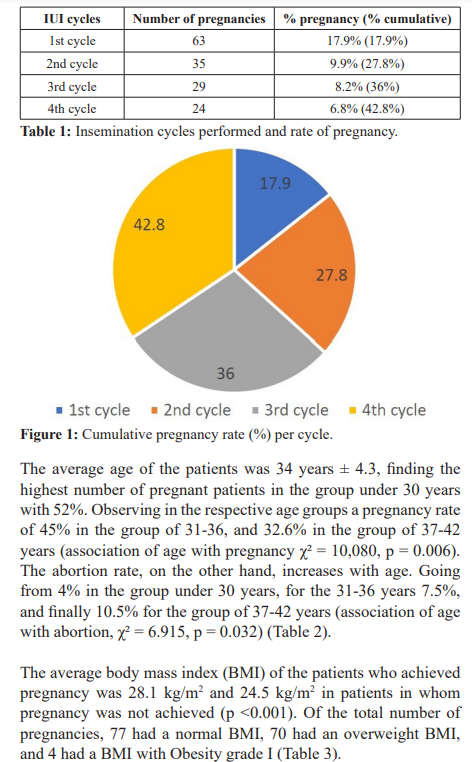
Of the total of 352 patients, a total of 151 patients were pregnant (42.9%). Of the total pregnancies, 63 were obtained in the first insemination cycle (17.9%), 35 in the second cycle (27.8%), 29 in the third cycle (36%) and 24 in the fourth cycle (42.8%) (Table 1, Figure 1).
Discussion
Regarding to homologous IUI cycles performed, higher pregnancy rates were observed in the first cycle of IUI 17.8%, compared with the European Society of Human Reproduction and Embryology of 12.4% [4], while Dinelli et al reported 14.8% per cycle [10]. By accumulating the 4th cycle of continuous stimulation, we can see a cumulative pregnancy rate of 42.8%.
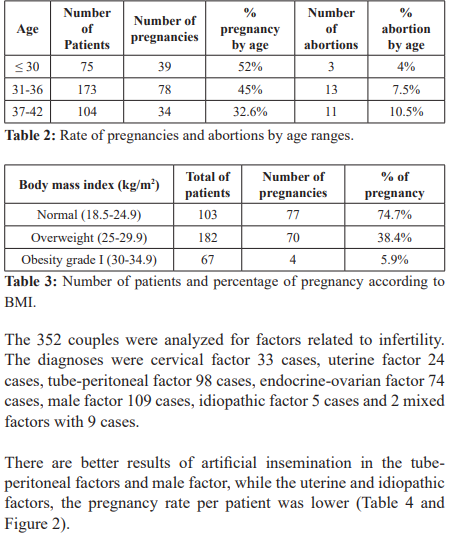
We found a higher rate of pregnancies in younger women (<30 years), 52% with respect to women over 37 years with 32.6%, age is one of the main prognostic factors for the success of the procedures of Assisted reproduction, regardless of the scheme used in ovarian hyperstimulation. According to different reports, higher pregnancy rates are obtained in women under 35 compared with older women, with cycles of ovarian hyperstimulation plus intrauterine insemination, due to the decrease in the quality of the oocytes and the reduction of receptivity. endometrial [11]. In this study, the age under 36 years benefited women undergoing intrauterine insemination treatment.
A study conducted on 512 patients, with a total of 1,101 cycles of IVF and ET, showed a significant increase (p<0.01) in the pregnancy rate in women under 30 years of age, compared with women older than 37 years (26 and 9%, respectively), and a decrease in the incidence of abortion in patients under 30 years of age, compared to women over 40 years (50 and 22%, respectively) [12-14]; possibly due to a decrease in the quality of oocyte in association with a gradual increase in the circulating level of FSH and decreased circulation of the antimüllerian hormone and inhibin B [15].
As age increases, the risks of other disorders that may adversely affect fertility, such a leiomyomas, tubal disease, and endometriosis, also increase [15]. The age-related decline in fertility is accompanied by significant increases in the rates of aneuploidy and spontaneous abortion [16].
The body mass index was evaluated in the patients who achieved pregnancy, being higher the pregnancy rate with an ideal BMI (18.5-24.9 kg/m2) with 74.7%, followed by 38.4% in pregnancy rate with overweight BMI (25-29.9 kg/m2), finally lower pregnancy rate in patients with Obesity I (30-34.9 kg/m2) with 5.9%. Allowing to corroborate that normal BMI benefits women subjected to homologous intrauterine insemination, this is because obese women are more likely to have an ovulatory dysfunction due to dysregulation of the hypothalamic-pituitary-ovary axis. On the other hand, the excess of free fatty acids can have a toxic effect on the reproductive tissues, leading to cellular damage and a low-grade chronic inflammatory state [10]. Women in fertile age who present fertility problems, the probability of conceiving spontaneously decreases by 5% for each unit that increases the BMI above 29kg/m2 [12] which is why we subject our patients start diet and exercise to increase the possibility of pregnancy.
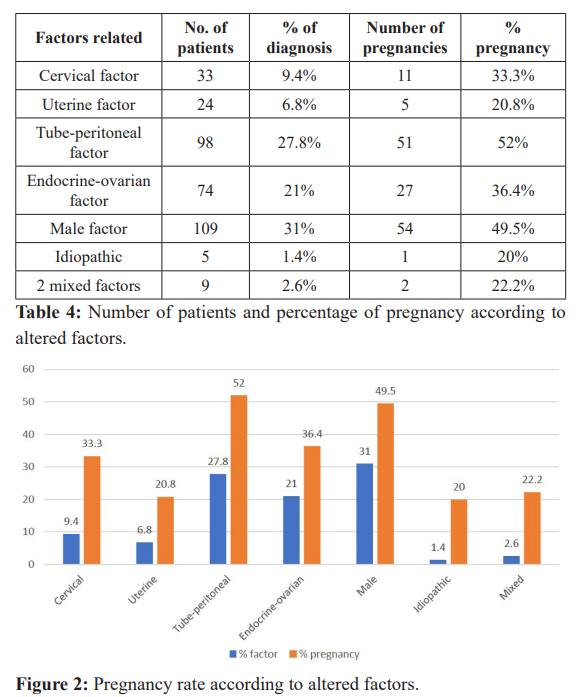
Regarding the most frequent factors causing infertility, it was observed that the tube-peritoneal factor, followed by the male factor, and endocrine-ovarian with an average of 42% of pregnancy, presented better response to ovarian stimulation treatment and insemination; compared with Barros-Delgadillo and Col, where the cause of the most frequent infertility was of mixed origin (58.5%) followed by endocrine-ovarian causes (22.2%) [13].
The success of pregnancy in our study is benefited by the sperm capacitation technique and the restoration of fertility through clinical management and hormonal stimulation of endocrine- ovarian and tube-peritoneal factors.
Conclusion
Intrauterine artificial insemination continues to be a reliable technique in assisted reproductive procedures, with lower cost and complexity compared to in vitro fertilization. The correct indication of this technique will directly impact the success, the etiology, the age of the patient, the number of cycles performed and the weight are the crucial factors.
References
- Serena H Chen. Misconceptions about conception: Clinical considerations in the diagnosis and management of female infertile. Medscape Ob/Gyn Women´s Health. 2007.
- Mateo HA, Mateo E, y Hernández L, et al. Tratamiento de pacientes con endometriosis e infertilidad. Ginecología y Obstetricia de México. 2012; 80: 705-711.
- Vasconcellos R, Clapauch R. Infertilidad femenina de origen endocrina. Arq Bras Endocrinol Metab. 2014; 58: 144-152.
- Dinelli L, Courbiere B, Achard V, et al. Prognosis factors of pregnancy after intrauterine insemination with the husband’s sperm: conclusions of an analysis of 2,019 Fertil Steril. 2014; 101: 994-1000.
- Barros-Delgadillo JC, Trejo-Castañeda H, Ormsby C, et al. Diferencia de respuesta a los antagonistas de GnRH en ciclos de hiperestimulación ovárica más inseminación intrauterina. Ginecol Obstet Mex. 2010; 78: 15-28.
- Barros Delgadillo JC, Rojas Ruiz JC, Molina MunguiÌa AC, et al. Factores pronóstico de embarazo en inseminación intrauterina. Ginecol Obstet Mex. 2006; 74: 611-625.
- Barros J, Fiszman R, Santibañez A, et al. Resultados preliminares del estudio de eficacia de dos esquemas de hiperestimulación ovárica controlada con hormona folículo estimulante recombinante en ciclos de inseminación intrauterina. Ginecología y Obstetricia de México. 2012; 80: 61-72.
- Arcaini L, Bianchi S, Baglioni A. Superovulation and intrauterine inseminations. Superovulation alone in the treatment of unexplained infertility. A randomized study. J Reprod Med. 1996; 41: 614.
- Kably A, Carrera E, Carballo E, et al. Resultados de Inseminación intrauterina en el centro especializado para la atención de la mujer. Ginecología y Obstetricia de México. 2011; 79: 280.
- Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017; 107: 840-847.
- Valdez Ortega José, MariÌn Romero Olivia, Hinojosa Cruz Juan Carlos, et al. Tasa de embarazos en pacientes sometidas a inseminación intrauterina en una unidad médica de alta especialidad. Revista Mexicana de Medicina de la Reproducción. 2009; 1: 135-138.
- Fontana R, della Torre S. The Deep Correlation between Energ Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. 2016; 8: 1-34.
- Amézquita-Vizcarra LE, Barros-Delgadillo JC, Muñoz- Manrique CG. Tasas de embarazo en el primer ciclo de inseminación intrauterina en pacientes estimuladas con gonadotropinas según el desarrollo folicular y edad. Ginecol Obstet Mex. 2017; 85: 659-666.
- ESHRE Capri Workshop Fertility and ageing. Human Reproduction Update. 2005; 11: 261-276.
- ASRM Female age-related fertility decline. Committee Opinion No. 589. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014; 123: 719-721.
- Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol. 2012; 24: 187-193.
- EndaMcVeigh, John Guillebaud, Roy Homburg. Polycystic ovarian syndrome. obstetrics and gynecology: an evidence- based text for MRCOG. London: Oxford University Press. 2004; 588-593.