First Line Mono-Chemotherapy using Methotrexate in Post-Molar Patients with Gestational Trophoblastic Neoplasia
Author'(s): Vo Minh Tuan1*, Phan Nguyen Nhat Le2
1University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam.
2TuDu Hospital, Ho Chi Minh City, Vietnam.
*Correspondence:
Vo Minh Tuan MD, PhD, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam, Tel: +84 909727199.
Received: 04 February 2018 Accepted: 06 March 2019
Citation: Vo Minh Tuan, Phan Nguyen Nhat Le. First Line Mono-Chemotherapy using Methotrexate in Post-Molar Patients with Gestational Trophoblastic Neoplasia. Gynecol Reprod Health. 2019; 3(2): 1-5.
Abstract
Objective: To determine the successful rate of Methotrexate/Folinic Acid chemotherapy in treatment of low risk gestational trophoblastic neoplasia (GTN) from the post-molar patients at Tu Du Hospital.
Methods: A retrospective cohort study based on 341 clinical records with low-risk GTN (as FIGO 2002 defined) from post-molar patients. Those patients were treated with Methotrexate/Folinic acid (MTX/FA) at Tu Du Hospital between 11/2011 and 10/2014. Outcome measures included the successful rate of MTX/FA and the factors related to the result of MTX/FA treatment.
Results: The successful rate of MTX/FA chemotherapywas 65.63% (CI 95%: 0.61-0.70). The failure rate of the patients with high level βhCG before chemotherapy > 100,000mUI/mL was 5.2 times greater than those of low level βhCG <1,000mUI/mL (P=0.007). The failure rate of the patients with FIGO risk score ≥ 4 was 6.48 times greater than those of FIGO risk score≤ 1(P=0.001). The failure rate of the patients had previous prophylactic chemotherapy was 2.34 times greater than those who did not (P=0.003).
Conclusions: MTX/FA chemotherapy was an effective treatment for low risk gestational trophoblastic neoplasia (GTN) from the post-molar patientswith the successful rate of 65.63%. Three factors asociatied with MTX/FA treatment result was βhCG level before chemotherapy, GTN risk score and previous prophylactic chemotherapy.
Keywords
Introduction
The term gestational trophoblastic neoplasia (GTN) only indicates the invasive and malignant gestational trophoblastic diseases which includes invasive hydatidiform mole (HM), choriocarcinoma, placental-site trophoblastic tumor and epithelioid trophoblastic tumor. GTN may developed after or without hydatidiform mole, and not related to the fetus position or gestational age. There haven’t been many report about the incidence rate of post Hydatidiform mol GTN in Vietnam. According to a research from Hao Pham (Vietnam National Ob-Gyn Hospital), the rate was 20.2% [1]. There is also a report from Tu Du Gynecology
Department shows that the incidence rate was 16.8% in 2013 and 13.2% in 2014. Current treatments for low-risk GTN is mostly Methotrexate and Actinomycin D. Although these treatments have been applied for years, the dominant effectiveness of these two is still controversial. The 2012 Cochrane review on GTN included 5 Randomized Controlled Trial (RCT) proved that Actinomycin D had better successful rate (5 RCT with 513 patients, RR 0.64, CI 95%: 0.54 – 0.76) [2]. No serious side-effect was reported in both Methotrexate and Actinomycin D group.
Vietnam has a pretty high GTN incidence rate, comparing to other countries, and Tu Du Hospital is responsible for treatment and management of most GTN case in Southern Vietnam. Every year, we has roughly 1000 Hydatidiform mole and about 150 GTN new case. Most of these patients were low-income, poor- educated and lived in the contryside, therefore, It is essential to find an efficiency, time-appropriated treatment with reasonable side-effect that comes with low price. Treatment of GTN depends on risk assessment and local circumstance in order to select an appopriate protocol. However, under objectively circumstances, including the shortness of Actinomycin D supplement, especially in the early 2014, Tu Du Hospital runs out of Actinomycin D, therefore Methotrexate/Folinic acid is currently the firstlline and the only options in single-agent chemotherapy. This pose a lot of problems to doctors to decide whether to use single or multi agents chemotherapy in order to achieve the best result with as less side- effect as possible. Until now, there hasn’t been a research about the effectiveness of Methotrexate/ Folinic Acid in GTN treatment at Tu Du Hospital [3].
That is why we decided to do this research: The effectiveness of MTX/FA in GTN treatment at Tu Du Hospital. With the Research Question is: What is the successful rate of MTX/FA in GTN treatment?
Study Objectives
To determine the successful rate of MTX/FA chemotherapy in treatment of GTN from post-molar patients at Tu Du Hospital.
To determine correlation factors of treatment results of MTX/FA chemotherapy.
Methods
Study design
Prospective cohort study.
Target population
Gestational trophoblastic neoplasia (GTN) from the post-molar patients treated with MTX/FA.
Research population
Gestational trophoblastic neoplasia (GTN) from the post-molar patients treated with MTX/FA at Tu Du Gynecology Department.
Sampling population
Sampling population from November 2011 to October 2014.
Inclusion criteria
Medical record clearly determined:
- Patients had been diagnosed low-risk GTN post-molar, stage I,II,III as FIGO 2002 defined.
- MTX/FA chemotherapy was indicated by Chief/Vice Chief of Department or doctors had been certificated in chemotherapy according to Vietnam Health Minister Rule.
- Medical record ends after the last MTX/FA treatment.
Exclusion criteria
- Insufficient information for data collecting.
- Patients with history of failed chemotherapy.
- Patients were not managed at Tu Du from the beginning.
- Patients don’t fully follow the regimen (patients did not revisit every 2 weeks) or withdraw from treatment.
Sample size
Estimates proportional with absolute precision formular:
n = Z21-α/2P(1-P)/ d2
α = 0,05; d: 5%; McNeish research [8] was P = 0,668
n = 341.
Study Procedure
Step 1: Screening
From Tu Du Outpatients and Gynecology Department patients records in 2011 – 2014, we select those patients that had been diagnosed GTN post-molar treated with MTX/FA. Through these chosen records, we will have the name, medical record number/ code, date in and out. From these information, we will search for the medical records of patients at Data Reserve Room at Tu Du Hospital.
Looking for all the medical record that has enough information from the inclusion criteria and exclusion criteria. Selecting all medical records that meets the inclusion criteria from 2014 backward until we get enough samples – according to the sample size.
Step 2: Grouping
These medical records will be divided into 2 group: Success and Failed with MTX/FA chemotherapy, after that we determine the success rate. Through this grouping, we analyze to determine whether this is a different in factors such as age, risk factors or hCG level between the two groups. From there, we can figure out those correlation factors to the result of MTX/FA.
Step 3: Data Collecting
From the patient records, we have collected information about clinical presentation, laboratory results before and after each cycle. After that, we turn these information into variables and fill in the data collecting form. The variable included: background variable, independent variable and dependent variable.
Step 4: MTX/FA chemotherapy data recording and monitoring
- MTX/FA Chemotherapy patients will be in the hospital 8days for each cycle.
- MTX/FA regimen includes MTX 1mg/kg/d IM in day 1, 3, 5, 7 ; and FA 1 mg/kg/d in day 2, 4, 6, 8.
- If no side-effect was observed, patients can go home and comeback for check-up every 2 weeks. This check-up includes full examination, hCG level test, pre-chemotherapy test and the next cycle will be continued, if qualified. In case of failed treatment, another regimen(different agent/s) will be indication.
- Side-effect will be examinated and recorded by doctor each day in medical record including signs and symptoms such as: mouth ulcers, nausea, vomitting, hair-loss, urine, defecation, platelet count, Corpus luteum cyst, metastasis signs… CBC and liver function, renal function was also indicated after each cycle or when a side-effect is noticed.
Definition of main factors
Result
Binary variable: success or failed.
According to Goldstein vaÌ? Berkowitz definition (2007) and (1996) [4,5].
Success: Includes all these criteria:
- hCG graph: hCG level decrease at least 1 log after cycle with MTX/FA.
- Clinical progress: No new metastasis signs.
- Laboratory result: no new metastasis signs on ultrasound scan,X ray or MRI.
Failed: Include any of these criteria:
- hCG graph: hCG level do not decrease at least 1 log after 2 consecutive MTX/FA cycle or had been indicated different regimen.
- Clinical progress: new metastasis sign.
- Laboratory result: new metastasis signs on ultrasound scan,X ray or MRI(if available).
- Bone marrow, liver, renal, GI tract poisonous signs was not improved after 2 resting weeks and supporting treatment.
βhCG:
Continuous variable, Tu Du hospital is having the complete βhCG laboratory system from Abbott, using the immunofluorescence method, measurement unit is mUI/ml. Negative is defines as <5mUI/ml. Recorded before and after each cycle, and divided into quartile 25-75% and risk scale with the following level: <1,000, 1,000-10,000, 10,000-100,000, >100,000 mUI/ml, to analyze.

From November 2011 to the end of October 2014 there are 450 patients had been diagnosed GTN. 392 patients are post-molar, or not stage I,II,III, low risk was using MTX/FA regimen, the others was treated with Actinomycin D, multi-agents chemotherapy or withdraw from treatment. With 392 GTN post-molar medical records, we had selected 341 records that met the requirements for our research.
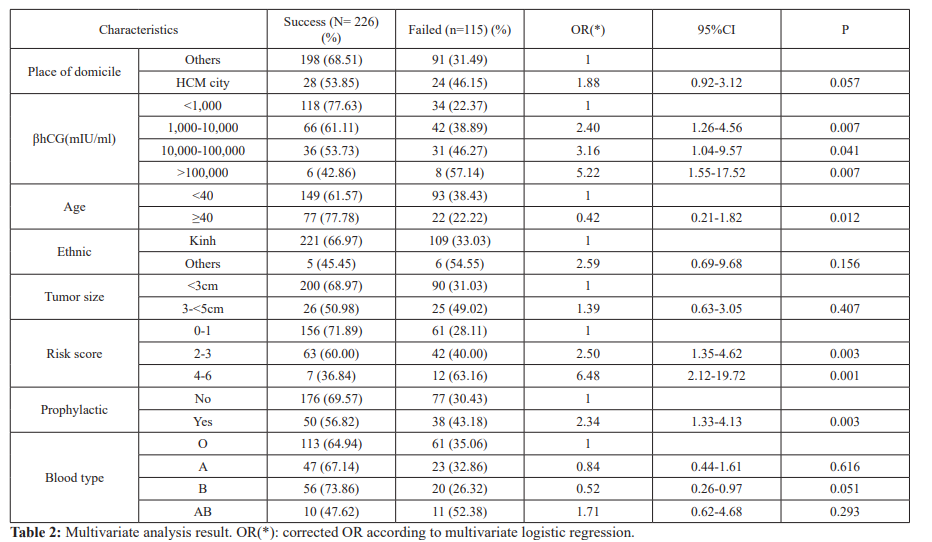
Table 1 desribed the demographic characteristics and history of patients in our research. Chi-squared test showed that there is no correlation factors to the result.
In order to control interference and correlation factors, we performed a multivariate analysis to find the relationship between factors and the MTX/FA treatment results. There are 3 correlation factors to the result is serum βhCG before MTX/FA chemotherapy, GTN risk score and previous prophylactic chemotherapy.
Dissicusion
Result of MTX/FA chemotherapy
Our data shows MTX/FA is effective to treat low-risk gestational trophoblastic neoplasia (GTN) from the post-molar patients with the success rate is 66.28%. The success rate of MTX/FA chemotherapy in GTN post-molar patients is 66.28% [CI 95% : 0.61-0.71]. This result shows similarity to research from McNeish (2002) [8] 66.8% and Jafari (2014) [6] 61.3%. However, this is
lower than researchs from Lertkhachonsuk (2009) [7] 73.6% and Phuong Le (2009) [2] 78%, it could be expained by the differents in failed definition, sample selection criteria from our research.
Correlation factors to MTX/FA chemotherapy result: After multivariate analysis, we have controlled interference factors and found three correlation factors to the MTX/FA treatment outcome, which include βhCG level before MTX/FA treatment, GTN risk score and prophylactic chemotherapy. The failure rate of patients with βhCG before chemotherapy >100,000mUI/mL increase 5.22 times to those had βhCG before chemotherapy <1,000mUI/mL (P<0.05). The failure rate of the patients with FIGO risk score ≥ 4 was 6.48 times greater than those of FIGO risk score≤ 1(P=0.001). The failure rate of the patients had previous prophylactic chemotherapy was 2.34 times greater than those who did not (P=0.003). We also have the corrected OR, which higher the original OR by more than 10%: 5.22 compare to 4.62 for the βhCG level before MTX/FA treatment factor, 6.48 compare to 4.38 for the GTN risk score factor and 2.34 compare to 1.73 for the prophylactic chemotherapy factor.
Side-effect: We only recorded 3 case (0.87%) with severe elevated liver enzyme, 1 of 3 was not improved after supporting treatments and had to changed the regimen, 3.81% has mouth ulcers and 12.32% had anemia, these are mild to moderate level, and mostly do not require specific treatments. Side effect is unremarkable and mostly not required specific treatments.
Conclusion
In the current circumstances including shortness of Actinomycin D and remarkable side effect of multi-agents chemotherapy, MTX/ FA is still an appropriate choice in GTN post-molar treatment.
Hydatidiform mole patients with high risk and took MTX as an prophylactic chemotherapy should not be treated with MTX (again).
GTN patients with βhCG level before treatment >100,000mUI/mL or risk score ≥ 4 should not be treated with single-agent MTX/FA. Future research should focus on RCT to compare the effectiveness between MTX/FA and Actinomycin D as a beginning treatment in GTN patients.
Acknowledgement
We are indebted to the participants for making this research possible and to all physicians of Oncology unit of Tu Du hospital.
References
- Hao Serum beta hCG in management post- hydatidiform mole, GTN treatment and relapse factors PhD Thesis. Hanoi Medical University. 2004; 20-55.
- Phuong Le, Toan Le, Khoa Pham. Evaluate the efficacy of MTX/FA regimen in low-risk GTN treatment and resistane factors. HCMC Medical Journal. 2009; 13: 53-62.
- Alazzam M, Tidy J, Hancock BW, et First-line chemotherapy in low-risk gestational trophoblastic neoplasia. The Cochrane Database of Systematic Reviews. 2012; 7.
- Goldstein DP, Berkowitz RS. Gestational trophoblastic disease. Berek and Novak's 1996; 12: 1276- 1278.
- Goldstein DP, Berkowitz Gestational Trophoblastic Disease. Berek and Novak's Gynecology. 2007; 14: 1581-1603.
- Jafari SM, Vejdani R, Sayyah MH. Comparison of Methotrexate-Folinic acid versus pulsed Actinomycin D in treatment of stage I, low risk gestational trophoblastic neoplasia: a randomized clinical trial. Iranian J of Obstetrics, Gyneocology and Infertility. 2014; 17: 1-11.
- Lertkhachonsuk AA, Israngura N, Wilailak S, et al. Actinomycin D versus methotrexate-folinic acid as the treatment of stage I, low-risk gestational trophoblastic neoplasia: a randomized controlled Int J Gynecol Cancer. 2009; 19: 958-988.
- McNeish IA, Strickland S, Holden L. Low-Risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose Methotrexate anf Folinic acid from 1992 to 2000. J Clin Oncol. 2002; 20: 1838-1844.