Nab-Paclitaxel/Carboplatin Superior to Solvent-Based Paclitaxel\Carboplatin in Treatment of Advanced Stage of Cervical Cancer
Author'(s): Olimova Zilola*, GOFUR-OKHUNOV Mirza-Ali and Abdullaev Aziz
Tashkent Cancer Center, Outpatient Chemotherapy Department, Tashkent, Uzbekistan.
*Correspondence:
Olimova Zilola, Head of Outpatient department of Tashkent City Cancer Hospital, Tashkent, Uzbekistan, Tel: +998909285373, E-mail: bzilolaxon05@inbox.ru.
Received: 12 August 2017 Accepted: 02 September 2017
Citation: Olimova Zilola, GOFUR-OKHUNOV Mirza-Ali, Abdullaev Aziz. Nab-Paclitaxel/Carboplatin Superior to Solvent-Based Paclitaxel\Carboplatin in Treatment of Advanced Stage of Cervical Cancer. Gynecol Reprod Health. 2017; 1(3): 1-4.
Abstract
Background: In Uzbekistan, women with cervical cancer are often diagnosed in advanced stages of the disease. Cervical cancer is the leading cause of death among patients with malignant tumors in Uzbekistan. In the randomized study comparing nab-paclitaxel\carboplatin scheme with solvent-based paclitaxel\carboplatin in our patients with metastatic cervical cancer. Nab-Paclitaxel\carboplatin demonstrated a significantly higher overall response rate than solvent-based paclitaxel\Carboplatin. The primary endpoint was investigator assessed overall response rate and assessment of safety and tolerability. Secondary endpoints included time to tumor progression (TTP), survival and pharmacokinetics.
Materials and Methods: Seventy-six patients were randomized from 20 June 2015 through 10 August 2016. The median age was 50 years, 87 percent of participants were less than 65 years old and 64 percent of participants were postmenopausal. Nab-Paclitaxel and Carboplatin administered intravenously on days 1, 8, and 15 of each 28-day cycle, with a starting dose of 100 mg/m2.
Results: Nab-paclitaxel\carboplatin significantly improved the investigator assessed overall response rate versus solvent-based paclitaxel\carboplatin (49% vs. 21%; P =0.001). The median time to progression was longer for nabpaclitaxel\ carboplatin than for solvent-based paclitaxel\carboplatin, the difference was significant (13.3 months vs.6.2 months p=0.078). Nab-paclitaxel\carboplatin also achieved a 29 percent improvement in progression free survival when compared to solvent-based paclitaxel (8.7 months vs. 5.1 months; P = 0.118). Nab-paclitaxel\ carboplatin arm was also superior in tolerability and safety. There were fewer adverse events in the nab-paclitaxel\ carboplatin arm - 10.6 versus 17.2 percent with solvent-based paclitaxel\carboplatin. There were fewer infections with nab-paclitaxel\carboplatin - 1.3 versus 6.1 percent with solvent-based paclitaxel\carboplatin. In addition, hematologic toxicities were approximately the same about 15 percent in each arm.
Conclusion: Chemotherapy for advanced stage of cervical cancer with nab-paclitaxel/carboplatin was associated with less toxicity and significant OR, TTP and survival superiority compared with solvent-based paclitaxel/ carboplatin.
Keywords
Introduction
In Uzbekistan, women with cervical cancer are often diagnosed in advanced stages of the disease. Cervical cancer is the leading causeo f death among patients with malignant tumors in Uzbekistan. Most women with metastatic cervical cancer or local recurrence after radiotherapy are candidates for palliative chemotherapy [1].
Gynecologic Oncology Group (GOG) protocol 204 established double therapy of cisplatin and paclitaxel as the standard of care.
This combination had an overall response rate of 29.1 % [2]. Then, GOG-240 demonstrated an improvement in both PFS, and OS endpoints from this doublet with the addition of bevacizumab. By harnessing this anti-angiogenic agent, there was an addition of 3.4 months to OS [3]. However there are no established treatment options in recurrent cervical cancer.
Nano-particle albumin bound (NAB) paclitaxel is a 130-nanimeter, chremophor-free preparation of paclitaxel. This preparation eliminates the need for pre-medication, has a shorten infusion time, and increase tumor concentration as compared to standard preparation [4-5]. Nab-paclitaxel has considerable activity and moderate toxicity in the treatment of drug resistant, metastatic and recurrent cervix cancer [6].
Carboplatin has been reported to be a less effective platinum analog than cisplatin for cervical cancer [7-9]. Carboplatin induces milder nephropathy, less nausea/vomiting, and lower neuropathy than cisplatin [10]. The combination of carboplatin and paclitaxel allows for paclitaxel administration over 3 hours, and carboplatin requires no hydration. In the first multi-institutional phase II trial of paclitaxel and carboplatin for metastatic or advanced cervical cancer to our knowledge, we found an overall RR of 59% (95% CI, 43 to 75), median PFS of 5.3 months, and median OS of 9.6 months, suggesting that the combination had promising efficacy and feasibility for outpatient treatment [11].
Methods/Design
In the randomized study comparing nab-paclitaxel\carboplatin scheme with solvent-based paclitaxel\carboplatin in our patients with metastatic cervical cancer.
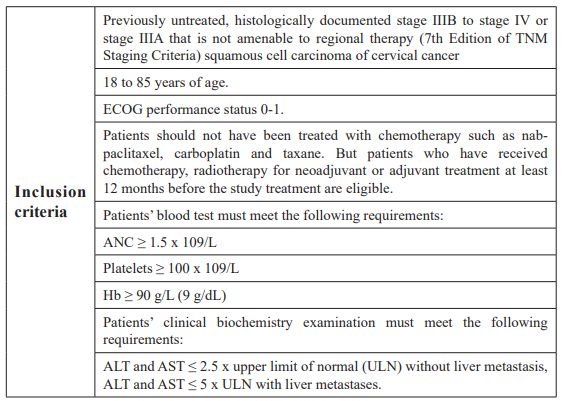
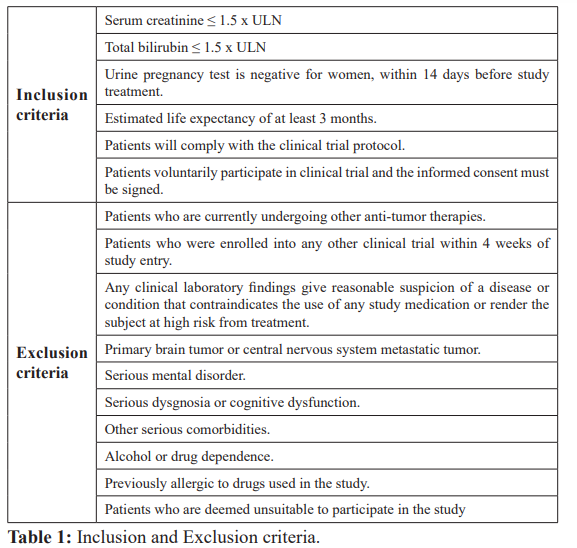
Seventy-six patients were randomized from 20 June 2015 through 10 August 2016. The median age was 50 years, 87 percent of participants were less than 65 years old. All patients in this study have locally advanced or metastatic cervical cancer which has been histologically confirmed. The inclusion and exclusion criteria are summarized in Table 1.
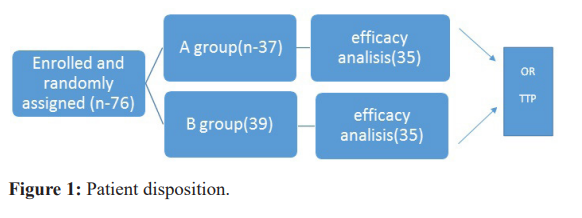
Randomly assigned patients included 76 patients. Patient disposition is shown in Figure 1. Group A: receiving nab-paclitaxel 100 mg/m2 on days 1, 8 and 15 of each 28-day cycle; or group B: receiving Paclitaxel 100 mg/m2, plus carboplatin AUC 5 on days 1, 8, and 15 of each 28-day cycle. Both group A and B receive up to six cycles of chemotherapy.
Study objectives
The primary endpoint was investigator assessed overall response rate and assessment of safety and tolerability. Secondary endpoints included time to tumor progression (TTP), survival and pharmacokinetics. Radiographic disease measurements were required at baseline and at least after every three cycles, using the same assessment technique (preferably computed tomography). Tumor response was evaluated according to RECIST (version 1.0) [12]. Before each treatment cycle and within 30 days after completing treatment, safety assessments were performed according to the Common Terminology Criteria for Adverse Events (version 3.0).
Statistics
The primary objective will be analyzed by chi-square test. Secondary endpoints of TTP and OS will be evaluated by Kaplan- Meier method with a 95% confidence interval [13]. The log-rank method will be used to compare the difference between the survival curves of two arms. Multifactorial Cox regression analysis will be used to determine the prognostic factors of the survivals including TTP and OS. Efficacy analysis was conducted for all eligible patients, and safety analysis was conducted for all treated patients.
Results
Median number of treatment cycles in all treated patients was six (range, one to six cycles) in both treatment groups. The proportion of patients who completed the entire treatment protocol was 70.9% in the A group and 72.2% in the B group. Median relative dose- intensities of nab-paclitaxel and carboplatin in the A group were 97.8% and 98.4%, respectively; those of solvent-based paclitaxel and carboplatin in the B group were 99.8% and 99.9%, respectively.
Nab-paclitaxel\carboplatin significantly improved the investigator assessed overall response rate versus solvent-based paclitaxel\ carboplatin (49% vs. 21%; P =0.001). The median time to progression was longer for nab-paclitaxel\carboplatin than for solvent-based paclitaxel\carboplatin, the difference was significant (13.3 months vs.6.2 months p=0.078). Nab-paclitaxel\carboplatin also achieved a 29 percent improvement in progression free survival when compared to solvent-based paclitaxel (8.7 months vs. 5.1 months; P = 0.118). Nab-paclitaxel\carboplatin arm was also superior in tolerability and safety. There were fewer adverse events in the nab-paclitaxel\carboplatin arm - 10.6 versus 17.2 percent with solvent-based paclitaxel\carboplatin. There were fewer infections with nab-paclitaxel\carboplatin - 1.3 versus 6.1 percent with solvent-based paclitaxel\carboplatin. In addition, hematologic toxicities were approximately the same-about 15 percent in each arm.
Only one patient died as a result of interstitial pneumonitis related to protocol treatment in the A group. Two patients in the B group died before completion of the treatment cycle, but the data and safety monitoring committee judged death unlikely to have been related to treatment.
Discussion
NAB-paclitaxel has three Foods and Drug Administration (FDA) approved indications: locally advanced or metastatic non-small cell lung cancer, metastatic adenocarcinoma of the pancreas, and metastatic breast cancer after failure of combination chemotherapy or relapse within 6 months of adjuvant therapy [14]. There have been limited evaluations of NAB-paclitaxel in cervical cancer. Here we review our experience in treating heavily pre-treated recurrent cervical cancer patients that have failed prior cytotoxic chemotherapy.
Previous researches have shown comparable efficacy and good safety profile of Nab-paclitaxel- based chemotherapy in treatment of advanced cervical cancer, compared to other standard platinum- based doublets [15].
In our trial it was noted the intriguing finding of pronged median overall survival in nab-paclitaxel–treated patients aged ≥ 65 years could have been related to better tolerability of the weekly nab-paclitaxel schedule. The Nab-paclitaxel regimen produced less severe neuropathy, neutropenia, myalgia, and arthralgia compared with solvent-based paclitaxel. The increased risk of thrombocytopenia and anemia in the nab-paclitaxel regimen was readily manageable. Taken together, the Nab-paclitaxel regimen has a favorable risk-benefit profile compared with that of solvent- based paclitaxel as a palliative therapy for all patients with advanced cervical cancer.
While this study provides evidence for the safety and tolerability of NAB-paclitaxel with Carboplatin in the setting of advanced stage of cervical cancer, there are several limitations to this data including: lack of patient reported outcomes, Response Evaluation Criteria in Solid Tumors (RECIST) response, and small study population.
Conclusion
Chemotherapy for advanced stage of cervical cancer with nab- paclitaxel/carboplatin was associated with less toxicity and significant OR, TTP and survival superiority compared with solvent-based paclitaxel/carboplatin. NAB-paclitaxel was overall well tolerated with no grade 4 or 5 adverse events. NAB-paclitaxel with Carboplatin is feasible and potentially active in treating recurrent and advanced stage of cervical cancer after failing chemo – and radiotherapy.
References
- Friedlander M, Grogan M. Guidelines for the treatment of recurrent and metastatic cervical cancer Oncologist. 2002; 7: 342-347.
- Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group J Clin Oncol. 2009; 27: 4649-4655.
- Tewari KS, Sill MW, Long III HJ, et al. Improved survival with bevacizumab in advanced cervical N Engl J Med. 2014; 370: 734-743.
- Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008; 14: 4200-4205.
- Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentration and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI- 007, compared with cremophor-based paclitaxel. Clin Cancer 2006; 12: 1317-1324.
- David S. Alberts, John A. Blessing, Lisa M. Landrum, et al. Phase II trial of nab-paclitaxel in the treatment of recurrent or persistent advanced cervix cancer: A gynecologic oncology group study Gynecologic Oncology, 2012; 127: 451-455.
- Arseneau J, Blessing JB, Stehman FB, et al. A phase II study of carboplatin in advanced squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study Invest New 1986; 4: 187-191.
- Weiss GR, Green S, Hannigan EV, et al. A phase II trial of carboplatin for recurrent or metastatic squamous carcinoma of the uterine cervix: A Southwest Oncology Group study Gynecol Oncol.1990; 39: 332-336.
- McGuire WP 3rd, Arseneau J, Blessing JA, et A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: A Gynecologic Oncology Group study J Clin Oncol.1989; 7: 1462– 1468.
- Anderson H, Wagstaff J, Crowther D, et al. Comparative toxicity of cisplatin, carboplatin (CBDCA) and iproplatin (CHIP) in combination with cyclophosphamide in patients with advanced epithelial ovarian cancer Eur J Cancer Clin 1988; 24: 1471-1479.
- Kitagawa R, KatsumataN, Ando M, et A multi-institutional phase II trial of paclitaxel and carboplatin in the treatment of advanced or recurrent cervical cancer Gynecol Oncol. 2012; 125: 307-311.
- Therasse P, Arbuck SG, Eisenhauer EA, et New guidelines to evaluate the response to treatment in solid tumors. European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst. 2000; 92: 205-216.
- Kaplan EL, Meier Nonparametric estimation from incomplete observations J Am Stat Assoc.1958; 53: 457-481.
- Gradishar WJ, Tjudlandin S, Davidson N, et Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. J Clin Oncol. 2005; 23; 7794-7803.
- Minion LE, Chase DM, Farley JH, et al. Safety and efficacy of salvage nano-particle albumin bound paclitaxel in recurrent cervical cancer: a feasibility study, Gynecologic Oncology Research and Practice. 2016; 3: 4.