Novel Methods of Improving Fecundity and Various Pathological Disorders Based on a Hypothetical Model of Embryo Implantation
Author(s): Diane L Check1 and Jerome Check H1,2*
1Cooper Institute for Reproductive Hormonal Disorders, P.C., MT. Laurel, NJ, USA.
2Cooper Medical School of Rowan University, Dept. Ob/Gyn, Div. Repro. Endo. & Infertility, Camden, NJ, USA.
*Correspondence:
Jerome H. Check, M.D., Ph.D., Cooper Institute for Reproductive Hormonal Disorders, P.C., MT. Laurel, NJ, 7447 Old York Road, Melrose Park, PA 19027, USA, Tel: 215-635-4400; Fax: 215-635-2304.
Received: 04 December 2020 Accepted: 27 December 2020
Citation: Check DL, Check JH. Novel Methods of Improving Fecundity and Various Pathological Disorders Based on a Hypothetical Model of Embryo Implantation. Gynecol Reprod Health. 2020; 4(4): 1-15.
Abstract
Progesterone (P) is very involved in achieving successful embryo implantation. It aids in the creation of thinwalled spiral arteries which are needed for nutrient exchange between mother and fetus, by creating a cellular immune response, which removes the thick-cell walls of some of the uterine arteries thus creating spiral arteries. This uterine artery remodeling requires 5 days, but the embryo reaches the uterine cavity by day 3. Thus, P inhibits implantation at that time by stimulating a glycoprotein called mucin-1, which lines the uterine cavity and prevents the embryo from attaching until the mucin-1 barrier is finally breeched, on day 5. Progesterone also inhibits dopamine, which normally serves to decrease cellular permeability. Thus, by blocking dopamine, irritants infuse into the uterine tissue leading to an inflammatory response (70% natural killer cells). These natural killer cells would attack the fetal semi-allograft. However, P again serves to inhibit immune rejection of the fetal semi-allograft, by inducing cells of the fetal-placental unit to make a unique immune modulatory protein called the progesterone induced blocking factor (PIBF), which suppresses cellular immunity. This hypothetical model explains the beneficial effect of creating a greater inflammatory response by endometrial irritation (endometrial scratch), to improve number of spiral arteries, which may be deficient. In addition, this model explains the potential beneficial effect of luteal and first trimester supplementation of P, in improving fecundity and also the possibility of sympathomimetic amines releasing dopamine to similarly improve fecundity by diminishing excessive cellular permeability. Excessive cellular permeability may be the cause of various chronic medical conditions, since they seem to respond very well to dextroamphetamine treatment. Cancer cells also use the PIBF mechanism to escape immune surveillance. Thus, it is no surprise that P receptor antagonists can improve quality and length of life to patients with a variety of cancers.
Keywords
Introduction
Based on our own research, and others, the following hypothetical model is proposed to explain the events of implantation into the endometrium at the time of invasion of the fetal-placental unit [1- 4]. Step 1 – Estradiol (E2), made by the dominant follicle induces progesterone (P) receptors in the endometrium. Step 2 – E2 in some way causes a significant increase in endometrial dendritic cells. Step 3 – P, made from luteinization of the dominant follicle after the luteinizing hormone (LH) surge, suppresses the biogenic amine dopamine. Since dopamine functions to diminish cellular permeability, this allows irritants to infuse into the endometrium. Step 4 – Infusion of irritants into the endometrium causes an inflammatory response. By one week after ovulation the cellular immune constituents are natural killer (NK) cells (70%), macrophages (20%), and a variety of T and B cells, making up the last 10%.
Step 5 – One of the main functions of this cellular immune reaction is to enable uterine artery remodeling, to prepare for implantation. This is needed to remove thick walls of some uterine arteries and create thin-walled spiral arteries. Step 6 – During the post-ovulation period, and prior to blastocyst attachment, the endometrium is coated with a glycoprotein, known as mucin-1. This mucin-1 layer prevents the embryo from adhering to the endometrium when it reaches the uterine cavity on day 3, because it will take five days to achieve proper uterine remodeling. Progesterone plays a role in mucin-1 production.
Step 7 – Certain chemicals and enzymes, possibly made by the increased number of dendritic cells that were attracted by the high E2 levels at peak follicular maturation, help to create a rift in the mucin-1 layer to expose bare endometrium.
This hole is over the area of the endometrium that has had the appropriate uterine artery remodeling.
Step 8 – Some white blood cells secrete chemokines, attracting the blastocyst in the uterine cavity to attach at this prepared site with adequate spiral arteries. Step 9 – Cells from the extra-villous trophoblast, of the invading fetal-placental unit, will form a single layer of trophoblast cells around the denuded blood vessels. This will provide a one-cell thick cell wall for support for these vessels, so they do not collapse. Step 10 – These thin-walled vessels will allow nutrient exchange between mother and fetus. They are called spiral arteries.
Step 11 – Once P is secreted by the luteinized follicle and the subsequent corpus luteum, the P causes the production of a unique immunomodulatory protein in the cytoplasm of the following fetal-placental cells: mesenchymal cells, embryonic cells, and trophoblast cells. This unique protein is known as the progesterone induced blocking factor (PIBF) [5-7].
Step 12 – Exposure of P to circulating gamma/delta T cells causes an abrupt rise of PIBF in the serum of post-ovulatory women, which continues during the pregnancy p [8]. Step 13 – Based on studies showing that the P receptor modulator, mifepristone, suppresses intracytoplasmic PIBF, but not serum PIBF, leads to the hypothesis that: it is the local release of intracytoplasmic PIBF that prevents the maternal host from immune rejection of the conceptus (which is a semi-allograft), by inhibiting the killing effects of white blood cells in the fetal microenvironment [4].
Step 14 – PIBF suppresses immune function in many ways. For example, one mechanism by which NK cells “kill their prey” is by releasing a toxic chemical called perforin. PIBF suppresses perforin degranulation [9].
Step 15 – Thus, the main immunosuppressive effect of P induced PIBF is local, related to release from the intracytoplasmic increase in PIBF [4]. Step 16 – It is not clear what is the function of high levels of circulating PIBF but fortunately, it does not suppress generalized immune function, which would leave the pregnant woman at greater risk for infections and possibly cancers.
Step 17 – In animals parturition is preceded by a drop in serum P, leading to a drop in serum PIBF. In humans parturition is not preceded by a drop in P. However, it is associated with less sensitivity of the gamma/delta T cells making PIBF to P, so parturition in humans is preceded by a drop in serum PIBF.
Based on this hypothetical model, which is formulated from previous experimental research, there are several endometrial factors that could inhibit proper implantation. Improper implantation may lead to infertility, miscarriage, preterm labor, or other complications, e.g., pre-eclampsia.
Endometrial factors that could lead to embryo implantation defects
Possibility #1 – inadequate uterine remodeling, related to inadequate permeability. This could lead to insufficient entry of irritants, leading to inadequate post-ovulatory inflammatory response, resulting in subpar uterine remodeling.
Since P blocks dopamine, and dopamine diminishes cellular permeability, one of the benefits of supplementing the luteal phase with P may be to improve the post-ovulation inflammatory response. But what if for some reason even raising the P dosage is insufficient to cause adequate inflammation?
There is the possibility that mechanical irritation, such as by an endometrial biopsy (subsequently referred to as an endometrial scratch), may allow the appropriate post-ovulatory cellular immune environment to develop.
A study to determine the efficacy of an endometrial scratch performed in the late luteal phase on subsequent live delivered pregnancy rates in three subsequent months, in women with unexplained infertility, in natural cycles, was performed [10]. This was a prospective matched controlled study. The criterion for selection was a minimum two years of unexplained infertility, but no limit on maximum length of infertility. In addition, a minimum of three cycles of follicle stimulating drugs, with intrauterine insemination (IUI), or in vitro fertilization (IVF) performed in another infertility center. There was no maximum for number of previous treatment cycles.
Both the control group and the experimental group received both vaginal P (Crinone®, endometrin®, or compounded P vaginal suppositories, according to their insurance) and oral micronized P 200mg at bedtime on an empty stomach, throughout the luteal phase. A mid-luteal phase injection subcutaneous of 1mg leuprolide acetate was given based on evidence that it may possibly increase implantation rates, possibly by stimulating endometrial gonadotropin releasing hormone (GnRH) receptors [11].
The experimental group received an endometrial scratch, using the pipelle de cornier, in the late luteal phase of the cycle preceding the first cycle in the study. Both control and experimental groups were required to attain a mature dominant follicle (mean diameter >18 mm, minimal serum E2 200 pg/mL), in each of the three cycles of investigation. The endometrial scratch was only performed the one time.
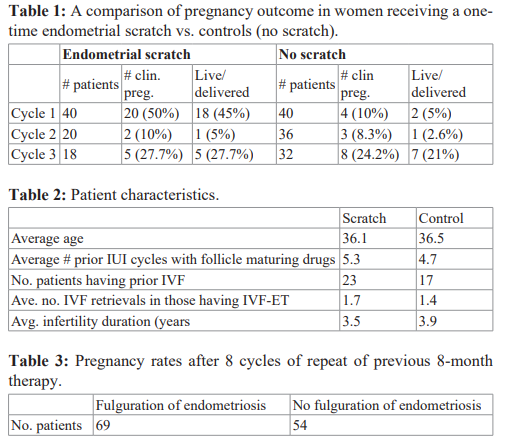
A comparison of pregnancy rates for scratch vs. no scratch is seen in Table 1. The miscarriage rate was: controls – 33.3% (5/15), scratch – 11% (3/27). The live delivered pregnancy rate was 9x higher in the first cycle after the scratch, compared to controls (45% vs. 5%). Yet, there was no real difference in the experimental group vs. control group in cycles 2 and 3. There were no confounding variables as seen in Table 2.
Actually, considering the fact that the controls treated just with P without the scratch had a 25% live delivered pregnancy rate in just three months of P treatment, despite an average of 3.9 years length of infertility, with an average of 4.7 cycles with follicle maturing drugs and IUI, and with 45% of them having an average of 1.4 previous IVF cycles also, supports the possibility that supplemental P, by inhibiting dopamine may help to induce a greater inflammatory response by increasing cellular permeability. Yet, through the PIBF mechanism, inhibits those increased NK cells from attacking the fetal semi-allograft.
Although the live/delivered pregnancy rate was significantly higher in the scratch group, and the miscarriage rate lower than those just receiving P, the 25% live delivered pregnancy rate, in just 3 cycles of taking P, in patients with long-term infertility previously failing to conceive with fertility drugs, IUI, and IVF, suggests that P deficiency can be a cause of unexplained infertility.
Thus, if inadequate uterine remodeling can be the cause of unexplained infertility, and the 25% live delivered pregnancy rate in the controls taking P supplementation was related to improving inadequate permeability, adding further irritation by the endometrial biopsy, seems to be significantly more effective, than the use of P supplementation alone.
To be sure the positive benefit of endometrial irritation for a group with long-term unexplained infertility, was not merely fortuitous, we offered failures, either in the scratch group, or controls, if they would like to try another cycle with the endometrial scratch in the late luteal phase, immediately prior to the observation cycle. The results after a second scratch cycle found that 12 of 26 (46.1%) achieved a clinical pregnancy, and 10 of 26 (38.4%) had a live delivery. These data show, however, that the benefit of the scratch only lasts for the menstrual cycle immediately following the scratch. It has no lingering benefits.
Other research has also found a benefit for performing the endometrial scratch in natural cycles, for unexplained infertility. The study from Gibreel et al, found a 6-month clinical pregnancy rate of 25.9%, in those receiving the scratch, vs. 9.8% in the control group [12]. However, without supplemental P, the pregnancy rates were a lot lower in the Gibreel et al. study [12]. It should be noted that the criteria for selection of patients with unexplained infertility was not as rigorous as the patient selection from our group.
A meta-analysis of eight clinical trials, found that a follicular phase endometrial scratch, followed by IUI improved pregnancy rates. In our studies all patients had natural cycles without IUI, but some did have mild follicular stimulation [13].
We have demonstrated in IVF cycles that the endometrial scratch seemed to improve live delivered pregnancy rates following fresh embryo transfer (ET), but seems to decrease the live delivered pregnancy rates following frozen ET. This could suggest that one of the reasons that correcting an ovulatory cycle with follicle maturing drugs produces lower pregnancy rates per cycle than would be expected compared to fertile women, was that these follicle maturing drugs may decrease cellular permeability leading to decreased natural killer cells. Thus, women taking follicle maturing drugs may benefit from the endometrial scratch to improve uterine remodeling.
However, we have found that performing an endometrial scratch seems to lower pregnancy rates in women with diminished oocyte reserve. Perhaps embryos may be less hearty, and therefore cannot negate an increased cellular immune response induced by the scratch, leading to subsequent immune rejection.
Endometrial factors that could lead to embryo implantation defects
Possibility 2 – excessive cellular immune response causing rejection of the fetal semi-allograft. As seen in the example of the scratch having a negative impact on frozen ETs, and embryos from women with diminished oocyte reserve, the endometrial scratch can be a double-edged sword. In some instances, where there is adequate uterine remodeling, because there is no diminished cellular permeability defect, the endometrial irritation and subsequent increase in endometrial cellular immunity may encourage immune rejection of the fetal-placental unit.
Excessive cellular immune response could be related to a defect of increased cellular permeability. Though by the model established, adding supplemental P in the early luteal phase can lead to an increase in immunosuppressive proteins, e.g., PIBF, the possibility exists in some instances the cellular immune excess is too great to be fully inhibited by an increase in PIBF production.
Theoretically, a medication that would release more dopamine from sympathetic nerve fibers, could act to diminish excessive cellular permeability, and thus inhibit excessive infusion of endometrial irritants. This would lead to a reduction in the excessive cellular immune response. Such a drug does exist: dextroamphetamine sulfate, or other sympathomimetic amines that release dopamine from sympathetic nerve fibers.
Pelvic pain has been associated with infertility. Generally, dyspareunia, mittelschmerz, dysmenorrhea (especially pre- menstrual worsening with age) or chronic pelvic pain has been considered probably related to endometriosis (or adenomyosis). Laparoscopic ablation, or excision of endometriotic implants, will reduce pain both at 6 months and 12 months after the procedure [14]. However, dextroamphetamine sulfate is more effective and the benefit longer lasting.
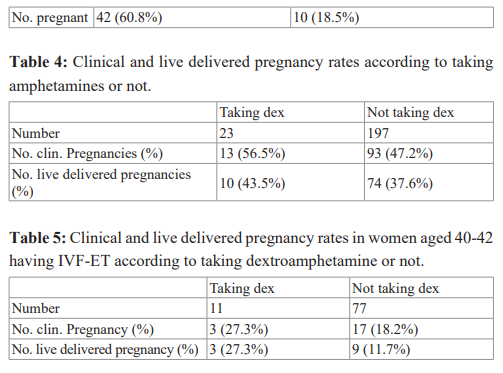
Our group actually performed the first study evaluating whether the laparoscopic removal of mild endometriosis could help correct infertility issues. The study published in the Int. J. Fertil, in 1987, randomly assigned 123 patients who failed to conceive after eight months of seemingly all infertility factors corrected. They were grouped into 69 patients whose implants were fulgurated and 54 patients who had diagnostic laparoscopic only [15]. The pregnancy rates after repeating the same therapy for eight cycles that failed for the first eight cycles prior to laparoscopy, according to whether endometriosis was removed or not, is seen in Table 3. A three- fold increase in pregnancy rates were seen in the group where endometriosis was removed. However, the study most quoted to support the concept that laparoscopic extirpation of endometriotic implants helps fecundity was published ten years later by Marcoux et al. [16]. Not all studies agree, e.g., the Gruppo Italiano Study in 1999 [17].
Despite the possibility that laparoscopic removal of endometriosis may improve fecundity, there are several reasons for not favoring laparoscopic approach for pelvic pain and infertility. The main reason favored by the authors, is treatment with dextroamphetamine sulfate is far more efficacious than surgery, in both relieving pain and in improving fecundity. There is evidence supporting favoring sympathomimetic amine therapy over surgery. Many women, who failed to gain any lasting relief of pain from surgical treatment, including more risky excisional surgery, respond extremely well to dextroamphetamine treatment [18-21]. A recent research presentation at the 2020 American Society for Reproductive Medicine, by Carpentier et al., found that dextroamphetamine sulfate, given to 25 women who still had significant pelvic pain, despite standard surgical, or medical treatment, provided marked improvement of pelvic pain in 68% of patients, within three months of treatment. Overall, 76% reported marked or moderate relief [22].
Other advantages of dextroamphetamine treatment over surgery include 1) no surgical risk, 2) because of excessive inflammation, including the ovaries, women with pelvic pain, whether endometriosis is demonstrated or not, are more prone to diminished oocyte reserve. Surgery can speed up development of diminished oocyte reserve, by directly damaging the ovaries or blood supply to the ovaries.
Frequently, gynecologists recommend medical therapy post- op, to help maximize success for a period of time. Delays in scheduling surgery and post-surgical medical management (e.g., oral contraceptives, GnRH agonists or antagonists or impeded androgens), may contribute to reducing oocyte reserve based on extra time of treatment in women of advanced age. One assumption of this model is that when a woman has the normal post- ovulation inflammation, she has no pelvic pain symptoms. Thus, pelvic pain may suggest excessive inflammation. The dosage of dextroamphetamine is titrated to eradicate the pain, assuming that this is the dosage that will be ideal for achieving live deliveries. Sometimes side effects may result in a compromise.
What advantage is there for using dextroamphetamine over other medical therapies? Dextroamphetamine sulfate is more effective in relieving pain without delaying getting pregnant. Medical therapy, if it suppresses pain only, delays procreation without improving fecundity when the therapy is stopped.
If increased cellular permeability is the mechanism causing pain, by causing excessive inflammation, why do other medications besides dextroamphetamine sulfate provide some relief from pain? One hypothesis is that P, by suppressing dopamine, increases cellular permeability. Estrogen induces P receptors. Blocking estrogen effect by GnRH agonists, or antagonists, or aromatase inhibitors, may block the increased permeability by suppressing estrogen, and thus inhibits development of P receptors in the endometrium.
If this hypothetical model is accurate, and if P would seem counter- productive by blocking dopamine and thus causing increased cellular permeability, then why do progestin’s improve pain? One could hypothesize that since P increases permeability, synthetic progestin (e.g., norethindrone or 19 nor-testosterone derivatives), may not inhibit dopamine as does P, yet counteract the effect of estrogen on increasing cellular permeability, or act as a competitive inhibitor to prevent P from inhibiting dopamine.
It may be hypothesized that the presence of ectopic endometrial implants, either exacerbates the cellular permeability defect, or in some other way increases inflammation, explaining the mild to moderate benefit of removing these implants surgically.
Possible benefit of dextroamphetamine sulfate improving fecundity
There is anecdotal evidence that dextroamphetamine sulfate can help women to have a live baby, despite multiple miscarriages, even following IVF-ET [23]. Case one presented at age 40 with a history of miscarriage at age 25 and subsequent infertility x 3.5 years. She failed to conceive at other infertility centers. After six cycles with follicle stimulating drugs + IUI and two cycles of IVF- ET. She was considered as having unexplained infertility. During her initial consult she preferred IVF, so her wishes were granted.
She conceived after her first IVF cycle at our IVF facility but had a miscarriage (triploidy). She conceived again on her second cycle of IVF but miscarried again (no chromosome analysis). Her third cycle was a frozen ET. She conceived, but miscarried (anembryonic gestational sac). She conceived again on her fourth IVF (second at our center) but had another miscarriage (chromosome analysis showed a normal male). Her fifth ET was a frozen ET, and she was treated with dextroamphetamine sulfate extended release capsule 30mg/day. She delivered a live baby, but pre-term related to pre- eclampsia. She never exhibited any clinical manifestation of pelvic pain, or the increased cellular permeability syndrome, so that is why we didn’t treat her with dextroamphetamine from the start.
Case two had ten years unprotected intercourse (unexplained infertility). She had 12 cycles of IUI, at one infertility center. She subsequently had three IVF-ET cycles at another center, where she conceived all three times, but miscarried each time. She consulted our infertility center and wanted IVF, because she had only ever conceived with this procedure (3 out of 3 IVF-ET cycles). She had mild diminished oocyte reserve. We started her on dextroamphetamine sulfate, 9.4mg extended release capsules. She only produced five metaphase II oocytes, of which three fertilized and two cleaved to day three and were transferred. She conceived and delivered a full-term healthy baby. She stayed on the same dose of dextroamphetamine sulfate during the whole pregnancy.
Our group performed a prospective patient option-controlled comparison study, to evaluate dextroamphetamine sulfate on pregnancy rates following IVF-ET, in a group of infertile patients who also had severe pelvic pain [24]. There were 23 women aged 35 and under with severe pelvic pain, treated with dextroamphetamine sulfate in first IVF-ET cycle with serum anti-mullerian hormone (AMH) >1.06ng/mL. They were matched to 197 historical controls, who did not take dextroamphetamine (they may or may not have had pelvic pain). All transfers were on day three.
The pregnancy outcome is seen in Table 4. Though chi-square analysis was not significant, there was a trend for higher pregnancy rates, despite the bias of probable endometriosis, in all 23 patients taking dextroamphetamine sulfate. It is important to note that all 23 patients had one month of dextroamphetamine sulfate before starting IVF, and all patients reported moderate to marked improvement in dysmenorrhea and other types of pelvic pain [24].
Another study that we performed to evaluate the efficacy of dextroamphetamine sulfate to improve fecundity, was providing the drug for patients with potentially fewer hearty embryos, i.e., women of advanced reproductive age, but with normal oocyte reserve. The study was a prospective patient option-controlled study [25]. The study group patients were women aged 40-42, with normal oocyte reserve (serum follicle stimulating hormone (FSH) <11 mIU/mL, day 3 serum AMH greater than 1.06 ng/ mL). All patients had moderate to severe dysmenorrhea. Their pregnancy rates were compared to historical controls (pelvic pain not a requirement).
There were 12 women recruited and 11 made it to ET. The average number of embryos transferred on day 3, was 2 in the study group and 2.1, in the historical controls. The pregnancy outcome is seen in Table 5. The live delivered pregnancy rates were 27.3% in the amphetamine treated group vs. 11.7% in the controls. The implantation rate was 18.2% for those treated with amphetamines vs. 11.8% for controls. All 11 patients reported marked improvement in pelvic pain.
There has been anecdotal experience finding sympathomimetic amine therapy to improve fecundity for infertile women not undergoing assisted reproductive techniques. One case was a 30-year-old, with one year of infertility. A hysterosalpingogram was not performed. She had regular menses; eight cycles documented where she made mature follicles and released the eggs, had a normal post-coital test and she received P support in the luteal phase. Her husband had a normal semen analysis.
She did have 1 cycle of IVF-ET, transferred two good embryos, and froze six, but failed to conceive. She did an endometrial scratch. She was treated with 9.4mg dextroamphetamine sulfate. She had not been on this therapy before because she had no symptoms suggesting the increased cellular permeability syndrome.
She conceived naturally while taking dextroamphetamine in her next cycle. The dextroamphetamine was continued, as was the P support (started in luteal phase) through the first trimester and had a full-term healthy baby.
The same woman came back at age 33, to try for another baby. She was placed back on dextroamphetamine sulfate, with P support in the luteal phase. She conceived in her first natural cycle and delivered another healthy full-term baby.
Another anecdotal case showed the potential benefit of sympathomimetic amine therapy to prevent recurrent miscarriage, in natural cycles. A 30-year-old woman presented with a history of four miscarriages, out of five pregnancies. Her one live baby was at age 28 (her second pregnancy). She was treated with P from early luteal phase in pregnancies four and five.
One loss was documented to be chromosomally normal (pregnancy five). Her symptoms were consistent with the increased cellular permeability syndrome, manifesting with premenstrual migraine headaches. Dextroamphetamine sulfate, 9.4mg, extended release capsules were started, and luteal phase P was given. She conceived and had a full-term healthy baby.
She was treated again with dextroamphetamine sulfate for her next pregnancy, with P support in the luteal phase. She conceived again and delivered a full-term baby.
Another anecdotal case vividly demonstrates the potential of using dextroamphetamine sulfate for very unexplained infertility. The patient started trying to conceive at age 21. She complained of heavy periods and severe cramps. She was trying for one year without success. She had a laparoscopy at another infertility center and endometriosis was found. The laparoscopy, with laser vaporization of endometriosis, did not help her severe pain. Her periods were regular. Empirically, the other infertility center gave her clomiphene citrate three cycles. She consulted our infertility group. Not only did she have severe dysmenorrhea and endometriosis, but also had treatment refractory Crohn’s disease. She was treated with dextroamphetamine sulfate, but because of side effects she was switched to lisdexamfetamine dimesylate, 40 mg, which she tolerated much better. Her pelvic pain immediately disappeared, as did her symptoms of Crohn’s disease [26].
She had six natural cycles with IUI, because the post-coital test was only fair. She was supplemented with vaginal and oral P in the luteal phase and mid-luteal phase injection of leuprolide acetate 1mg. She failed to conceive in these six cycles.
During breaks from treatment, she attempted to conceive naturally. She eventually did nine ETs, both fresh and frozen, including four retrievals with transfers (one at another infertility center). She had five frozen ETs also. She conceived on her last frozen transfer.
On the last frozen ET cycle, the dosage of lisdexamfetamine dimesylate was increased from 40mg to 60mg. She conceived at age 29. Also, she had lymphocyte immunotherapy three times before that did not work [27].
This case exemplifies the possibility that perhaps the dosage of dextroamphetamine sulfate that relieves pelvic pain symptoms and other co-morbidities, e.g., Crohn’s disease, may not be enough to reduce excessive cellular immune response in the endometrium. Thus, one could consider empirically raising the dosage of amphetamine, if successful pregnancies have not ensued in a reasonable length of time.
Another interesting anecdotal case shows that one may prevent a miscarriage that appeared imminent, by instituting therapy with dextroamphetamine sulfate. A nulliparous 33-year-old woman, with a history of four first trimester miscarriages was studied. She had never been on P support. Her follicle was mature, so she was treated with oral and vaginal P, without follicular maturation drugs. She conceived and her human chorionic gonadotropin (hCG) levels were appropriate and fetal viability was demonstrated. However, her gestational sac stopped growing, despite an appropriate crown- rump length, and there was more than a week discrepancy in sac/ crown rump length sizes. This is generally a poor prognosis [28]. Furthermore, the fetal heart rate increased to 196, a sign of fetal stress.
The patient was a nurse, and against her husband’s objection, she allowed us to treat her with 9.4mg dextroamphetamine sulfate extended release capsules. Her sac size subsequently caught up to normal size. The fetal heart rate decreased to normal and she delivered a live healthy baby.
There is also the possibility that treatment with dextroamphetamine sulfate may be able to prevent unexplained fetal demise in the last trimester. A nulliparous 34-year-old woman had two last trimester miscarriages. What was most frustrating is that the pathologist was unable to determine the cause of death in either one. Two other infertility centers suggested IVF, with transfer of embryos into a gestational carrier.
She could not afford a gestational carrier, so she consulted our infertility practice to see if we had a different suggestion. We suggested a natural cycle, P support in the early luteal phase until delivery, and dextroamphetamine sulfate. She conceived on her second treatment cycle and delivered a healthy baby full-term. She returned for baby two and she was treated the same way. She conceived on her first cycle. Once again, she delivered a healthy full-term baby.
Treatment with dextroamphetamine sulfate also helped a woman with secondary infertility and a history of frequent miscarriage. A 31-year-old woman presented with a 1 year of infertility. Her first pregnancy took six months to achieve, and she delivered a healthy baby at age 28. Subsequently, she had four first trimester miscarriages, with the last two pregnancies supported with P. She had no cramps, or other symptoms, to consider using dextroamphetamine sulfate from the start.
It was determined that she no longer made mature follicles and thus, she was treated with clomiphene citrate, 50mg x 5 days. She made a mature follicle but the post-coital was poor, so IUI performed. She conceived, but had a chemical pregnancy, with maximum b-hCG level of 111 mIU/mL.
Dextroamphetamine sulfates, 9.4mg extended release capsules, were given empirically. She conceived again with clomiphene and P in the luteal phase and 1mg leuprolide acetate in the mid-luteal phase [11]. She delivered healthy twins.
Dextroamphetamine treatment may have also helped an infertile woman with diminished oocytes to have a successful pregnancy, in a natural cycle, with P in the luteal phase. What is even more impressive is that she had failed to conceive despite four IVF-ET cycles at another infertility center. She had a history of premenstrual migraine headaches, nausea, vomiting and diarrhea, which completely abated with dextroamphetamine therapy. She delivered a healthy full-term baby.
In the discussion, so far, and the medical history of the case reports, the increased cellular permeability may not only lead to premenstrual medical conditions involving pelvic tissue, but other areas of the body, including the brain and bowel, leading to pain related to excessive inflammation in response to excessive absorption of toxic elements into the tissue [26,29]. The association of some of these conditions premenstrual is probably related to further increased cellular permeability, related to the hypothetical blocking of dopamine by progesterone. The permeability defect can not only lead to inflammation and pain related to infusion of irritants into tissue, but can lead to irritating agents leaking out, e.g., histamines, leading to premenstrual urticaria and anaphylaxis [30].
However, the permeability disorder does not necessarily have to occur only during the premenstrual time [31-34]. Hypothetically, infusion of unwanted chemicals into mitochondria may cause skeletal muscle dysfunction, leading to chronic fatigue or even paresis [31,35,36]. Infusion of toxic elements into smooth muscle mitochondria may lead to bowel dysfunction, e.g., achalasia, gastroparesis, pseudointestinal obstruction, and constipation [37- 41].
Evidence to support this hypothesis, is that they all responded very well to dextroamphetamine sulfate (whose main function is to release dopamine from sympathetic nerve fibers) despite becoming recalcitrant to standard therapies.
There have been too many published reports, or published abstracts, from national meetings to list them all in the publications. Many of them can be found in a few summary articles [42-45]. A listing of the various treatment refractory medical conditions that have responded very well to treatment with dextroamphetamine sulfate is seen in Tables 6 to 14
Thus far, the hypothetical model has presented the possibility that in some instances implantation failure can be related to inadequate uterine remodeling because of insufficient inflammation that could possibly be improved by an endometrial scratch. On the other hand, the model proposes that in some instances implantation failure may be related to excessive inflammation with immune rejection of the fetal semi-allograft that may be improved by diminishing excessive permeability and thus excessive cellular immune response by treating with sympathomimetic amines.
Progesterone is the center for this hypothetical model. The model proposes that the increased permeability results from P blocking dopamine. However, the increased cellular immunity, especially related to an increase in NK cells, may then cause immune rejection of the fetal semi-allograft.
Based on this hypothetical model, the NK cells and macrophages are neutralized by an effect of P. The theory maintains that this is mainly related to the production and secretion of an immunomodulatory protein from embryonic, mesenchymal, and trophoblast cells, and circulating gamma/delta T cells, known as PIBF [8]. One other possible cause of implantation defect is insufficient neutralization of the killing effects of the cellular immune system by insufficient production of PIBF, even with the absence of excessive NK cells.
Thus, one way to theoretically improve implantation defects is to treat with extra P in the luteal phase to increase serum PIBF production. Evidence will subsequently be provided that luteal phase P supplementation can improve fecundity, not only by improving pregnancy rates, but also to reduce risk of miscarriage. Before proceeding with evidence to support the efficacy of P therapy, some of the experimental data concerning PIBF will be presented.
The progesterone induced blocking factor
A brief description of experimental data, concerning the PIBF protein, will be presented. For a more detailed description of this protein the readers are referred to four publications [4,7,8,46].
Progesterone induced blocking factor is a protein not found in the cells of normal tissue, but is present in mesenchymal, embryonic, and trophoblast cells of the fetal-placental unit. One exception may be circulating gamma/delta T-cells which make this protein when exposed to P (male or female) [8].
There are two forms of PIBF. The first, or “Parent form”, has a molecular mass of 90 kDa (757 amino acid residues) and is associated with the centrosome. The PIBF gene has been identified in chromosome 13 in the vicinity of BRCA1 and 2 and p53 genes. The second, which are splice variants of the nuclear protein, are found in the cytoplasm and these have a smaller molecular mass, e.g., 35 kDa, found in the cytoplasm of leukemia cells and 57 kDa and 67 kDa found in glioblastoma multiforme cells. The 34- 35 kDa splice variant is found in mesenchymal, embryonic, and trophoblast cells of the fetal-placental unit [7].
Using leukemia cell lines, which contain the 35 kDa intracytoplasmic splice variant of PIBF, that is similar size to the one found in embryonic, mesenchymal, and trophoblast cells, we found that all 10 cell lines tested had more messenger RNA for the PIBF protein than any other known protein these cancer cells make [47].
When P was added to the media there was an up-regulation of the mRNA for the nuclear parent form of PIBF measuring 90 kDa. There was far more mRNA dedicated to making the PIBF protein than any other mRNA dedicated to production of other proteins, in these 10 leukemia cell lines. Progesterone was added to the media of four cell lines in which the intracytoplasmic 35 kDa splice variant protein had been identified. Adding P to the media significantly increased the amount of intracytoplasmic PIBF in all four cell lines. [47].
Mifepristone is a P receptor modulator. It interferes, or blocks, some P receptors, but not all. Adding mifepristone to the media of 4 leukemia cell lines making PIBF protein caused a significant decrease in intracytoplasmic PIBF.
Mifepristone also decreased the 57 kDa intrcytoplasmic PIBF in glioblastoma multiforme cells [48]. Not only does serum PIBF significantly rise after ovulation, but it will precipitously rise in menopausal women, or males, given intramuscularly or oral P [8,49].
In contrast, the following progestins do not increase serum PIBF: dydrogesterone, 17-OH progesterone (17-OHP) (Makena®), medroxyprogesterone acetate, and 19 nor-testosterone derivatives [8].
Mifepristone administration does not decrease the serum PIBF, as long as the serum P is increased [50]. Yet, taking mifepristone for just one day has a high rate of causing fetal demise. Based on these experimental studies, one may hypothesize that if inadequate production of PIBF plays a role in first trimester miscarriages, or implantation failure, by early immune rejection of the conceptus, it would seem that it is inadequate intracytoplasmic levels that may be more important than circulating levels in women who have adequate serum P levels. Certainly, low serum P levels may play a role in miscarriage, when the serum P is low.
Theoretically, a miscarriage might occur if: there is a normal early luteal phase inflammatory response, but inadequate intracytoplasmic PIBF to neutralize it. Therefore, the suggestive treatment should be supplemental P – vaginal and/or intramuscular because oral P is metabolized during the first pass through the liver and may not concentrate in the endometrium [8].
However, a miscarriage may theoretically occur if there is excessive inflammation, despite a normal PIBF response. In this case, the treatment should be increasing the progesterone levels, to increase intracytoplasmic PIBF levels above normal, to negate the excessive inflammatory response. However, another option would be to add dextroamphetamine sulfate to release more dopamine from sympathetic nerve fibers to diminish excessive cellular permeability.
One could also face both excessive inflammation and diminished PIBF expression. Ideal therapy would be both supplemental P and dextroamphetamine sulfate. The question arises as to whether there is a method to determine if despite adequate serum P level, or histologic changes on endometrial biopsy, there is insufficient PIBF secretion to suppress cellular immune rejection? Unfortunately, the answer is no. Serum PIBF only correlates with serum P, and the important area to measure is intracytoplasmic PIBF. However, that would not be possible in the intact human pregnant state.
It is not clear what the role circulating PIBF, made from gamma/ delta T cells, plays. One possibility is that it prevents early parturition. In animals, a drop in P would also cause a drop in PIBF, which immediately precedes parturition. In humans, a drop in serum P does not precede parturition. However, sensitivity of the gamma/delta T cells to produce PIBF seems to wane even when exposed to normal P levels since a drop in serum PIBF precedes labor and delivery in humans [8].
One must recall that 17-OHP (Makena®) does not increase PIBF secretion from gamma/delta T cells. Thus, it is likely that P may be superior to 17-OHP in preventing pre-term deliveries.
Progesterone supplementation to treat infertility
Can merely an inadequate secretion of P be a cause of infertility? If so, how common is it?
Over 40 years ago, Georgeanna Jones coined the term luteal phase defect, and she considered this a cause of infertility. For over 40 years, up to the present, there are some who believe P insufficiency can cause infertility, whereas others do not agree. No one challenges the concept that at least some progesterone is needed to allow a pregnancy to proceed to a live birth. However, various studies have failed to find any molecular marker that is found in the luteal phase that becomes subnormal, as long as the mid-luteal phase serum P exceeds 5 ng/mL [8].
Based on inconsistent clinical studies, but especially because not one study found a putative luteal phase molecule that is deficient, which, a priori would be needed for implantation, despite what seemingly is adequate serum P levels, led to the conclusion by the practice committee of the ASRM that “there is no evidence that luteal phase defects are a cause of infertility” [51].
Of nearly 100 molecular markers evaluated in various studies, not one evaluated if a lack of intracytoplasmic PIBF can be a cause of infertility [7]. Of course, there is no practical method at the present time to evaluate intracytoplasmic PIBF. What is a clinician supposed to do when faced with a couple with infertility, that has patent fallopian tubes, apparent normal ovulation based on regular menses, adequate mid-luteal serum P, demonstration of oocyte release by ultrasound, normal semen parameters, and normal post-coital tests?
Patients do not want to wait until the one uncontroversial study is performed and there is universal agreement on the efficacy of therapy, e.g., with P. The treating physician must decide based on the literature, lectures at meetings, and teachings from more experienced mentors, on a treatment plan that would be the appropriate therapy that best fits their patient population. For example, a good percentage of infertility specialists may recommend IVF-ET for unexplained infertility, for economic reasons that would be best for the treating physician, rather than the patient. There is no question that IVF-ET will be an effective treatment for many patients that fit the above description. However, its success could merely be related to providing a very expensive way to provide P.
To compare the efficacy of ovulation inducing drugs, versus P therapy in luteal phase defects, a study was conducted about 30 years ago [52]. In the time era that we started this study, the pervading concept was that P deficiency was the result of not attaining a mature follicle. Thus, the most common suggested treatment for women with out-of-phase endometrial biopsies was follicle maturing drugs, especially clomiphene citrate. In this study, 100 women who had unexplained infertility (at least one- year duration) and an out-of-phase late luteal phase endometrial biopsy were enrolled. Fifty-eight of these patients were found to make a mature dominant follicle (18-24 mm average diameter by ultrasound and a serum E2 >200pg/mL).
The 58 women were randomly assigned to either follicle maturing drugs (clomiphene citrate or human menopausal gonadotropin), or vaginal P in the luteal phase. Those treated with vaginal P were more likely to have a six-month clinical pregnancy (77.3%) than those treated with follicle maturing drugs (11.1%). The results are seen in Table 15. Twenty-five failing on follicular maturing drugs without P during the first six months were given luteal P during second six months, and 10 of 25 conceived, with only one miscarriage. The live delivered six-month pregnancy rate was 3.7% with follicle maturing drugs vs. 74.2% with P. Thus, for women with luteal phase defects, who make mature follicles, P was clearly superior to follicle maturing drugs [52,53].
Because a late luteal phase endometrial biopsy, that was in phase showing histologically that there was adequate P effect would not necessarily determine that an adequate amount of intracytoplasmic PIBF was being made, we decided to change our policy and provide luteal phase support to all women aged 30 or over. Because pelvic pain may be associated with increased NK cells, and then a need for a higher level of intracytoplasmic PIBF to suppress these NK cells, P support was also given to women under 30 with pelvic pain. We found that empirical use of P, for all infertile women over age 30, or under age 30 but with pelvic pain produced an 80% live delivered pregnancy rate, in 6 months [7].
Related to continued controversy about the existence of luteal phase defects, and the fact that some physicians kept doubting benefits of exclusive P in the luteal phase as a treatment of infertility, we decided to re-evaluate the efficacy of P treatment for women with unexplained infertility (no endometrial biopsy) [54]. Patient characteristics of this study are seen in Table 16. The results found clinical pregnancies in 27 of 32 women (84.3%), in an average of 4.5 treatment cycles. There were four miscarriages (15%). The live delivered pregnancy rate was 70% in six cycles of treatment. Most of these patients had been seen in other infertility clinics and had been treated with follicle maturing drugs (clomiphene, letrozole, or gonadotropins) and IUI, or IVF.
What if a woman with regular menses releases the egg before the follicle is mature? In a study conducted by our practice over 30 years ago we found a live delivered pregnancy rate of 25% in patients treated exclusively with P, 30% when treated exclusively with follicle maturing drugs and 65% when treated with both (see Table 17). Our policy is to use follicular maturation drugs in women with infertility, or recurrent miscarriage, if they release the egg before the follicle attains an E2 level of 200 pg/mL (is important in inducing P receptors in the endometrium) [53].
Thus, though a follicle maturing drug in this study seemed superior to luteal phase P alone, the group treated with exclusive follicle maturing drugs seemed to have a much higher miscarriage rate without supplemental luteal phase support with P. Though the number of patients for the aforementioned study was small, the data suggested that luteal phase P may help to reduce the chance of a miscarriage, in the women with a luteal phase defect and immature follicles treated with follicle maturing drugs [53].
Though supplemental P may help to establish a pregnancy in infertile women, and lower the risk of miscarriage in those who conceive, the possibility exists that women who have no trouble conceiving, but have miscarriages, may have a different etiology, than merely the need for extra P. The importance of P supplementation as a method to reduce miscarriage risk has been a subject of debate. Small studies can sometimes lead to erroneous conclusions. Most researchers would agree that a proper randomized prospective study, that has a great deal of power, that is multi-centered, and published in an extremely well-respected journal, may produce the final word.
Such a study was published in the New England Journal of Medicine (NEJM), The PROMISE study [55]. There were 35 authors and more than 4000 women recruited. Though published in 2015, it was recently mentioned again in an issue of the NEJM that features new studies that refute old concepts. It was featured on CNN News in May, 2019 with the title “Progesterone therapy prevents miscarriage for only some women, study finds: 75% of the P group had a live baby vs. 72% of the controls”!!
But what good is a properly randomized, multi-center study, with a large study population if the experimental design is flawed [56]?. Based on our experimental model, the most critical time to suppress immune damage to the fetal placental unit is at the time of invasion of the fetal placental unit, six days after ovulation. Thus, the most critical time to start P therapy is immediately after ovulation. This allows adequate PIBF production by the embryonic cells, mesenchymal cells and trophoblast cells. In the PROMISE study, the P was started shortly after the first positive beta-hCG level, or at least within the first two weeks from confirmation of pregnancy. Thus, a big flaw in the study’s experimental design was starting the progesterone much too late!!
Other clinicians have, subsequent to the PROMISE study, found that supplemental P in the early luteal phase decreases miscarriage rates [57]. Stephenson, et al., had the same criticism of the PROMISE study, i.e., the late use of P. Stephenson’s study was an observational cohort study using prospectively collected data. Possibly women with elevated glandular nuclear cyclin E expression (>70%), as determined by endometrial biopsy, may be more prone to repeat miscarriage. This was the group receiving supplemental P in the early luteal phase. Controls were those with normal glandular nuclear cystin E (<20%). Most did not receive P, but some insisted on it, so they were in the treated group.
Overall, 68.5% were successful using P treatment vs. 51% not using it. The authors stated that they are planning a randomized controlled “well-designed” study to further evaluate the efficacy of P in reducing miscarriage. So, the question arises as to whether be it ever worth starting P during a pregnancy, if the level is already low? Is it futile?
Timothy Yeko found that 17 of 18 pregnant women, whose serum P was less than 15 ng/mL, had a miscarriage [58]. However, we found that with aggressive P treatment (IM and vaginal) that 70% of pregnant women whose serum P was <15 ng/mL and 60% of women whose serum P was <8 ng/mL, had a live baby [59,60].
Not only is the PIBF protein found in the cytoplasm of fetal embryonic cells, mesenchymal cells, and trophoblast cells, it is also found in the cytoplasm of many different types of cancer cells [61]. If the PIBF protein is important in helping the fetal-placental unit, and also cancer cells, to escape immune surveillance, AND if supplementing P can successively treat infertility, miscarriage, and even pre-term delivery, THEN using a P receptor antagonist to suppress intracytoplasmic PIBF in cancer cells should be able to provide increased longevity and palliation for patients with cancer.
Indeed, oral mifepristone, the P receptor modulator/antagonist, has been found to stop many cancers from progressing (see Table 18) [62-70]. Many of these cancers are not associated with the classic nuclear P receptor. The possible role of PIBF and membrane P receptors has been summarized in several publications [7,46,71,72]. It should be noted, however, that the concept that PIBF protein utilized by the fetal placental unit to evade immune surveillance, may be sometimes used by cancer cells to evade immune surveillance, was presented as a hypothesis in 2001, and it was predicted that this could lead to unique methods to treat cancer [73,74].
Summary
A model of embryo implantation, based on experimental data, has been presented. Successful implantation requires methods to induce a proper inflammatory response, to create spiral arteries needed for nutrient exchange between mother and fetus. Insufficient inflammation can be one cause of implantation failure. This may be seen more commonly when taking follicle maturing drugs. One way to improve the defect is to create a greater inflammatory response, by performing an endometrial scratch.
However, excessive inflammation can also be a cause of implantation failure, with cellular immune rejection of the fetus. Excessive inflammation may result from increased cellular permeability, possibly related to inadequate release of dopamine from sympathetic nerve fibers. Infertility, or miscarriage issues, can be achieved by using drugs, e.g., sympathomimetic amines, that release more dopamine from sympathetic nerve fibers.
Excessive permeability, leading to excessive infiltration of toxic elements into pelvic tissue, can lead to pelvic pain. However, the permeability defect can extend to other tissues leading to a variety of chronic pathological conditions that despite being refractory to standard therapy, responds very well to sympathomimetic amines. The original concept of using dextroamphetamine sulfate was to release more dopamine, based on this model of implantation. Possibly, one could find with future studies that the efficacy of dextroamphetamine sulfate may be related to an entirely different mechanism.
Though experience finds that the dosages of amphetamines used does not lead to withdrawal, or dependence, there still exists widespread concerns about this class II drug and, its use is precluded by many governmental agencies. Evaluation of other drugs releasing dopamine, e.g., cabergoline, which is less controversial, are presently being evaluated.
The authors, based on their experience, find it hard to believe that there still remains controversy about the beneficial effects of P for improving fecundity. Based on the experimental model, the hypothesized mechanism for the main beneficial effects of P on fecundity is through the production of the immunomodulatory PIBF protein. The concept that its main effect is on the fetal microenvironment would explain why one cannot develop a test, as yet, that determines if P levels are adequate or not.
Thus, one should use P in situations of infertility in women of advancing reproductive age, diminished oocyte reserve, or those with pelvic pain. With no down-side, it should also be given to women with a history of miscarriage.
Future studies could determine that the main beneficial effect of P is not through the PIBF protein. However, based on this implantation model, with the thought that cancer cells could also use the PIBF protein to escape immune surveillance, led to a model of potential therapy that can provide a highly effective novel therapy for treating advanced cancers. Of course, future studies could determine that P receptor antagonists work through a different mechanism. Unfortunately, similar to amphetamine, mifepristone is approved as an abortifacient, and thus to satisfy some anti-abortion groups, its widespread off-label use has been thwarted.
Based on this experimental model, unique therapies have been developed that are effective for a wide variety of clinical illnesses, despite failure to respond to standard therapies and unique effective treatment for advanced cancers.





References
- Szekeres-Bartho J, Wegmann A progesterone-dependent immune-modulatory protein alters the Th3/Th2 balance. J Reprod Immunol. 1996; 31: 81-95.
- Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004; 63: 1-12.
- Check JH, Aly J, Chang Improving the chance of successful implantation- part I-embryo attachment to the endometrium and adequate trophoblast invasion. Clin Exp Obst Gynecol. 2016; 43: 787-791.
- Check JH, Aly J. Improving the chance of successful implantation -part 2-Circumventing immune rejection and the fetal semi-allograft. Clin Exp Obst Gyn. 2018; 45: 9-13.
- Check JH, Szekeres Bartho J, O'Shaughnessy Progesterone induced blocking factor seen in pregnancy lymphocytes soon after implantation. Am J Reprod Immunol. 1996; 35: 277-280.
- Check JH, Arwitz M, Gross J, et al. Evidence that the expression of progesterone-induced blocking factor by maternal T-lymphocytes is positively correlated with conception. Am J Reprod Immunol. 1997; 38: 6-8.
- Check JH, Cohen R. The role of progesterone and the progesterone receptor in human reproduction and Exp Rev Endocrinol Metab. 2013; 8: 469-484.
- Cohen RA, Check JH, Dougherty MP. Evidence that exposure to progesterone alone is a sufficient stimulus to cause a precipitous rise in the immunomodulatory protein the progesterone induced blocking factor (PIBF). J Assist Reprod Genet. 2016; 33: 221-229.
- Faust Z, Laskarin G, Rukavina D, et Progesterone-induced blocking factor inhibits degranulation of NK cells. Am J Reprod Immunol. 1999; 42: 71-75.
- Chang E, Check JH, Liss JR, et al. An endometrial scratch can improve pregnancy rates in natural cycles of women with unexplained infertility given luteal phase Fertil Steril. 2017; 108: e369.
- Check JH, Choe JK, Cohen R, et Effect of taking a onetime injection of one mg leuprolide acetate three days after embryo transfer on pregnancy outcome and level of first beta human chorionic gonadotropin (beta-hCG) level. Clin Exp Obstet Gynecol. 2015; 42: 568-570.
- Gibreel A, Badawy A, El-Refai W, et endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res. 2013; 39: 680-684.
- Vitagliano A, Noventa M, Saccone G. Endometrial scratch injury before intrauterine insemination: is it time to re- evaluate its value? Evidence from a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2018; 109: 84-96.
- Duffy JMN, Arambage K, Correa FJS, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014; 4: CD011031.
- Nowroozi K, Chase JS, Check JH, et al. The importance of laparoscopic coagulation of mild endometriosis in infertile women. Int J Fertil. 1987; 32: 442-444.
- Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild N Eng J Med. 1997; 337: 217-222.
- Parazzini F. Ablation of lesions or no treatment in minimal- mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum 1999; 14: 1332-1334.
- Check JH, Wilson C. Dramatic relief of chronic pelvic pain with treatment with sympathomimetic amines – case report. Clin Exp Obstet Gynecol. 2007; 34: 55-56.
- Check JH, Cohen R. Chronic pelvic pain- traditional and novel therapies: Part II medical therapy. Clin Exp Obst Gyn. 2011; 38: 113-118.
- Check JH, Jaffe A. Resolution of pelvic pain related to adenomyosis following treatment with dextroamphetamine sulfate. Clin Exp Obstet Gynecol. 2015; 42: 671-672.
- Check Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obst Gynecol. 2016; 43: 109-111.
- Carpentier PA, Meier B, Check JH, et al. Sympathomimetic amine treatment very effective for relieving pelvic pain in women even when hormonal therapy and surgery were not sufficient. Fertil Steril. 2020; 114: e203.
- Check JH, Chern R, Katsoff B. Prevention of first-trimester miscarriage with dextroamphetamine sulfate treatment in women with recurrent miscarriage following embryo transfer- case report. Clin Exp Obstet Gynecol. 2014; 40: 471-472.
- Check JH, Check DL, Cohen R, et al. The use of dextroamphetamine sulfate to alleviate pelvic pain does not lower live delivered pregnancy rates following IVF-ET in younger women. Fertil Steril. 2019; 112: e322.
- Check JH, Cohen R, Check D, et Sympathomimetic amine therapy may improve live delivered pregnancy rates following IVF-ET in women of advanced reproductive age-a pilot study. Fertil Steril. 2019; 112: e325.
- Check JH. Increased tissue permeability and sympathetic nervous system hypofunction may be the common link between dysmenorrhea, chronic pelvic pain, Mittelschmerz, and Crohn’s disease. Clin Exp Obst Gynecol. 2016; 43: 112-
- Check JH, Liss JR, Check ML, et al. Lymphocyte immunotherapy can improve pregnancy outcome following embryo transfer (ET) in patients failing to conceive after two previous ET. Clin Exp Obstet Gynecol. 2005; 32: 21-22.
- Nazari A, Check JH, Epstein R, et Relationship of small-for-dates sac size to crown-rump length and spontaneous abortion in patients with a known date of ovulation. Obstet Gynecol. 1991; 78: 369-373.
- Check JH, Cohen The triad of luteal phase ocular migraines, interstitial cystitis, and dyspareunia as a result of sympathetic nervous system hypofunction. Clin Exp Obst Gyn. 2014; 41: 575-577.
- Check JH, Dougherty MP. Use of sympathomimetic amines to correct premenstrual urticaria and anaphylaxis. Clin Exp Obstet Gynecol. 2019; 46: 309-312.
- Check DL, Check JH, Katsoff Dextroamphetamine sulfate therapy markedly improves the chronic fatigue syndrome. J Nurs Occup Health. 2020; 2: 146-148.
- Check JH, Katsoff B, Cohen R. A novel highly effective medical treatment of severe treatment refractory Crohn’s disease using sympathomimetic amines- case report. Inflam Bowel Dis. 2010; 16: 1999-2000.
- Check JH, Katsoff B, Cohen A case report showing that a woman with ulcerative colitis refractory to standard therapy responded well to the sympathomimetic amine dextroamphetamine sulfate. Inflam Bowel Dis. 2011; 17: 870- 871.
- Check JH, Cohen R, Check D. Evidence that migraine headaches in women may be related to a common defect in the sympathetic nervous system as evidenced by marked improvement following treatment with sympathomimetic amines. Clin Exp Obst Gyn. 2011; 38: 180-181.
- Check DL, Check JH, Citerone T, et al. Sympathomimetic amine therapy markedly improves severe fatigue that diminishes quality of life in patients with cancer-A case Cancer Sci Res. 2020; 3: 1-3.
- Potestio CP, Check JH, Mitchell-Williams Improvement in symptoms of the syndrome of mitochondrial encephalopathy, lactic-acidosis, and stroke-like symptoms (MELAS) following treatment with sympathomimetic amines- possible implications for improving fecundity in women of advanced reproductive age. Clin Exp Obstet Gynecol. 2014; 41: 343-345.
- Leskowitz SC, Shanis BS, Check JH. Resolution of atypical chest pain during treatment for idiopathic orthostatic edema. Am J Gastroenterology. 1990; 85: 621-622.
- Boimel P, Check JH, Katsoff D. Sympathomimetic amine therapy may improve refractory gastroparesis similar to its effect on chronic pelvic pain-case report. Clin Exp Obstet Gynecol. 2007; 34: 185-187.
- Check JH, Cohen R. Marked improvement of severe gastroparesis following high dosage, but very well tolerated, dextroamphetamine Clin Exp Obst Gynecol. 2017; 44: 611-612.
- Check JH, Cohen R. Successful treatment of a female with chronic pseudo-intestinal obstruction with sympathomimetic amines and thyroid hormone replacement. Clin Exp Obst Gyn.2010; 37: 115-116.
- Check JH, Katsoff The use of sympathomimetic amines for the treatment of severe constipation refractory to conventional therapy- case report. Clin Exp Obst Gyn. 2013; 40: 284-285.
- Check JH, Katsoff D, Kaplan H, et al. A disorder of sympathomimetic amines leading to increased vascular permeability may be the etiologic factor in various treatment refractory health problems in Med Hypothesis. 2008; 70: 671-677.
- Check JH, Cohen R, Katsoff B, et al. Hypofunction of the sympathetic nervous system is an etiologic factor for a wide variety of chronic treatment-refractory pathologic disorders which all respond to therapy with sympathomimetic amines. Med Hypoth. 2011; 77: 717-725.
- Check JH. Changing the name of a syndrome: Sympathetic neural hyperalgesia edema syndrome becomes – the increased cellular permeability Clin Exp Obst Gyn. 2017; 44: 819-823.
- Check DL, Check JH. Various presentations of the increased cellular permeability syndrome in males responding very well to sympathomimetic amine therapy – possible treatment for end-stage Covid-19 complications. J Med Clin Res & Rev. 2020; 4: 1-7.
- Check JH, Check D. Therapy aimed to suppress the production of the immunosuppressive protein progesterone induced blocking factor (PIBF) may provide palliation and/ or increased longevity for patients with a variety of different advanced cancers – A review. Anticancer Res. 2019; 39: 3365-3372.
- Srivastava, Thomas A, Srivastava BI, et al. Expression and modulation of progesterone induced blocking factor (PIBF) and innate immune factors in human leukemia cell lines by progesterone and mifepristone. Leuk Lymphoma. 2007; 48: 1610-1617.
- Zamora-Sanchez CJ, Hansberg-Pastor V, Salido-Guadarrama I, et al. Allopregnanolone promotes proliferation and differential gene expression in human glioblastoma cells. Steroids. 2017; 119: 36-42.
- Check JH, DiAntonio A, Check DL, et A study to determine if estrogen (E) is needed to induce de novo progesterone (P) receptors on gamma/delta T-cells as evidenced by determining the degree of rise of progesterone induced blocking factor (PIBF) following P exposure in males. Clin Exp Obst Gyn. 2020; 47: 419-420.
- Check JH, DiAntonio G, DiAntonio A, et The progesterone receptor antagonist mifepristone does not lower serum progesterone induced blocking factor (PIBF) in the presence of progesterone. Clin Exp Obst Gynecol. 2016; 43: 189-191.
- Check JH. An Editor’s opinion of the recent committee opinion of the American Society for Reproductive Medicine that the luteal phase deficiency as a clinical entity causing infertility has not been proven. Clin Exp Obst Gynecol. 2016;43: 479-483.
- Check JH, Nowroozi K, Wu CH, et al. Ovulation inducing drugs versus progesterone therapy for infertility in patients with luteal phase defects. Int J Fertil. 1988; 33: 252 256.
- Check JH. Progesterone therapy versus follicle maturing drugs - possible opposite effects on embryo Clin Exp Obst Gyn. 2002; 29: 5-10.
- Check JH, Liss J, Check D. The beneficial effect of luteal phase support on pregnancy rates in women with unexplained infertility. Clin Exp Obstet Gynecol. 2019; 46: 447-449.
- Coomarasamy A, Williams H, Truchanowicz E, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med. 2015; 373: 2141-2148.
- Check Pros and cons of the use of progesterone to reduce miscarriage rates. Clin Exp Obst Gyn. 2018; 45: 652-655.
- Stephenson, McQueen D, Winter M, et Luteal start vaginal micronized progesterone improves pregnancy success in women with recurrent pregnancy loss. Fertil Steril. 2017; 107: 684-690.
- Yeko TR, Gorrill MJ, Hughes LH, et al. Timely diagnosis of early ectopic pregnancy using a single blood progesterone measurement. Fertil Steril. 1987; 48: 1048-1050.
- Check JH, Winkel CA, Check ML. Abortion rate in progesterone treated women presenting initially with low first trimester serum progesterone levels. The Am J Gyn Health. 1990; 2: 33-34.
- Choe JK, Check JH, Nowroozi K, et al. Serum progesterone and 17-hydroxyprogesterone in the diagnosis of ectopic pregnancies and the value of progesterone replacement in intrauterine pregnancies when serum progesterone levels are low. Gynecol Obstet Invest. 1992; 34: 133-138.
- Lachmann M, Gelbmann D, Kalman E, et al. PIBF (progesterone induced blocking factor) is overexpressed in highly proliferating cells and associated with the Int J Cancer. 2004; 112: 51-60.
- Check JH, Dix E, Sansoucie L, Check Mifepristone may halt progression of extensively metastatic human adenocarcinoma of the colon – case report. Anticancer Res. 2009; 29: 1611- 1613.
- Check JH, Dix E, Cohen R, et Efficacy of the progesterone receptor antagonist mifepristone for palliative therapy of patients with a variety of advanced cancer types. Anticancer Res. 2010; 30: 623-628.
- Check JH, Wilson C, Cohen R, et al. Evidence that mifepristone, a progesterone receptor antagonist can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme grade Anticancer Res. 2014; 34: 2385-2388.
- Check JH, Check D, Wilson C, et al. Long-term high-quality survival with single-agent mifepristone treatment despite advanced cancer. Anticancer Res. 2016; 36: 6511-6513.
- Check JH, Check D, Poretta T. Mifepristone extends both length and quality of life in a patient with advanced non-small cell lung cancer that has progressed despite chemotherapy and a check-point Anticancer Res. 2019; 39: 1923-1926.
- Check DL, Check Significant palliative benefits of single agent mifepristone for advanced lung cancer that previously failed standard therapy. Med Clin Sci. 2019; 1: 1-5.
- Check DL, Check JH, Poretta T, et Prolonged high-quality life in patients with non-small cell lung cancer treated with mifepristone who advanced despite osimertinib. Cancer Sci Res. 2020; 3: 1-5.
- Check DL, Check JH, Poretta T. Conservative laparoscopic surgery plus mifepristone for treating multifocal renal cell carcinoma. Cancer Sci Res. 2020; 3: 1-4.
- Check JH, Check D, Srivastava, et al. Treatment with mifepristone allows a patient with end stage pancreatic cancer in hospice on a morphine drip to restore a decent quality of life. Anticancer Res. 2020; 40: 6997-7001.
- Check The role of progesterone and the progesterone receptor in cancer. Expert Review Endo Metab. 2017; 72: 187-197.
- Check JH, Check DL. Progesterone and Glucocorticoid Receptor Modulator Mifepristone (RU-486) as Treatment for Advanced Cancers. Intech Open. 2020.
- Check JH, Nazari P, Goldberg J, et al. A model for potential tumor immunotherapy based on knowledge of immune mechanisms responsible for spontaneous abortion. Med Hypoth. 2001; 57: 337-343.
- Check JH, Dix E, Sansoucie Support for the hypothesis that successful immunotherapy of various cancers can be achieved by inhibiting progesterone associated immunomodulatory protein. Med Hypoth. 2009; 72: 87-90.