Ovarian Stimulation Increases the Risk of Fetal Cardiac Defects of Pups Exposed to Severe Maternal Hyperglycemia
Author'(s): Rolanda Lister MD1, Etoi Garrison MD1, Francine Hughes MD2, Scott Baldwin MD, PhD3, Bin Zhou MD, PhD4
1Vanderbilt University Medical Center, Department of Obstetrics and Gynecology, B-1100 Medical Center North, TN, US.
2New York University, Department of Obstetrics and Gynecology, 150 East 32nd Street, New York, NY, US.
3Vanderbilt University Medical Center, Department of Pediatrics, 2200 Children's Way, 5230 Doctors' Office Tower, TN, US.
4Albert Einstein College of Medicine, Department of Pediatrics, Department of Genetics, Department of Medicine, Michael F. Price Center, 1301 Morris Park Avenue, NY, US.
*Correspondence:
Rolanda Lister, Vanderbilt University Medical Center, Department of Obstetrics and Gynecology, B-1100 Medical Center North, Nashville, TN, US, E-mail: Rolanda.l.lister@vumc.org.
Received: 02 January 2018 Accepted: 23 January 2019
Citation: Rolanda Lister, Etoi Garrison, Francine Hughes, et al. Ovarian Stimulation Increases the Risk of Fetal Cardiac Defects of Pups Exposed to Severe Maternal Hyperglycemia. Gynecol Reprod Health. 2019; 3(1): 1-5.
Abstract
Objectives: To study the incidence of congenital heart defects (CHD) in offspring born to hyperglycemic mothers with and without ovarian stimulation.
Design: Reproductive biology
Setting: Mouse model
Patients: N/A
Intervention: Hyperglycemia was induced in CD-1 wild type female mice using a single intraperitoneal dose of 150 mg/kg of streptozotocin. Stimulated dams (SD); (n=3) were injected with pregnant mare serum and human chorionic gonadotropin 48 hours apart. Non-stimulated dams (NSD); (n=4) were not injected. Both groups were mated with normal male CD-1 mice for timed pregnancies. Fetal hearts were extracted on embryonic day 16.5 and histological analyses was performed. Student's t-tests were employed to compare the incidence of cardiac defects in the SD and NSD groups. P ≤ 0.05 was significant.
Main outcome measure: The incidence of CHD in progeny of diabetic dams with and without ovarian hyperstimulation.
Results & Conclusions: The average litter size was higher in SD compared to NSD. The average blood glucose for the SD and NSD was similar. Overall, the incidence of cardiac malformations did not differ between the two groups. However, in severe maternal hyperglycemia (>400 mg/dL), there was a higher incidence of fetal cardiac malformations in the pups born to SD vs NSD.
Conclusion: High-grade lesions are the fundamental step in the evolution of cervical dysplasias to cervical cancer. The management of these lesions is essential in the prevention of cervical cancer.
Keywords
Introduction
Assisted reproductive technology (ART) utilization has tripled over the last two decades [1]. Approximately 1.6% of all pregnancies in the United States are conceived with the use of (ART) [1]. ART includes the handling of oocytes, in vitro fertilization (IVF) and embryo transfer and intracytoplasmic sperm injection (ICSI) [1]. It is often preceded by ovulation induction. Infertile couples employ one or any combination of these ART techniques.
Diabetes impairs reproductive function and is associated with subfertility and may lead to an increased uptake of ART [2]. The most common cause of infertility in women is anovulation due to poly-cystic ovarian syndrome (PCOS) [3]. Obese women are more likely to have polycystic ovarian syndrome, insulin resistance, sub-fertility and diabetes. IVF is a well-described independent risk factor for the development of CHD [4-6]. Because of this association, it is standard practice to refer patients who have undergone IVF to a fetal echocardiogram to screen for CHD [7].
Diabetes affects 2% of all pregnancies amounting to over three million women in the U.S. alone [8]. Diabetes in mothers who undergo fertility treatments are often not optimally controlled preconception prior to ART[9]. The obesity epidemic has led to the increase of Type 2 Diabetes Mellitus (T2DM) and consequently an increase in the incidence of diabetes-related heart defects in the offspring [10,11]. Infants of mothers with diabetes are five times more likely to have CHD [12-14]. Survivors suffer long- term morbidity such as valve disease, cognitive delay, and life- threatening arrhythmias even after surgical correction of CHD [15]. The incidence of congenital anomalies is directly and positively correlated to first trimester hemoglobin A1C during the time of embryogenesis and sharply rises with A1C >12 corresponding to severe hyperglycemia regardless of Type 1 or Type 2 DM status [16]. Excess maternal glucose passing through the placenta to the fetal circulation leads to enhanced mitochondrial activity, production of reactive oxygen species and oxidative stress provoking apoptotic cell death in the development of diabetic embryopathy [17]. Hyperglycemia alters gene expression at various stages of heart development. However, it is unclear if the combination of ovarian stimulation and pre-gestational diabetes increases the incidence of CHD. Thus, our objective is to compare the rates of CHD in mouse pups born to diabetic mothers with and without ovarian stimulation.
Our model utilizes the streptozotocin (STZ) induced diabetes mouse model because it is a well-established and highly effective way to induce severe maternal hyperglycemia by selectively eliminating the insulin producing beta islet cells of the pancreas [18]. Ovarian stimulation using pregnant mare serum (PMS) and human chorionic gonadotropin (HCG) recapitulates the preimplantation stimulation technique used clinically whereby, women are administered gonadotropins to stimulate ovarian follicle development and then administered HCG to induce ovulation [19]. It is not known known which level (ovarian stimulation, intracytoplasmic sperm injection or embryo transfer) of the ART leads to increased CHD. This study aims to examine the role of ovarian stimulation in the development of CHD in the setting of maternal hyperglycemia or diabetes. We sought to explore whether allowing the diabetic female mice that undergo ovarian stimulation further increases the incidence of CHD.
Methods
Animal husbandry
Animals were housed in micro-isolation colorless cages and given food and water ad libitum, except when fasting prior to blood glucose measurements. Housing rooms were temperature controlled with 12 hours alternating light and dark cycle. All mouse experiments were performed according to the guidelines of the National Institutes of Health and the protocol approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Induction of maternal diabetes and ovarian stimulation Hyperglycemia was induced in 8-week-old CD-1 wild type female mice using a single intraperitoneal dose of 150 mg/kg of STZ that acts to delete beta-islet cells of the pancreas. After fasting animals for 6 hours, hyperglycemia was confirmed in all mice (defined as >200 mg/dl blood glucose) using a One Touch commercial glucometer. Glucose levels were measured in weekly intervals for 2 weeks before mating and on the day of cesarean/sacrificing the dams. The glucose values (mg/dL) reported represent an average of pregestational hyperglycemia and the glucose level at the time of cesarean for each mouse. Ovarian stimulation of experimental animals (n=3) consisted of injecting each mouse with 8 IU/kg pregnant mare serum (PMS) and human chorionic gonadotropin (HCG) 48 hours apart. Control animals with hyperglycemia (n=4) were not injected.
Timed mating
At 10 weeks of age, both hyperglycemic stimulated dams (SD; n=3) and hyperglycemic non-stimulated dams (NSD; n=4) were mated with normal male CD-1 mice for timed pregnancies. Noon on the day of observing vaginal plugs was designated as embryonic day (E) 0.5. The pregnant dams were euthanized at embryonic day (E) 16.5. A cesarean section was performed to extract the fetuses from the uterine horns of the SD and NSD. The fetuses of the SD and NSD were designated stimulated pups (SP) and non-stimulated pups (NSP) respectively. Thoracotomies were performed on the fetuses in both groups to retrieve the hearts.
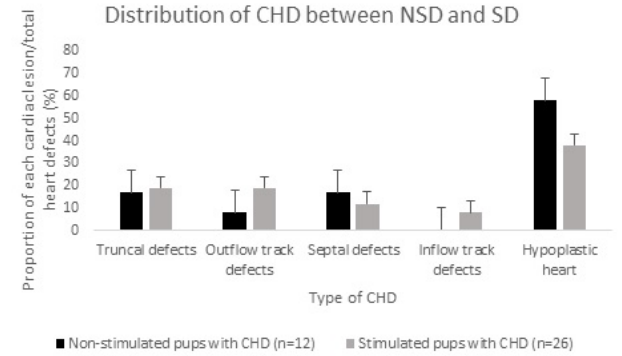
Figure 1: Proportion of each cardiac lesion in both Non-stimulated pups (NSP) and Stimulated pups (SP) over total cardiac defects.
As compared to NSP, the incidence of truncal defects (DORV, truncus arterios, transposition of great vessels) in the SP were increased (2/12 vs. 5/26; *p=0.05). The incidence of outflow track defects (aortic stenosis, pulmonary stenosis), septal defects (ventricle septal defects), inflow track defects (endocardial cushion, mitral valve stenosis) were not different (p=0.61, 0.37,and 0.42 respectively). However, the incidence of hypoplastic heart (hypoplastic left or right heart) was increased in the NSP compared to the SP (7/12 vs 10/26; *p=0.04). Data is presented in standard error of the mean.
Histological analysis
On E 16.5, fetal hearts were collected from hyperglycemic SD and NSD. Gross morphological analysis was done at the time of dissection. The hearts were isolated from the fetal thorax, formalin fixed and paraffin embedded and processed for histology. Slides were deparaffinized with xylene prior to staining with hematoxylin and eosin (H&E) and imaged using Olympus DXS-high-resolution microscope at 5x, 10x and 20-x magnification. The morphology of each heart was determined adequate if the following structures were identified: left ventricle, right ventricle, outflow tracts, mitral valve, tricuspid valves and interventricular septum. Heart defects were classified into the following groups: inflow track, outflow track, septal, truncal and hypoplastic heart. If there were a combination of defects (i.e ventricle septal defect plus double outlet right ventricle), that defect would be assigned the most appropriate single diagnosis (i.e double outlet right ventricle) and not counted twice (once for ventricle septal defect and once for double outlet right ventricle). A single blinded examiner performed the interpretation of morphology and a second blinded examiner, a co-investigator, determined any uncertainty with diagnosis.
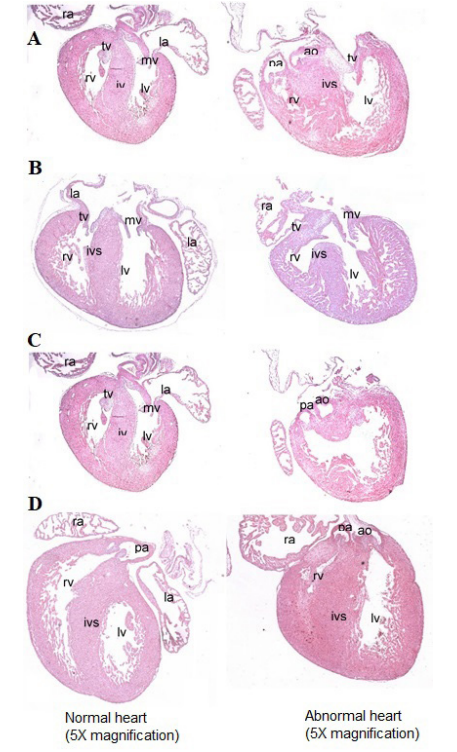
Figure 2: Hematoxylin & Eosin Staining 5x magnification of abnormal histology examples (right side) in both pup hearts from SD and NSD in hyperglycemic mice compared to normal hearts (left side).
A.Truncal defect i.e. Double outlet right ventricle. B. Inflow track defect (i.e endocardial cushion defect). C. Septal defects (i.e ventricular septal defect). D. Hypoplastic heart (i.e hypoplastic right heart syndrome). Not shown is pulmonary and aortic stenosis. Hematoxylin & Eosin staining 5 X magnification. Abbreviations: Pulmonary artery (Pa), aorta (ao), aortic valve (av), mitral valve (mv), pulmonary valve (pv), right ventricle (rv), left ventricle (lv).
Statistics
The data is presented as mean ± standard deviation (SD) and standard error of the mean (SEM). We used the program Microsoft Excel to generate the mean, SD, SEM and Student’s t-tests for each group. Student’s t-tests were employed to compare the incidence of cardiac defects in the SP and NSP respectively. A p-value ≤ 0.05 was significant.
Results
Induction of maternal diabetes & ovarian stimulation Maternal hyperglycemia was successfully attained with the single injection of 150 mg/kg STZ in all 7 mice that were injected. The average blood glucose for the SD and NSD did not differ significantly (452 ± 200 vs 413 ± 124 mg/dL; p= 0.76) shown in Table 1. After injecting the 3 dams with PMS and HCG 48 hours apart, the average litter size was significantly higher in SD dams compared to NSD (18.69 ± 1.15 vs 12 ± 2.94; p=0.015).
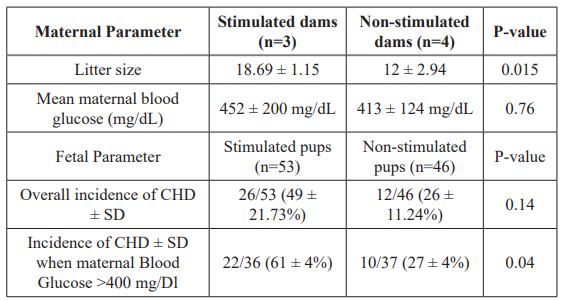
Table 1: The litter size was significantly higher for the stimulated diabetic group indicating ovarian response to pregnant mare serum and HCG. The mean glucose concentrations ± SD were similar in both groups.
Overall, the incidence of CHD were similar between both the stimulated pups and non-stimulated pups. However stimulated pups born to severely hyperglycemic mothers with glucose concentrations >400 mg/dL, there was a significant increase in the incidence of CHD. Data is presented as mean percentage between all litters ± SD.
Histological analysis
Overall, the incidence of cardiac malformations showed a non- significant increasing trend between the SD and the NSD (46%+/- 21 vs 26%+/-11; p=0.16) respectively. However, in severe maternal hyperglycemia (>400 mg/dL), there was a greater than a two-fold increase in the incidence of fetal cardiac malformations in the pups born to the SD. Twenty-two of 36 SD pups demonstrated cardiac malformations (61 ± 4%) vs 10 of 37 for NSD pups (27 ± 4%); (p=0.04).
Maternal data is presented in Table 1. Fetal data is presented in Table 2 and is represented as stimulated pups (SP) and non- stimulated pups (NSP). As compared to NSP, the incidence of truncal defects (DORV, truncus arterios, transposition of great vessels) in the SP were increased (2/12 vs. 5/26; *p=0.05). The incidence of outflow track defects (aortic stenosis, pulmonary stenosis), septal defects (ventricle septal defects), inflow track defects (endocardial cushion, mitral valve stenosis) were not different (p=0.61, 0.37,and 0.42 respectively). However, the incidence of hypoplastic heart (hypoplastic left or right heart) was increased in the NSP compared to the SP (7/12 vs 10/26; p=0.04).
While severe hyperglycemia (maternal blood glucose>400mg/dL) is associated with an increase in the incidence of CHD, the severity of the CHD is however variable between the SD and NSD and no distinct pattern is observed based on ovarian stimulation status.
Discussion
In summary, our data show that the use of PMS and HCG is successful in increasing the yield of embryos in a diabetic cohort. Although our data does not show that ovarian stimulation in diabetic dams significantly increased the incidence of cardiac defects overall, there is a significant increase in the incidence of CHD of severely hyperglycemic dams >400 mg/dL. Currently, there is no standard approach to management of diabetic patients prior to ART. Because of the known association of maternal diabetes and CHD, patients are often advised to control their blood glucose levels and normalize A1C prior to achieving spontaneous pregnancy. Perhaps normalization of A1C prior to ovarian stimulation should be an additional goal to further reduce the incidence of CHD. This observation highlights the importance of glycemic control prior to ovarian stimulation in a diabetic cohort.
Our data demonstrate a heterogeneous array of CHD associated with pregestational diabetes. While overall, the incidence of CHD is not modified in the presence of ovarian stimulation, the severity of the CHD is variable. Although the presence of hyperglycemia is associated with cardiac defects the underlying reason for the heterogeneous nature of the defects is still a question. For example, the types of heart defects range from ventricle septal defects to severe lesions such as double outlet right ventricle and hypoplastic left and right heart syndrome. It is not well understood why littermates exposed to the same hyperglycemic environment and ovarian hyperstimulation have different types of cardiac lesions ranging from normal to complex cardiac lesions.
We have created a mouse model of STZ induced pregestational maternal diabetes followed by ovarian stimulation that produces significant increases in abnormal cardiac phenotypes as the severity of hyperglycemia exceeds 400mg/dL. Additionally, we were able to stratify the incidence of CHD with the severity of pregestational diabetes and identify how ovarian stimulation affects this incidence. Our goal was to identify if ovarian stimulation modified the incidence of CHD in embryos exposed to maternal diabetes. However, we do acknowledge the absence of ovarian stimulation in non-diabetic animals thus we cannot make any determinations on the role of ovarian stimulation alone in the development of CHD but will make this a future direction.
Additionally, the proposed mechanism of increased CHD in the setting of ART is largely unknown. However, imprinting disorders leading to abnormal methylation, such as Beckmann Weidman Syndrome are known to be more common in IVF pregnancies[20]. Because of this association, some have suggested that abnormal DNA methylation leading to abnormal gene expression may be a potential culprit. Future experimentation will address the differences in DNA methylation and differential expression between stimulated and non-stimulated pup hearts exposed to maternal hyperglycemia.
Conclusion
In a murine model of severe maternal hyperglycemia, ovarian stimulation increases litter size and the propensity of developing CHD in their offspring. However, this observation may have important clinical and fetal implications for patients who have pre- gestational diabetes and receive ART to achieve pregnancy.
Financial support
- Reproductive Scientist Development Program Scholarship- 5K12HD000849-26.
- Vanderbilt’s Diabetes Research Training Center-Pilot and Feasibility Grant 4042241451.
- National Institutes of Health: NIH-K01HL140278-01
- Building Interdisciplinary Careers in Women’s Health 2K12HD043483-17.
References
- Sunderam S, Kissin DM, Crawford SB, et al. Assisted Reproductive Technology Surveillance- United States, 2014. MMWR Surveill Summ. 2017; 66: 1-24.
- Amaral S, Oliveira PJ, Ramalho-Santos Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008; 4: 46-54.
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995; 333: 853-861.
- Sala P, Ferrero S, Buffi D, et Congenital defects in assisted reproductive technology pregnancies. Minerva Ginecol. 2011; 63: 227-235.
- Buckett WM, Chian RC, Holzer H, et al. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007; 110: 885-891.
- Fedder J, Loft A, Parner ET, et al. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Hum Reprod. 2013; 28: 230-240.
- Nayak K, Naveen Chandra GS, Ranjan Shetty, et Evaluation of fetal echocardiography as a routine antenatal screening tool for detection of congenital heart disease. Cardiovasc Diagn Ther. 2016; 6: 44-49.
- Gabbay-Benziv R, Reece EA, Wang F, et al. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015; 6: 481-488.
- Lazaridou S, Dinas K, Tziomalos Prevalence, pathogenesis and management of prediabetes and type 2 diabetes mellitus in patients with polycystic ovary syndrome. Hormones (Athens). 2017; 16: 373-380.
- Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008; 57: 1-5.
- Lawrence JM, Contreras R, Chen W, et al. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care. 2008; 31: 899- 904.
- Lisowski LA, Verheijen PM, Copel JA, et Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz. 2010; 35: 19-26.
- Hoffman JI, Kaplan The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39: 1890-1900.
- Correa A, Gilboa SM, Besser LM, et Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008; 199: 237.e1-9.
- Sever L, Lynberg MC, Edmonds The impact of congenital malformations on public health. Teratology. 1993; 48: 547-549.
- Greene MF, Hare JW, Cloherty JP, et al. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic Teratology. 1989; 39: 225-231.
- Eriksson UJ, Wentzel P. The status of diabetic embryopathy. Ups J Med Sci. 2016; 121: 96-112.
- Radenkovic M, Stojanovic M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J Pharmacol Toxicol Methods. 2016; 78: 13-
- Ochin H, Ma X, Wang L, et al. Low dose clomiphene citrate as a mild stimulation protocol in women with unsuspected poor in vitro fertilization result can generate more oocytes with optimal cumulative pregnancy J Ovarian Res. 2018; 11: 37.
- Lazaraviciute G, Kauser M, Bhattacharya S, et al. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ ICSI compared with children conceived spontaneously. Hum Reprod Update. 2015; 21: 555-557.