Rectovaginal Carriage Rate of Group B Streptococcus and Its Associated Risk Factors among Pregnant Women in a Tertiary Hospital in Sourthern Nigeria
Author'(s): Ekerete Sunday Dan, Aniekan Monday Abasiattai* and Aniefiok Jackson Umoiyoho
Department of obstetrics/gynaecology, University of Uyo Teaching Hospital, Uyo, Nigeria.
*Correspondence:
Professor Aniekan Monday Abasiattai, Department of Obstetrics/Gynaecology, University of Uyo Teaching Hospital, Uyo-Nigeria, E-mail: animan74@yahoo.com.
Received: 02 November 2017 Accepted: 29 November 2017
Citation: Ekerete Sunday Dan, Aniekan Monday Abasiattai, Aniefiok Jackson Umoiyoho. Rectovaginal Carriage Rate of Group B Streptococcus and Its Associated Risk Factors among Pregnant Women in a Tertiary Hospital in Southern Nigeria. Gynecol Reprod Health. 2017; 1(4): 1-7.
Abstract
Background: Recto-vaginal colonization with Group B Streptococcus during pregnancy is a major cause of bacterial infection in the perinatal period resulting in urinary tract infection, premature rupture of membranes (PROM), chorioamnionitis, endometritis, bacteraemia, as well as sepsis, meningitis and pneumonia in neonates.
Objective: To determine the prevalence of Group B Streptococcus colonization and elucidate the risk factors associated with its rectovaginal colonization among pregnant women receiving antenatal care at the University of Uyo Teaching Hospital, Uyo, Nigeria.
Materials and Methods: This was a descriptive cross-sectional study conducted between May and August 2014. One hundred and fifty pregnant women who were between 35-40 weeks of gestation were purposively selected into the study. An interviewer administered questionnaire was administered to each consenting woman before vaginal and ano-rectal swab samples were collected. Standard microbiological methods were used to isolate and identify Group B Streptococcus from vaginal and ano-rectal swabs obtained.
Results: A total of 6 (4.0%) out of 150 pregnant women had recto-vaginal colonization with Group B Streptococcus. There was no significant statistical association between Group B Streptococcus colonization status and maternal sociodemographic characteristics including age (p>0.05), occupation (p>0.05), educational level (p>0.05), religion (p>0.05) and obstetric factors (obesity and gravidity) p>0.05.
Conclusion: This study revealed the prevalence of recto-vaginal Group B Streptococcus colonization among women obtaining antenatal care in our environment with the attendant risk to the fetuses in the affected population. Hence, there is need to conduct extensive epidemiological investigations in order to ascertain the actual Group B Streptococcus colonization rate, disease burden and to include routine screening of Group B Streptococcus during antenatal visits in our centre.
Keywords
Introduction
Group B streptococcus (GBS) is an encapsulated gram positive bacteria occurring either in short chains or in pairs that usually produces a narrow zone of beta-haemolysis on blood - agar and belongs to Lancefield group B [1]. They are found in the recto- vaginal area of 10-30% of healthy pregnant women [2-4], and colonization can be chronic, transient or intermittent [5]. The rate of GBS colonization in the vagina and/or rectum among pregnant women varies among communities, ethnic groups, socioeconomic status, geographic area and age [6]. More data on epidemiology of GBS are from Europe and North America [7], though cases of GBS infections and carriage have been reported from Nigeria [7-13], and other African countries [7,14-16]. Prevalence studies of GBS vaginal colonization in pregnant women conducted in different part of the country are as follows; Ile-Ife (11.3%) [7], Jos (6.6%) [11], Calabar (9%) [8,16], Maiduguri(9.8%) [10], Ibadan (17.6%) [12], Zaria (14%) [13], and Abeokuta (11%) [8]. A recent study done in Ibadan showed a decline in prevalence rate to 10% [9].
In studies carried out in other African countries, higher prevalence rates were obtained, such as: Malawi (16.5%) [14], Gambia (22.0%) [17], Zimbabwe (20-32%) [15], and Ivory Coast (19.0%) [18]. Studies from other non-African countries reported similar rates and these include; Korea (9.5%) [19], USA (12.2%) [20], Iran (9.1%) [21] and Hong Kong (10.4%) [22]. In multicenter studies conducted in the Netherlands, 29.0% was reported among African women, 13.0% among Asians and 21.0% among Europeans [23].
Others are 20.4% in Brazil [24] and 27.6% in Saudi Arabia [25].
Group B Streptococcus colonization of the vagina/rectum is associated with significant maternal peripartum diseases, including bacteraemia, endocarditis, chorioamnionitis, endometritis, urinary tract infections, arthritis and death [14,28-30]. The colonization of the vagina, perineum and rectum of pregnant women is a major risk factor for subsequent infection in their newborns [8,31,32]. About 50-60% of infants born to colonized mothers have positive GBS cultures from their skin and mucous membranes, and 1-2% of these colonized newborns develop early onset GBS Invasive Disease (EOD) [33,34]. In the newborns, GBS is responsible for serious infections such as pneumonia, septicemia and meningitis [2,35,36]. The neonates get colonized with GBS through the aspiration of infected amniotic fluid or by vertical transmission during passage through the colonized vaginal canal.
The Group B Streptococcal pathogen is the most prevalent infection in the first week of life which is associated with high mortality [37,38]. Neonates who survive are often left with developmental disabilities, including mental retardation, hearing or vision loss and speech problems.
Thus the center for disease control (CDC) and prevention, and the American College of Obstetricians and Gynecologists (ACOG) have recommended routine screening tests at 35-37 weeks of pregnancy to detect recto-vaginal colonization by GBS in all pregnant women and the use of intra-partum antibiotic prophylaxis if the screening test is positive. This recommendation has been shown to be an effective means of preventing neonatal transmission and the associated morbidity and mortality [39].
Since the establishment of our centre, there has been no known study on GBS and its risk factors. Hence there is need to estimate the prevalence and risk factors associated with GBS colonization among women receiving antenatal care at the University of Uyo Teaching Hospital as this will provide an opportunity to develop local screening and diagnostic protocols for our pregnant women. This will facilitate proper evaluation and management of these women during pregnancy and the intra-partum period to reduce the complications associated with GBS colonization of the vagina/ rectum.
Methodology
Study Area
This was a descriptive cross sectional study done between May and August 2014, in the antenatal clinic of University of Uyo Teaching Hospital, Uyo, Nigeria.
Sampling Technique
All pregnant women presenting in the antenatal clinic of the Teaching Hospital between 35 to 40 weeks gestation were assessed for eligibility to participate in the study.
Study Population
One hundred and fifty pregnant women who fulfilled the study criteria (who had not taken any antibiotics in the last 2 weeks before presentation and were in 35th to 40th week of gestation) and consented to the study were recruited.
Sample size calculation
In Calabar, the capital of Cross River State, which is located in the South-South geopolitical zone of Nigeria, the prevalence of recto- vaginal GBS colonization among pregnant women was 9.0% [8]. Uyo, in Akwa Ibom State belongs to the same geographical belt as Cross River State and are neighbouring states. The minimum sample size was calculated assuming a 95% confidence level using the formula below
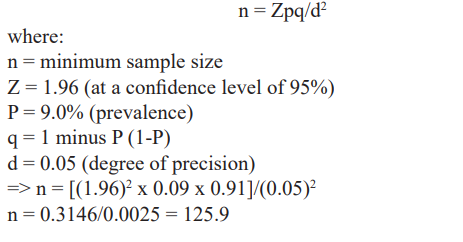
This was rounded off to 126 and 10% (approximately 13) of this number was added for attrition. Total minimum sample size was 126+13 = 139. Therefore, a total of 150 pregnant women are which is not less than 139 pregnant women’s were included in this study.
Ethical Consideration
The participation of patients was voluntary; the principle of patient confidentiality was strictly adhered to. Each participant was duly counselled and a prepared consent form was signed. Pregnant women with positive GBS culture were treated with intravenous antibiotics in labour. Neonates of these mothers were also observed for 72 hours for signs of infection before there were discharged home. Formal approval was obtained from the Ethical Research Committee of the University of Uyo Teaching Hospital, Uyo.
Specimen collection, handling and transport
Swabs were taken from both the lower vagina and rectum of each participant by inserting only 1 cm or less (of the cotton bud end) of the sterile swab stick into the vagina, and then, the rectum (inserting swab through the anal sphincter) using a different swab stick. The swabs were placed in Amies transport media and were immediately transported to the microbiology laboratory of university of Uyo teaching hospital for culture.
Socio-demographic and obstetric history about participants in the study were collected using an interviewer's administered questionnaire. The questionnaires covered suspected risk factors associated with colonization of the recto-vaginal tracts by GBS.
Culture and identification of GBS
In the laboratory, the vaginal and anorectal swabs were removed from Amies transport media and inoculated into Edward’s media supplemented with 10 microgram/ml colistin and 15 microgram/ml nalidixic acid to prevent growth of contaminants. The inoculated media were incubated for 18-24 hours at 35o-37oC in ambient air, then sub-cultured on 5% sheep blood agar plates and incubated overnight in 5% CO2 atmosphere for 18-24 hours. All suspected GBS colonies (pin point, with narrow beta-haemolysis) were subjected to gram stain and catalase test.
All gram positive and catalase negative cocci isolates were subjected to CAMP (Chritie, Atkins, Munch, Peterson) test. All CAMP test positive bacteria were subjected to latex agglutination test using PathoDXtra strep. Group B latex (oxoid, uk), for confirmation of GBS.
Data analysis
Data generated from the study were analysed using the Statistical
Package for Social Sciences (SPSS Inc; Chicago, IL, USA), version17.Descriptive Statistics was done for continuous variables while Chi-Square test was used to compare categorical variables. The level of significant was set at 5% (P<0.05).
Results
Socio-demographic characteristics
The sociodemographic characteristics of 150 pregnant women screened for GBS colonization is shown in table 1. All (100%) the respondents were Christians. Their mean age was 27.68 ± 4.76 with a range of 14-40 years; mean weight was 71.74 ± 13.34Kg with a range of 44 to 122Kg, and the mean gestational age 37.09 ± 1.58 weeks. The majority of the participants were between the ages of 25-29 years (40.0%).
The participants were from 3 major ethnic groups with the majority from Annang tribe (47.3%), followed by Ibibio (41.3%), Igbo (7.3%), and others (Oro, Hausa and Efik), which constituted 4.0%. Most study participants were in unskilled-employ group (53.3%), the rest were unemployed group (32.7%) and skilled- employ group (14.0%). The educational levels of participants were primary level of education (7.3%), secondary level of education (51.3%) and post-secondary level of education (41.3%). One hundred and twenty nine (86.0%) of the respondents were married, while single and cohabiting women constituted 3.3% and 10.7% of the study population respectively. Also, 44.7% of the participants were multigravida (carrying pregnancy for 3rd or more times), primigravida (1st pregnancy) were 24.7% and secundigravida (2nd pregnancy) were 30.7% (Table 1).
Only 16 (10.7%) of the study participants practiced one form of contraception or the other (Table 2).
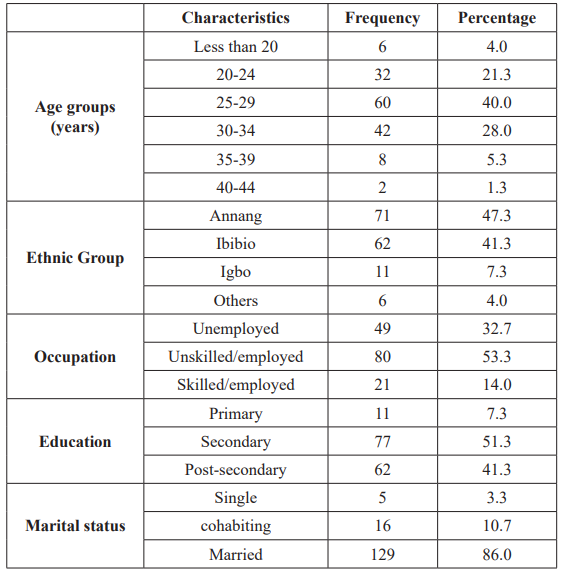
Table 1: The socio-demographic characteristics of the respondents and includes their ages, ethnic groups etc.

Table 2: the clinical/obstetric variables and here the last column (contraceptive type is not well arranged as the male condom is empty because the figures in this section are pushed up.
Overall Prevalence
A total of 6 (4.0%) out of 150 pregnant women studied were colonized with GBS (Figure 1).
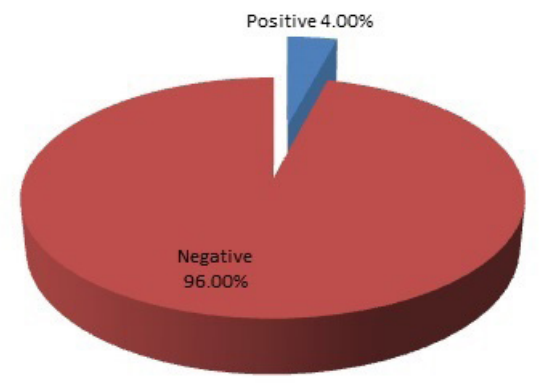
Figure 1: Prevalence of Group B streptococcus among pregnant women in UUTH.
Risk factors analysis
Different variables associated/not-associated with GBS colonization are outlined in Table 3. Among the 6 patients who were colonized with GBS, 6.3%, 3.3% and 4.8% were from age groups of 20-24, 25-29, and 30-34 years, respectively. The GBS colonization rate was higher in 20-24 years age group and least in 25-29 age groups. The frequency of GBS colonization among different age groups was not statistically significant (p=0.876).
Colonization of GBS was found to be high (6.1%) in those who were unemployed compared to those who were in unskilled-employ group(3.8%) while no GBS was isolated from those in skilled- employed group. However, the difference in GBS colonization rate among pregnant women who were unemployed and those who had jobs was not statistically significant (p=0.235).
Out of the 71 patients from Annang ethnic group, 2 (2.2%) had GBS isolated from them, while 4 (6.5%) were isolated from the participants (62) from the Ibibio ethnic group. The difference in GBS colonization was not statistically significant among different ethnic groups (p=0.183).
Among the 46 secundigravida, 4 (8.7%) were GBS positive, while 2 (3.0%) of the 67 multigravida (pregnancy of 3 or more time) were GBS colonized. No GBS was isolated among the 37 primigravida. No statistically significant association was observed for GBS colonization in the study subjects and gravidity.
Out of the participants that practiced contraception previously, none were colonized by GBS. However, the difference in GBS colonization between previous contraceptive users and non-users was not statistically significant (p=2.499)
Overall, no statistically significant association was observed for GBS colonization in the study subjects with any of the sociodemographic characteristics and clinical variables stated in table 3 on the next page.
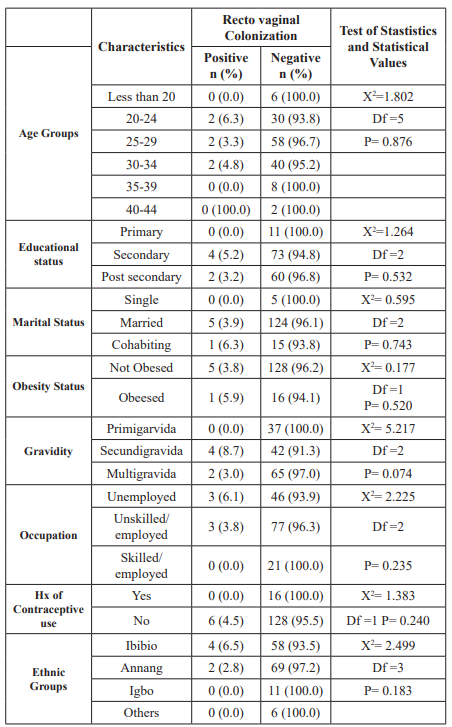
Table 3: Association between sociodemographic and clinical characteristics and recto vaginal colonization by GBS among respondents.
Discussion
In this study the overall prevalence of GBS colonization among pregnant women was 4.0%. This is low when compared to a study conducted among pregnant women in Calabar, Cross River State, which was 9.0% [8]. The prevalence of GBS colonization in this study is also lower than those obtained from other Nigerian centres [7-13]. The low colonization rate of GBS among pregnant women in this study is consistent with findings from studies done in Isreal (3.5%) [40], Togo (4.0%) [41], Jos, Nigeria (6.6%) [11], and Lima, Peru (6.0%) [42].
This low prevalence of GBS colonization is intriguing. Speculations include cultural, customs and personal hygienic habits. For instance, women in our environment are known to wash their vagina and perineal area thoroughly with water and soap regularly. This may influence the flora of the urogenital tract [43]. More so, uncircumcised men are more likely to contribute to GBS colonization44. In other countries with a high circumcision rate, such as Isreal and Turkey, GBS Carriage rates in pregnant women were also low, 3.5% [40] and 8.0% [45], respectively. This present study was carried out in a Christian population where all respondents and probably their spouses were Christians. All male Christians are circumcised. This could account for the low GBS colonization rate among their spouse in this study.
The lower prevalence rate found for GBS colonization in this study might also be due to the fact that all participants had formal education which could have had a positive impact on their awareness, sexual exposure and level of hygiene. Also, this study was conducted in a tertiary hospital where pregnant women receive routine health talks on nutrition, personal hygiene and other method of infection prevention by the midwives. This may have contributed to the low colonization rate of GBS.
The prevalence of GBS in our study is however, high when compared to the colonization rate from Maputo in Mozambique (1.8%) [46] and Rosario in Argentina (3.2%) [47]. The difference in colonization rate in the study reported from Mozambique and our study may be due to source of blood (human blood versus sheep blood agar) used to culture GBS among others like genetics and geographic differences.
Overall, the differences in colonization rate might be due to genetic differences, geographical location, the characteristics of the study population and laboratory diagnostic methods including time and site of sample collection [6].
The prevalence rate of 4.0% is low compared to the assumed prevalence of 9% [8], used in calculating the sample size. The difference in colonization rate might be due to methods of sample collection, laboratory diagnostic technique and characteristics of the population studied. The Calabar study used a neomycin sheep blood agar, while this study used Edward media with colistin. This study is tertiary hospital based while that of Calabar was a multicentre study (2 peripheral hospitals in addition to the teaching hospital). Also, the Calabar study used a larger sample size than that of this study. These could account for the difference in the prevalence rate between these two studies.
Single vaginal culture and lack of rectal culture has been documented as the cause of low prevalence in some studies [21]. However, the low yield of 4.0% in this study is not a result of single culture since both vaginal and rectal samples were cultured. Secondly, the CDC has recommended the use of selective enrichment broth to maximize the isolation of GBS, because when direct agar plating is used instead of selective enrichment broth, approximately 50% of women who are GBS carriers can be missed [48]. However, in this study despite using selective enrichment broth, (Edward media, modified), we observed GBS colonization only in a small number of pregnant women, suggesting that maternal colonization with GBS is low in U.U.T.H.
Knowledge about risk factors contributing to GBS colonization in pregnant women is relevant to minimize the morbidity and mortality associated with maternal and neonatal GBS infections. In the present study, no statistically significant association was observed for GBS colonization in the study subjects with any of the sociodemographic/obstetrics characteristics. Similar findings have been reported in previous studies [7,24,42,49,50]. However studies conducted in Athens (Greece) [51] and Hong kong [22] showed that GBS colonization rate was high among pregnant women who worked outside their home and those who had frequent visits to the antenatal clinic [22,51]. A study conducted in Maiduguri revealed significant statistical association between GBS colonization and sociodemographic variables among pregnant women [10].
It is observed in our study that secundigravid women were more often associated with GBS colonization, likewise women from the Ibibio ethnic group. However, there was no statistically significant association with these variables. Therefore, a prevention strategy in the antenatal population cannot safely rely on risk factor approach for identification of GBS colonized mothers, since there is no statistically significant association between GBS colonization and the socio-demographic / obstetric factors.
This study had some limitations: Sero-typing was not performed due to lack of group specific anti-sera, the antibiotic susceptibility pattern of the isolated GBS were not done due to short duration of the study and the sample size was small.
Conclusion
In conclusion, the low carriage rate of GBS and insignificant association with suspected risk factors in this study need to be further verified through an expanded study with many more women and at several sites within Uyo with the same study protocol. Therefore a higher sample size than that used in this study, may have yielded a larger number of GBS colonized women and probably a statistically significant relationship between GBS colonization and socio-demographic / obstetric factors. However, the establishment of GBS colonization in pregnant women calls for a review of the present hospital policy on antenatal care to include routine screening and reporting of GBS prevalence.
References
- Edward MS, Baker CJ. Streptococcus agalactiae (Group B Strep) in: Mandell GL, Bennett JE, Dolin R, Principles and practice of infectious disease. 7th ed. Elsevier: Churchill Livingstone; 2010.
- Motlova J. Strakova L, Urbaskova P, et al. Vaginal and rectal carriage of streptococcus agalactiae in the Czech Republic: Incidence, serotypes distribution and susceptibility to Indian Journal of Medical Research. 2004; 119: 84-87.
- Annie Rajaratnam, Thomas Kuruvilla, Beena Prevalence of group b streptococcal colonization among pregnant Women in a tertiary care hospital in coastal karnataka. Inter J Applied Biology and pharm Tech. 2013; 4: 308-310.
- Nandyal Update on GBS infection: Perinatal and neonatal periods. J. Perinate Neonatal nurs. 2009; 22: 2307.
- Turrentine MA, Ramirez Recurrence of Group B Streptococcus Colonization in subsequent pregnancy. Obstet Gynecol. 2008; 112: 259-264.
- Elbaradie SM, Mahmoub M, Farid M. maternal and neonatal screening for Group B Streptococcus by SCP B gene base PCR: a preliminary study. Indian J Med Microbial. 2009: 27:17-21.
- Onipede A, Adefusi O, Adeyemi A, et al. Group B Strept carriage during late preganancy in Ile-Ife, Afri Journal of Clinical and Experimental Microbiology. 2012; 13:135-143.
- Nwachukwu NC, Utsalo SJ, Kanu I, et Genital Colonization of Group B Streptococcus at Term Pregnancy in Calabar, Nigeria. The Internet Journal of Pediatrics and Neonatology. 2007; 7: 9.
- Donbraye-Emmanuel OOB, Okonko ID, Donbraye E, et al. Isolation and characterization of Group B Streptococcus and other pathogens among pregnant women in Ibandan South West Journal of Applied Biosciences. 2010; 29:1781- 1792.
- Okon KO, Usman H, Umar Z, et al. Prevalence of group B streptococcus colonization among pregnant women attending antenatal clinic of a tertiary Hospital in North Eastern American J of Research Communication. 2013; 1: 54-66.
- Nsagha DS, Bello CSS, Kahdakaiolukemi Maternal carriage in pregnancy of Group B Streptococcus in Jos: Relation of endocervical – anorectal colonization. Nig. Qt.J.Hosp.Med. 1997; 7: 53-56.
- Onile BA. Group B Streptococcus carriage in Nigeria. Trans. Sco. Trop.Med & Hyg. 1980; 74: 367-370.
- Uhiara JE. Group B Streptococcal carriage among parturient and their neonates in Zaria, Afr.J.Med. 1993; 22: 79- 83.
- Dzowela T, Komolafe OO, Igbigbi A. Prevalence of GBS colonization in antenatal women at the Queen Elizabeth Central Hospital, Blantyre – A preliminary study. Malawi medical journal. 2005; 17: 97-99.
- Moyo SR, Modzori J, Tswana SA, et al. Prevalence, capsular type distribution, anthropometric and obstetric factors of group B streptococcus (Streptococcus agalactiae) colonization in pregnancy. Central Africa Journal of Medicine. 2000; 40: 115-120.
- Shabayek SA, Abdalla SM, Abouzeid AM. Vaginal carriage and antibiotic susceptibility profile of group B Streptococcus during late pregnancy in Ismailia, J Infect Public Health. 2009; 2: 86-90.
- Suara RO, Adegbola RA, Baker CJ, et Carriage of group B streptococcus in pregnant Gambian mothers and their infants. J Infect Dis. 1994; 170: 1316-1319.
- Stoll J, Schuchat A. Maternal carriage of group B streptococcus in developing countries. Paediatrics Infectious Disease Journal. 1998; 17: 499-503.
- Uh Y, Jang IH, Hwang GY, et al. Emerging erythromycin resistance among group B streptococci in Korea. Eur J Clin Microbiol Infect Dis. 2001; 20: 52-54.
- Chohan L, Hollier LM, Bishop K, et al. Patterns of antibiotic resistance among group B streptococcus isolates: 2001-2004. Infect Dis Obstet 2006: 57492.
- Mansouri S, Ghasami E, Shahabi N. Vaginal Colonization of Group B Streptococci during Late Pregnancy in Southeast of Iran: Incidence, Serotype Distribution and Susceptibility to J Med Sci. 2008; 8: 574-578.
- Tsui NH, Ip N, Ng PL, et Streptococcal maternal colonization in Hong Kong. Hong Kong Med J. 2009; 15: 414-419.
- Vander Berg, Sprij AJ, Ootrvigel FM, et al. Prevalence of colonization with group B streptococcus in pregnant women of a multi-ethnic population in the European Journal of Obstetrics and Gyneacology and Reproductive Biology. 2006; 124: 178-183.
- Costa AL, Lany F, Chein MB, et Prevalence of colonisation by group B streptococcus in pregnant women from public maternity of northwest region of Brazil. Rev Bras Gynecol. Obstet. 2008; 30: 274-280.
- El-Kersh TA, Al-Nuaim LA, Kharfy TA, et al. Detection of genital colonization of group B streptococcus during late Saudi Med J. 2002; 23: 56-61.
- Joachim A, Matee MI, Massawe FA, et al. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial BMC Public Health. 2009; 9: 437.
- Moyo Studies on streptococcus agalactiae surface anchored makers with emphasis on strains and human sera from Zimbabwe Ph.D. Thesis. Norwegian University of Science and Technology. 2002; 45-48.
- Tazi A, Curedet T, Varon E, et al. Fluoroquinolone – resistant GBS in acute exacerbation of chronic bronchitis Emerging Infectious Diseases. 2008; 14: 349–350.
- Phares CR, lynfield R, Farley MM, et al. Epidemiology of invasive GBS disease in the United States 1999-2005. JAMA 299: 2056-2065.
- Sendi P, Johnasson L, Dahesh S, et al. Bacterial Phenotype Variants in Group B Streptococcus toxic shock syndrome. Emerging infectious disease. 2009; 15: 223-232.
- Larsen JW, Sever JL. Goup B Streptococcus and pregnancy. AM J obstet Gynecol. 2010; 198: 440-448.
- Petterson Perinatal infection with Group B Streptococcus Semin fetal Neonate M. 2010; 12: 193-197.
- Shet A, Ferrieri Neonatal and maternal Group B Streptococcus infections: A comprehensive review. Indian J. med. Res. 2004; 120: 141-150.
- Natarajan G, Johnson YR, Zhang F, et Real-time polymerase chain reaction for rapid detection of Group B Streptococcus Colonization in neonate. Paediatrics. 2006; 118: 14-22.
- Artz LA, Kempf VAJ, Autenvieth Rapid Screening for Streptococcus agalactiae in vaginal specimens of pregnant women by fluorescent in-situ hybridization. Journal of clinical microbiology. 2003; 41: 2170- 2173.
- Tor-Udom S, Tor-Udom P, Hiviote W. The prevalence of Streptococcus agalactiae group B colonization in pregnant women at Thammasat Journal of the Medical Association of Thailand. 2006; 89: 411-414.
- James DK, Steer PH, Weiner CP, et al. High Risk Pregnancy management option. 3rd ed. Philadelphia: Elsevier Saunders. 2005; 674-690.
- Behrman RE, Kliegman Nelson Textbook of paediatrics. 7th ed. Philadelphia: Elsevier Saunders. 2004; 879–883.
- Cunningham FG, Williams JW. Williams Obstetrics. 22nd New York, McGraw-Hill: Medical Pub. Division. 2005; 39–90.
- Eidelman AI, Rudensky B, Turgeman D, et al. Epidemiology of GBS colonization and disease in mother s and infants: update of ongoing 10-year Jerusalem Israel J of medical sciences. 1990; 26: 71-73.
- Stoll BJ, Schuchat Maternal carriage of GBS in developing countries. International Paed Disease J. 1998; 17: 499-503.
- Collins TS, Calderon M, Gilman RH, et al. GBS colonization in a developing country: its association with sexually transmitted diseases and socioeconomic factors. Am J Trop. Hyg. 1998; 59: 633-636.
- Cottrel Vaginal douching. J of Obst Gynaecologic Neonatal Nursing. 2003; 32: 12-18.
- Yamamoto T, Nagasawa I, Nojima M, et Sexual transmission and reinfection of GBS between spouses. The J of Obst and Gynae. Research. 1999; 25: 215-219.
- Barbaros I, Murat C, Mehmet V, et The colonization incidence of GBS in pregnant women and their newborns in Istanbul. Paediatrics International. 2005; 47: 64-66.
- De Steenwinkel DO, Tak Hu, Muller AE, et al. Low carriage rate of GBS in pregnant women in Maputo, Mozambique. Trop Med Int health. 2008; 13: 427-429.
- Toresani I, Limansky A, Bogado I, et al. Phenotypic and genotypic study of streptococcus agalactiae in vagina of pregnant women in Artigentia. MEDICINA. 2001; 61: 295-
- Schrag S, Gorwitz R, Fultz-Buttz K, et al. Prevention of perinatal GBS Revised Guidelines from Centre for disease control and prevention. Morbidity and mortality weekly report MMWR, Recommendation Report. 2002; 5: 11-12.
- Zusman AS, Baltimore RS, Fosica Prevalence of maternal GBS colonization and related risk factors in a Brazilian population. Braz J Infect Dis. 2006; 10: 242-246.
- Musa Mohammed, Daniel Asrat, Yimtubezinash Woldeamanuel, et GBS colonization among pregnant women attending antenatal clinic of Hawassa Health centre, Hawassa, Ethiopia. Ethiop. J Health Dev. 2012; 26: 36-42.
- Tsolia M, Psoma M, Gavrili S, et al. GBS colonization of Greek pregnant women and neonates: prevalence, risk factors and Clin Microbiol Ifect. 2003; 9: 832-838.